Development of Mucoadhesive Chitosan Derivatives for Use as Submucosal Injections
Abstract
:1. Introduction
2. Chitosan-Based Biomaterials as Biological Adhesives and Hemostats
3. Biomaterials for Submucosal Injection
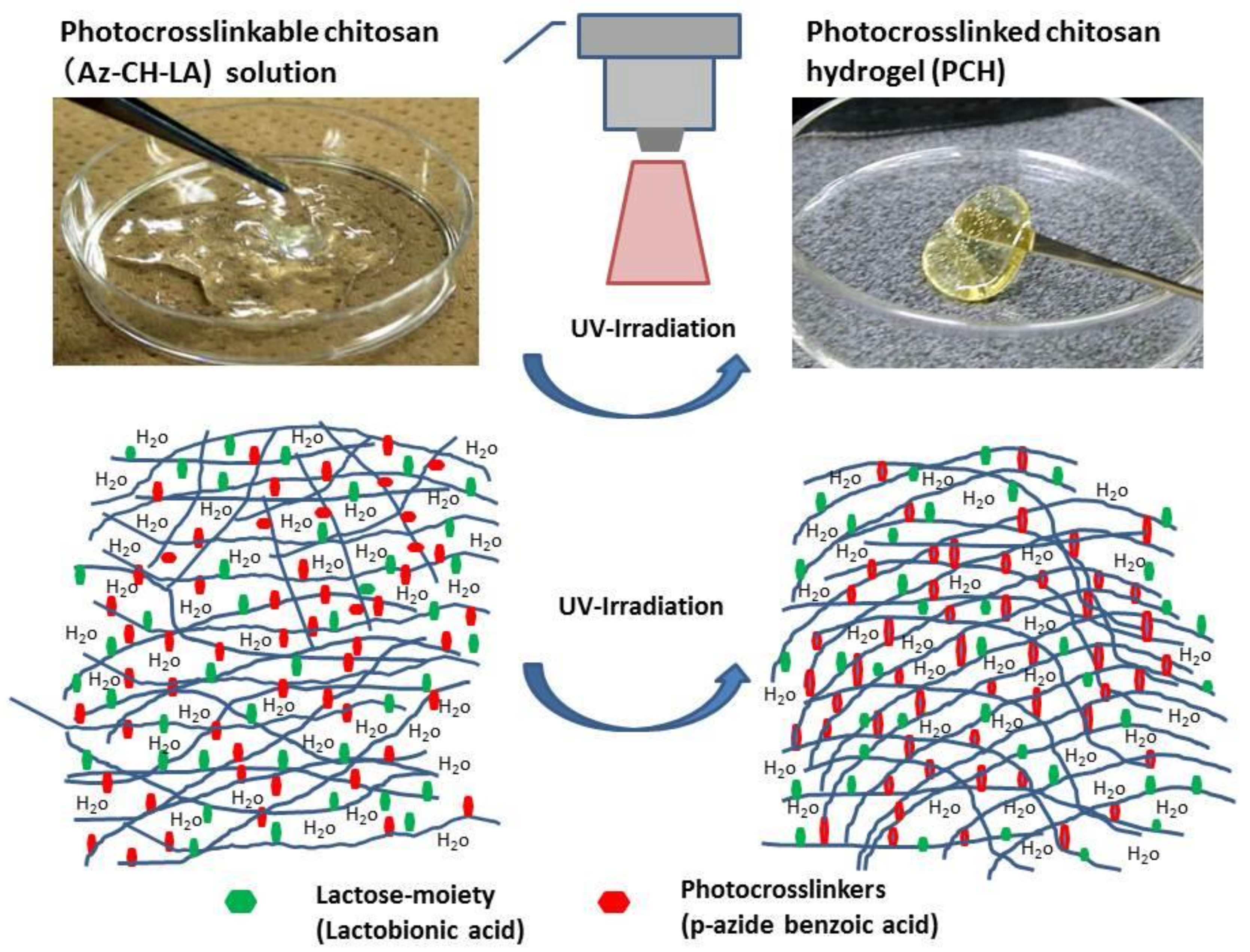
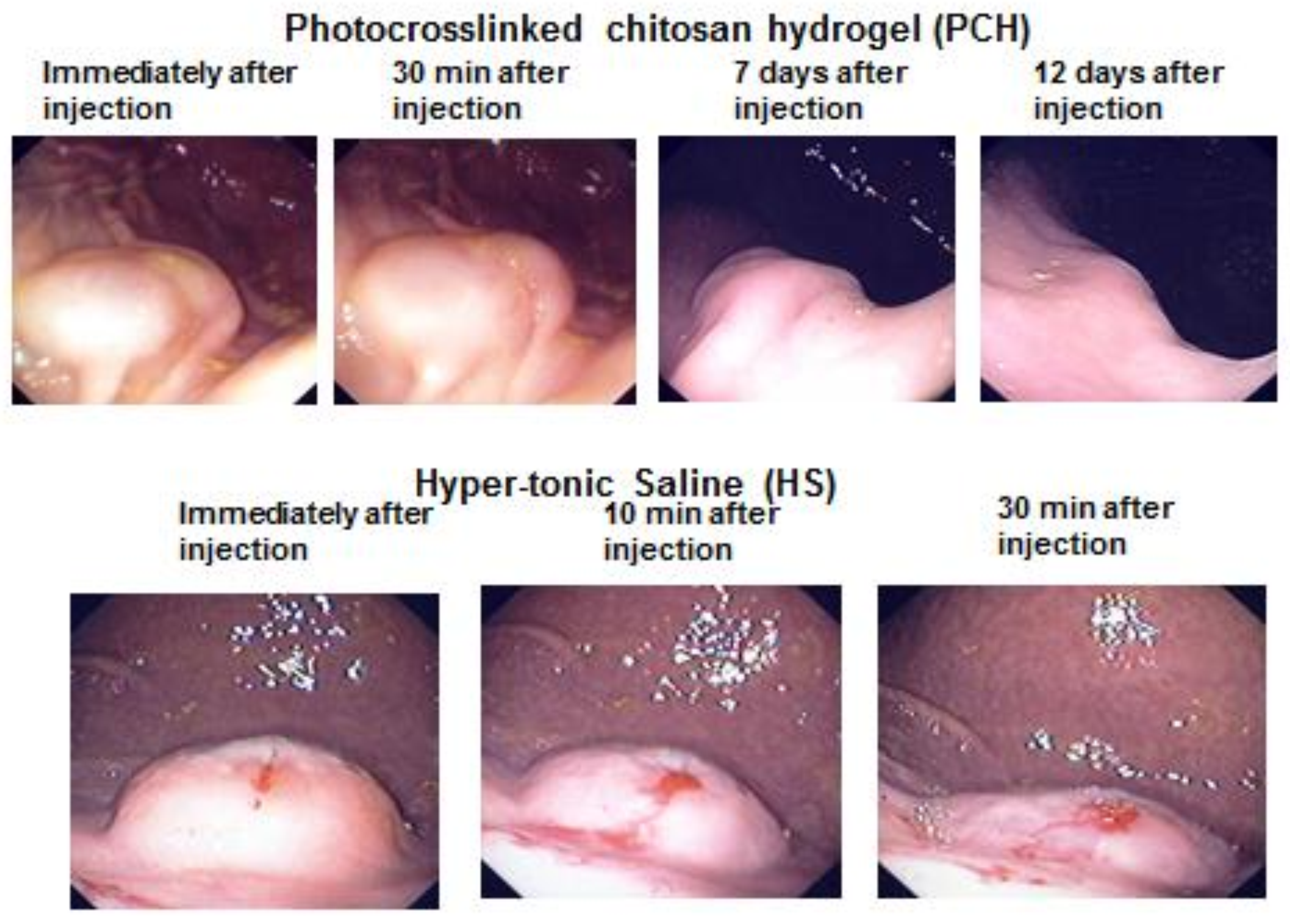
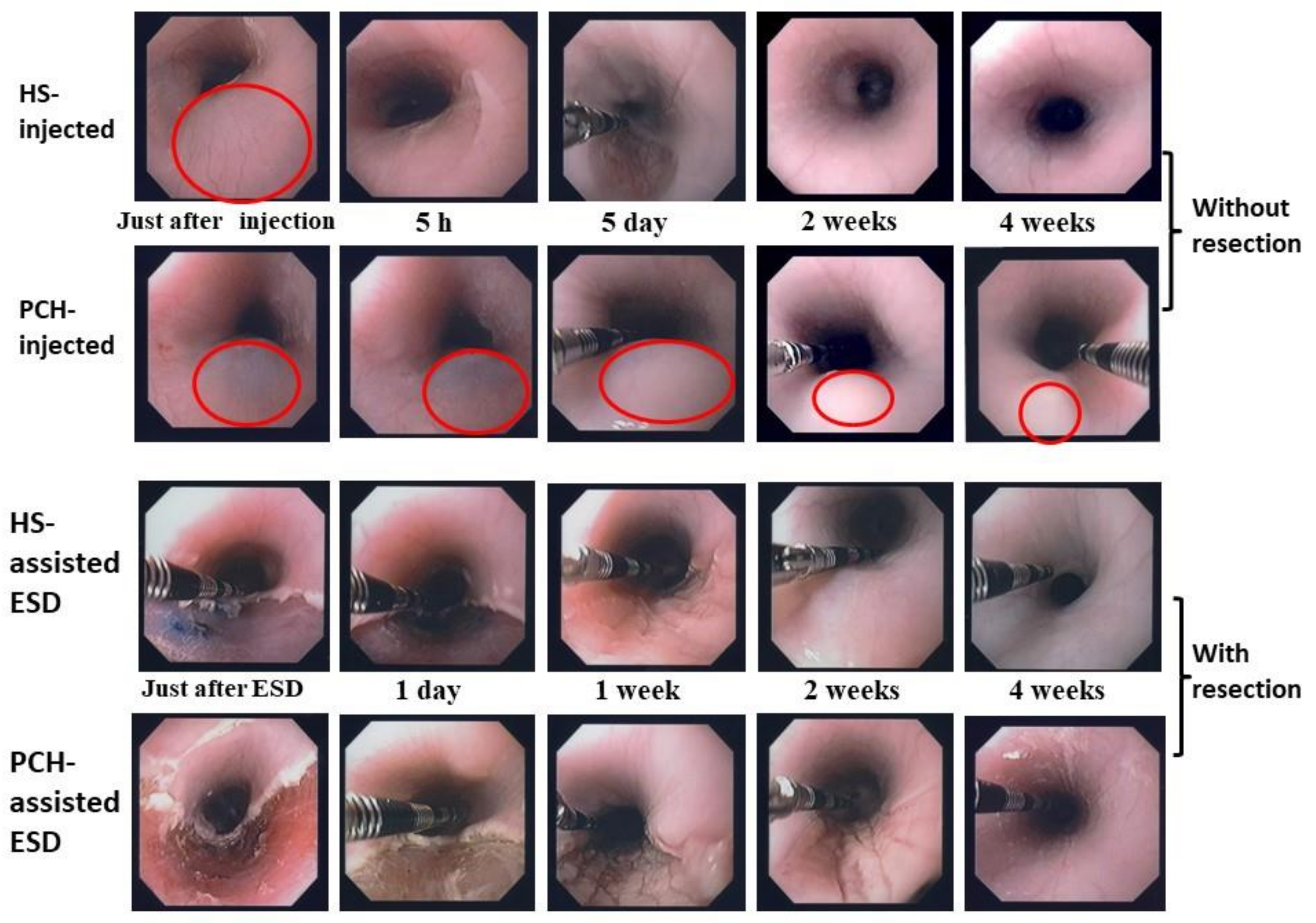
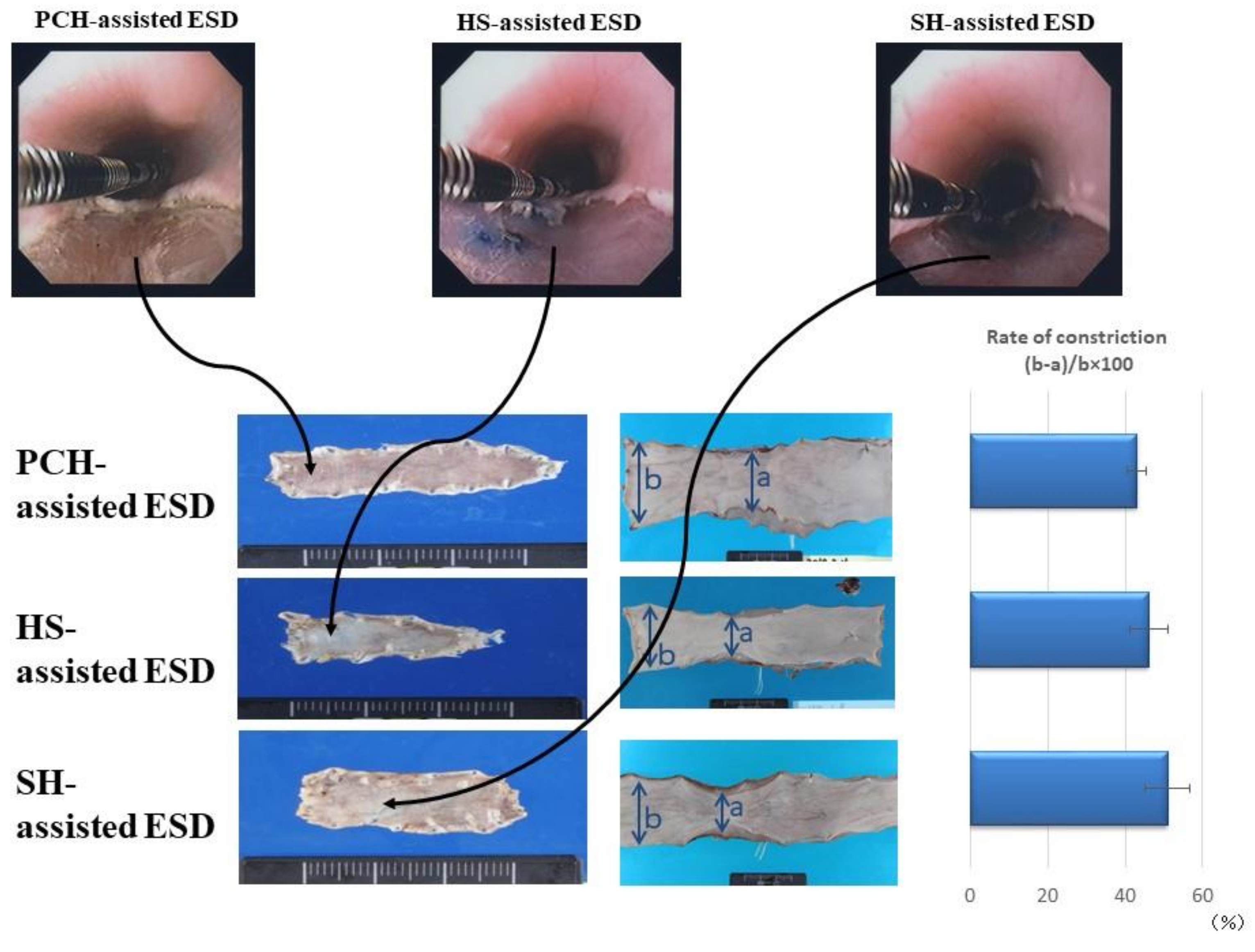
4. Photocrosslinked Chitosan Hydrogel (PCH) for Submucosal Injection
5. Structure of the GI Mucosa and Formation of Polyelectrolyte Complexes
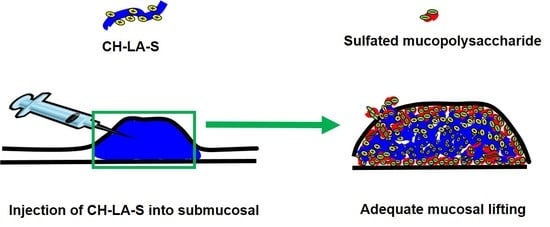
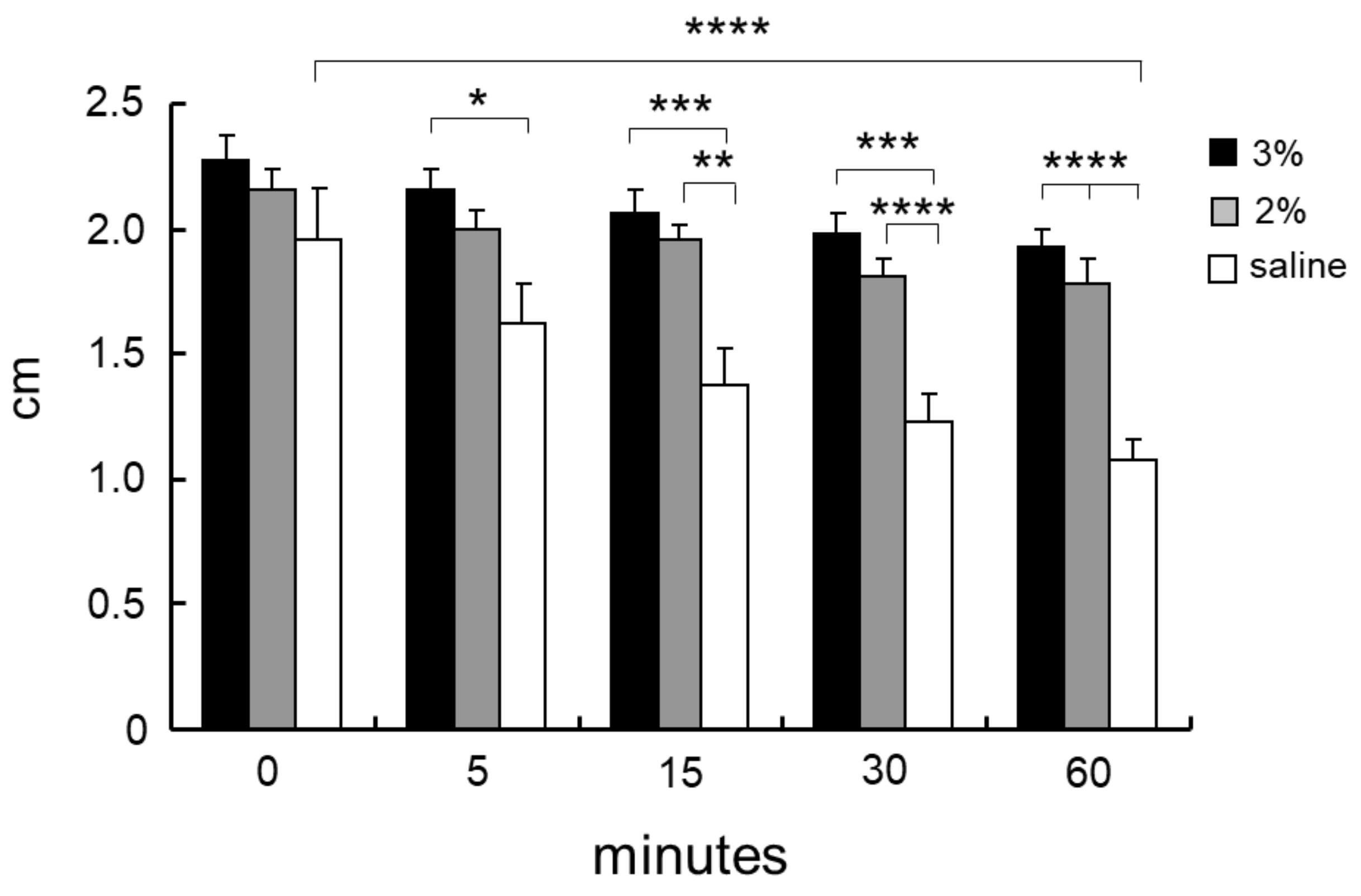
6. Soluble Chitosan Derivatives for Submucosal Injection
7. Summary
Author Contributions
Conflicts of Interest
References
- Uedo, N.; Takeuchi, Y.; Ishihara, R. Endoscopic management of early gastric cancer: Endoscopic mucosal resection or endoscopic submucosal dissection: Data from a Japanese high-volume center and literature review. Ann. Gastroenterol. 2012, 25, 281–290. [Google Scholar] [PubMed]
- Shim, C.S. Endoscopic mucosal resection: An overview of the value of different techniques. Endoscopy 2001, 33, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Abdelfatah, M.M.; Othman, M.O. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Ann. Cardiothorac. Surg. 2017, 6, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, M.; Yahagi, N.; Kashimura, K.; Matsuura, T.; Nakamura, M.; Kakushima, N.; Kodashima, S.; Ono, S.; Kobayashi, K.; Hashimoto, T.; et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest. Endosc. 2005, 62, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Ishihara, M.; Aiko, S.; Nogami, Y.; Nakamura, S.; Kanatani, Y.; Kishimoto, S.; Hattori, H.; Horio, T.; Tanaka, Y.; et al. Experimental evaluation of photocrosslinkable chitosan hydrogel as injection solution for endoscopic resection. Endoscopy 2009, 41, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.M.; Zhang, X.Q.; Chen, M.; Huang, S.; Zou, X.P. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J. Gastroenterol. 2014, 20, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Kumano, I.; Ishihara, M.; Nakamura, S.; Kishimoto, S.; Fujita, M.; Hattori, H.; Horio, T.; Tanaka, Y.; Hase, K.; Maehara, T. Endoscopic submucosal dissection for pig esophagus by using photocrosslinkable chitosan hydrogel as submucosal fluid cushion. Gastrointest. Endosc. 2012, 75, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Feitoza, A.B.; Gostout, C.J.; Burgart, L.J.; Burkert, A.; Herman, L.J.; Rajan, E. Hydroxypropyl methylcellulose: A better submucosal fluid cushion for endoscopic mucosal resection. Gastrointest. Endosc. 2003, 57, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Yongping, S.; Cheng, T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.J.; Chen, S.Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. B 2008, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Ishihara, M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015, 10, 015014. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ishihara, M.; Hattori, H.; Nakamura, S. A review on biomedical applications of chitosan-based biomaterials. Int. J. Pharm. Biol. Sci. 2015, 6, 162–178. [Google Scholar]
- Desbrières, J.; Martinez, C.; Rinaudo, M. Hydrophobic derivatives of chitosan: Characterization and rheological behavior. Int. J. Biol. Macromol. 1996, 19, 21–28. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Morimoto, M.; Saimoto, H.; Usui, H.; Okamoto, Y.; Minami, S.; Shigemasa, Y. Biological activities of carbohydrate-branched chitosan derivatives. Biomacromolecules 2001, 2, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Chou, C.C.; Li, C.F. Preparation, water solubility and rheological property of the N-alkylated mono or disaccharide chitosan derivatives. Food Res. Int. 2002, 35, 707–713. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.F.; Tan, H.M. Hydroxyethyl chitosan-g–poly (acrylic acid-co-sodium acrylate) super absorbent polymers. J. Appl. Polym. Sci. 2010, 117, 2233–2240. [Google Scholar]
- Peng, Y.F.; Han, B.Q.; Liu, W.S.; Xu, X.J. Preparation and antimicrobial activity of hydroxypropyl chitosan. Carbohydr. Res. 2005, 340, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Mura, C.; Nacher, A.; Merino, V.; Merino-Sanjuan, M.; Carda, C.; Ruiz, A.; Manconi, M.; Loy, G.; Fadda, A.M.; Diez-Sales, O. N-succinyl-chitosan systems for 5-aminosalicylic acid colon delivery: In vivo study with TNBS-induced colitis model in rats. Int. J. Pharm. 2011, 416, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Saito, Y.; Yura, H.; Ishikawa, K.; Kurita, A.; Akaike, T.; Ishihara, M. Photocrosslinkable chitosan as a biological adhesive. J. Biomed. Mater. Res. 2000, 49, 289–295. [Google Scholar] [CrossRef]
- Ishihara, M. Photocrosslinkable chitosan hydrogel as a wound dressing and biological adhesive. Trends Glycosci. Glycotechnol. 2002, 14, 331–341. [Google Scholar] [CrossRef]
- Ishihara, M.; Obara, K.; Nakamura, S.; Fujita, M.; Masuoka, K.; Kanatani, Y.; Takase, B.; Hattori, H.; Morimoto, Y.; Ishihara, M.; et al. Chitosan hydrogel as a drug delivery carrier to control angiogenesis. J. Artif. Org. 2006, 9, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Ishihara, M.; Fujita, M.; Kanatani, Y.; Hattori, H.; Matsui, T.; Takase, B.; Maehara, T. Acceleration of wound healing in healing-impaired db/db mice with photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2. Wound Repair Regen. 2005, 13, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Tsujimoto, H.; Hase, K.; Ishihara, M. Characterization of a water-soluble chitosan derivative and its potential for submucosal injection in endoscopic techniques. Carbohydr. Polym. 2017, 175, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ishihara, M.; Ozeki, Y.; Deguchi, H.; Sato, M.; Saito, Y.; Yura, H.; Sato, M.; Kikuchi, M.; Kurita, A.; et al. Experimental evaluation of photocrosslinkable chitosan as a biological adhesive with surgical application. Surgery 2001, 130, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Otani, Y.; Tabata, Y.; Ikada, Y. A new biological glue from gelatin and poly l-glutamic acid. J. Biomed. Mater. Res. 1996, 31, 158–166. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Hyon, S.H.; Ikada, Y. Modification of synthesis and investigation of properties for 2-cyanoacrylates. Biomaterials 1990, 11, 73–79. [Google Scholar] [CrossRef]
- Vanholder, R.; Misotten, A.; Roels, H.; Matton, G. Cyanoacrylate tissue adhesive for closing skin wounds: A double blind randomized comparison with suture. Biomaterials 1993, 14, 737–742. [Google Scholar] [CrossRef]
- Pederson, T.B.; Hongel, J.L.; Pilegaard, H.K.; Hasenkam, J.M. Comparative study of lung sealants in porcine ex vivo model. Ann. Thorac. Surg. 2012, 94, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Moy, O.J.; Peimer, C.A.; Koniuchi, M.P.; Hoeard, C.; Zielezny, M.; Katikaneni, P.R. Fibrin seal adhesive versus nonadsorbable microsuture in peripheral nerve repair. J. Hand Surg. 1988, 13, 273–278. [Google Scholar] [CrossRef]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapid curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hematostatic materials. Boimacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Obara, K.; Ishizuka, T.; Fujita, M.; Sato, M.; Masuoka, K.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled release of fibroblast growth factors and heparin from photocrosslinked chitosan hydrogels and subsequent effect on in vivo vascularization. J. Biomed. Mater. Res. A 2003, 64, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, K.; Ishihara, M.; Asazuma, T.; Hattori, H.; Matsui, T.; Takase, B.; Kanatani, Y.; Fujita, M.; Saito, Y.; Yura, H.; et al. Interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials 2005, 26, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ishihara, M.; Shimizu, M.; Obara, K.; Ishizuka, T.; Saito, Y.; Yura, H.; Morimoto, Y.; Takasem, B.; Matsui, T.; et al. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials 2004, 25, 699–706. [Google Scholar] [CrossRef]
- Hirao, M.; Masuda, K.; Asanuma, T.; Naka, H.; Noda, K.; Matsuura, K.; Yamaguchi, O.; Ueda, N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest. Endosc. 1988, 34, 264–269. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Cuy, J.L.; Beckstead, B.L.; Brown, C.D.; Hoffman, A.S.; Giachelli, C.M. Adhesive protein interactions with chitosan: Consequences for valve endothelial cell growth on tissue-engineering materials. J. Biomed. Mater. Res. A 2003, 67, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Katsinelos, P.; Kountouras, J.; Paroutoglou, G.; Chatzimavroudis, G.; Zavos, C.; Pilpilidis, I.; Gelas, G.; Paikos, D.; Karakousis, K. A comparative study of 50% dextrose and normal saline solution on their ability to create sbmucosal fluid cushions for endoscopic resection of sessile rectosigmoid polyps. Gastrointest. Endosc. 2008, 68, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Esparrach, G.; Cuatrecasas, M.; Rodríguez de Miguel, C.; Sánchez-Montes, C.; Córdova, H. Efficacy and safety of a combination of hyaluronic acid, chondroitin sulfate, and poloxamer 407 as a submucosal injection solution for endoscopic resection: Pilot study on a swine model. Endosc. Int. Open 2017, 5, E450–E454. [Google Scholar] [PubMed]
- Yamamoto, H.; Yube, T.; Isoda, N.; Sato, Y.; Sekine, Y.; Higashizawa, T.; Ido, K.; Kimura, K.; Kanai, N. A novel method of endoscopic mucosal resection using sodium hyaluronat. Gastrointest. Endosc. 1999, 50, 251–256. [Google Scholar] [CrossRef]
- Yamamoto, H.; Koiwai, H.; Yube, T.; Isoda, N.; Sato, Y.; Sekine, Y.; Higashizawa, T.; Utsunomiya, K.; Ido, K.; Sugano, K. A successful single-step endoscopic resection of a 40 millimeter flat-elevated tumor in the rectum: Endoscopic mucosal resection using sodium hyaluronate. Gastrointest. Endosc. 1999, 50, 701–704. [Google Scholar] [CrossRef]
- Matsui, Y.; Inomata, M.; Izumi, K.; Sonoda, K.; Shiraishi, N.; Kitano, S. Hyaluronic acid stimulates tumor-cell proliferation at wound sites. Gastrointest. Endosc. 2004, 60, 539–543. [Google Scholar] [CrossRef]
- Walker, K.J.; Madihally, S.V. Anisotropic temperature sensitive chitosan-based injectable hydrogels mimicking cartilage matrix. J. Biomed. Mater. Res. B 2015, 103, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Moon, H.J.; Park, J.H.; Shinde, U.P.; Ko, D.Y.; Jeong, B. PEG poly (l-alanine) thermogel as a 3D scaffold of bone-marrow derived mesenchymal stem cells. Macromol. Biosci. 2015, 15, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for dru delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, R.; Hua, X.; Chen, H.; Xu, J.; Wu, R.; Cen, L. Highly stretchable HA/SA hydrogels for tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Biencowe, A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Vesselov, L.M.; Whittington, W.; Lilge, L. Performance evaluation of cylindrical fiber optic light diffusers for biomedical applications. Laser Surg. Med. 2004, 34, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Amano, Y.; Nogami, Y.; Takase, B.; Ishihara, M. Hemostasis for severe hemorrhage with photocrosslinkable chitosan hydrogel and calcium alginate. Ann. Biomed. Eng. 2010, 38, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Amano, Y.; Nogami, Y.; Kawakami, M.; Yura, H.; Ishihara, M. Development of a novel emergency hemostatic kit for severe hemorrhage. Artif. Org. 2013, 37, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Kanatani, Y.; Ishizuka, T.; Nogami, Y.; Nakamura, S.; Tanaka, Y.; Maehara, T. Hemostatic effects of photocrosslinkable chitosan hydrogel-mixed photocrosslinked chitosan sponges (PCM-S) on hepatic bleeding in rats. Artif. Org. 2010, 34, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Allen, A. Structure and function of gastrointestinal mucus. In Physiology of the Gastroenterology Tract, 1st ed.; Johnson, L., Ed.; Raven Press: New York, NY, USA, 1981; pp. 617–639. [Google Scholar]
- Johansson, M.E.; Hansson, G.C. Mucus and the goblet cell. Dig. Dis. 2013, 31, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Rossen, J.; Buller, H.; Einerhand, A. The MUC family: An obituary. Trends Biochem. Sci. 2002, 27, 126–131. [Google Scholar] [CrossRef]
- Bell, S.; Xu, G.; Khatri, I.; Wang, R.; Rahman, S.; Forstner, J. N-linked oligosaccharides play a role in disulphide-dependent dimerization of intestinal mucin Muc2. Biochem. J. 2003, 373 Pt 3, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vilar, J.; Hill, R. The structure and assembly of secreted mucins. J. Biol. Chem. 1999, 274, 31751–31754. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.; Bhaskar, K.; Hadzopoulou-Cladaras, M.; LaMont, J. Cysteine-rich regions of pig gastric mucin contain von Willebrand factor and cysteine knot domains at the carboxyl terminal. Biochim. Biophys. Acta 1999, 144, 777–792. [Google Scholar] [CrossRef]
- Bell, S.; Xu, G.; Forstner, J. Role of the cystine-knot motif at the C-terminus of rat mucin protein Muc2 in dimer formation and secretion. Biochem. J. 2001, 357 Pt 1, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Kirkham, S.; Howard, M.; Woodman, P.; Kutay, S.; Brazeau, C.; Buckley, J.; Thornton, D.J. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J. Biol. Chem. 2004, 279, 15698–15705. [Google Scholar] [CrossRef] [PubMed]
- Schonherr, E.; Hausser, H.-J. Extracellular matrix and cytokines: A functional unit. Dev. Immunol. 2000, 7, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Prydz, K. Determinants of glycosaminoglycan (GAG) strucuture. Biomolecules 2015, 5, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Ishihara, M.; Takikawa, M.; Mori, Y.; Hattori, H.; Fujita, M.; Nakamura, S. Novel experimental and clinical therapeutic uses of low-molecular-weight heparin/protamine microparticles. Pharmaceutics 2012, 4, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kanatani, Y.; Kishimoto, S.; Nambu, M.; Ohno, C.; Hattori, H.; Takase, B.; Tanaka, Y.; Yura, H.; Kiyosawa, T.; et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J. Biomed. Mater. Res. A 2009, 91, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nakamura, S.; Kishimoto, S.; Kawakami, M.; Suzuki, S.; Matsui, T.; Ishihara, M. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int. J. Nanomed. 2010, 5, 147–155. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Wang, G.; Xiao, Y.; Zhang, B.; Qi, N. Biocompatibility evaluation of chitosan-based injectable hydrogels for culturing mice mesenchymal stem cell in vitro. J. Biomater. Appl. 2010, 24, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fernandes, A.B.; Pelosi, P.; Rocco, P.R. Bench-to-bedside review: The role of glycosaminoglycans in respiratory disease. Crit. Care 2006, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Huttner, W.B. Tyrosine sulfation and the secretory pathway. Ann. Rev. Physiol. 1988, 50, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Lipmann, F. Isolation of tyrosine-O-sulfate by pronase hydrolysis from fibronectin secreted by Fujinami sarcoma virus-infected rat fibroblasts. Proc. Natl. Acad. Sci. USA 1985, 82, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Iwamori, M.; Suzuki, H.; Ito, N.; Iwamori, Y.; Hanaoka, K. Lipid compositions of human gastric fluid and epithelium: The role of sulfated lipidsin gastric cytoprotection. J. Clin. Gastroenterol. 2005, 39, 129–133. [Google Scholar] [PubMed]
- Vos, J.P.; Lopes-Cardozo, M.; Gadella, B.M. Metabolic and functional aspects of sulfogalactolipids. Biochim. Biophys. Acta 1994, 1211, 125–149. [Google Scholar] [CrossRef]
- Chen, W.B.; Wang, L.F.; Chen, J.S.; Fan, S.Y. Characterization of polyelectrolyte complexes between chondroitin sulfate and chitosan in the solid state. J. Biomed. Mater. Res. A 2005, 75, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xiao, Q.; Zhang, L.; Zhao, Y.; Yang, Y. Nerve growth factor loaded heparin/chitosan scaffolds for accelerating peripheral nerve regeneration. Carbohydr. Polym. 2017, 171, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Gomez, A.J.; Palma, J.L.; Yap, W.T.; Shea, L.D. Heparin-chitosan nanoparticle functionalization of porous poly(ethylene glycol) hydrogels for localized lentivirus delivery of angiogenic factors. Biomaterials 2014, 35, 8687–8693. [Google Scholar] [CrossRef] [PubMed]
- Menchicchi, B.; Fuenzalida, J.P.; Bobbili, K.B.; Hensel, A.; Swamy, M.J.; Goycoolea, F.M. Structure of chitosan determines its interactions with mucin. Biomacromolecules 2014, 15, 3550–3558. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.K.; Cheng, K.M.; Hu, C.S.; Huang, Y.C.; Young, J.J. Novel protein-loaded chondroitin sulfate-chitosan nanoparticles: Preparation and characterization. Acta Biomater. 2011, 7, 3804–3812. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Elerbroek, S.; Martin, J. Matrix composition in opossum esophague. Dig. Dis. Sci. 2001, 46, 965–975. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, H.; Ishihara, M. Development of Mucoadhesive Chitosan Derivatives for Use as Submucosal Injections. Polymers 2018, 10, 410. https://doi.org/10.3390/polym10040410
Hattori H, Ishihara M. Development of Mucoadhesive Chitosan Derivatives for Use as Submucosal Injections. Polymers. 2018; 10(4):410. https://doi.org/10.3390/polym10040410
Chicago/Turabian StyleHattori, Hidemi, and Masayuki Ishihara. 2018. "Development of Mucoadhesive Chitosan Derivatives for Use as Submucosal Injections" Polymers 10, no. 4: 410. https://doi.org/10.3390/polym10040410