Active Ester Containing Surfmer for One-Stage Polymer Nanoparticle Surface Functionalization in Mini-Emulsion Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of p-(11-Acrylamido)undecanoyloxyphenyl Dimethylsulfonium Methyl Sulfate (AUPDS) Surfmer
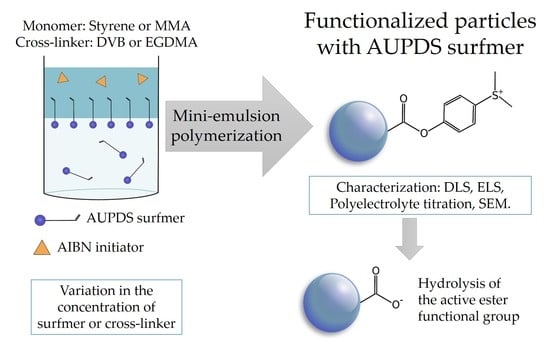
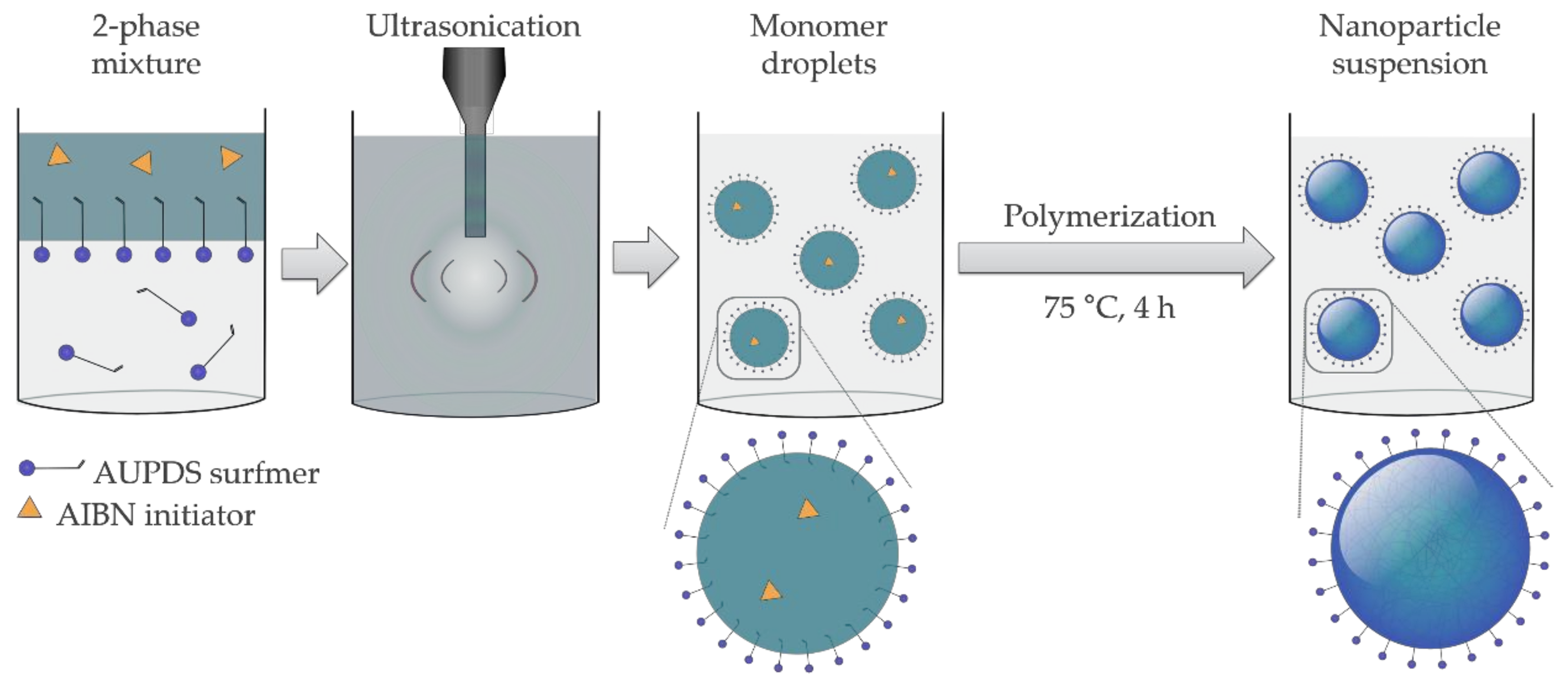
2.4. Preparation of PMMA and PS Particles Copolymerized with AUPDS Surfmer Through Mini-Emulsion Polymerization
2.5. Particle Characterization: Size, Polydispersity and Surface Charge
3. Results and Discussion
3.1. Synthesis of p-(11-acrylamido)undecanoyloxyphenyl Dimethylsulfonium Methyl Sulfate (AUPDS) Surfmer
3.2. Choice of Reagents for Mini-Emulsion Polymerization
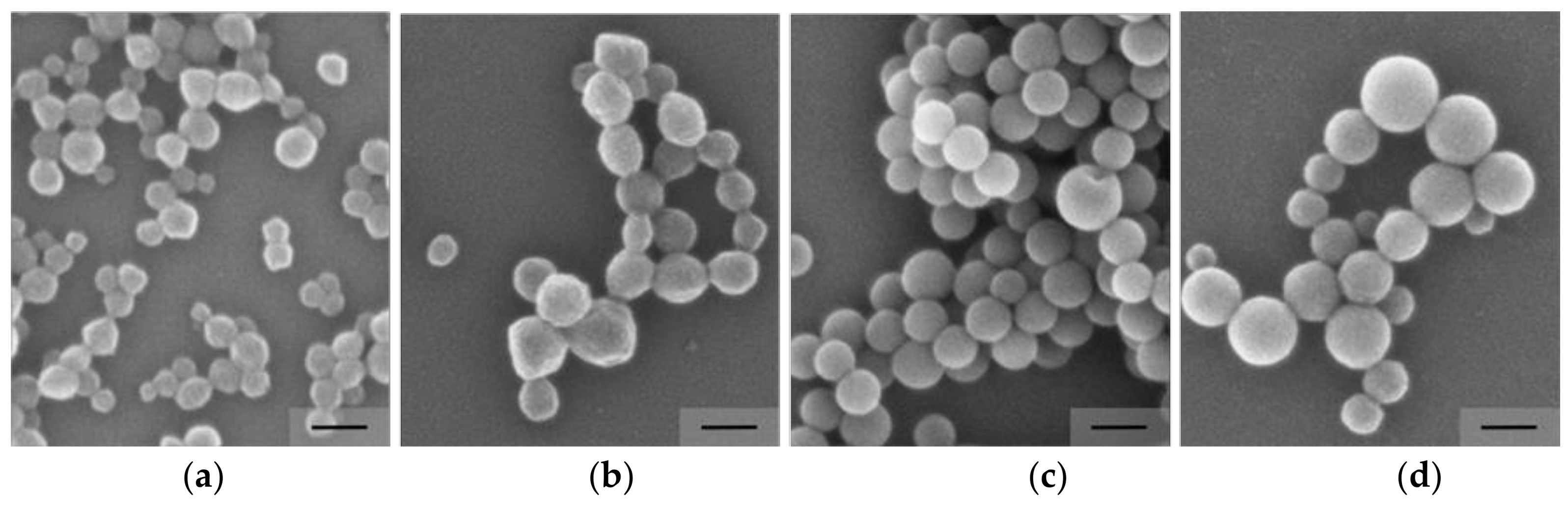
3.3. Influence of the Surfmer Concentration on NP Formation
3.4. Variation of the Cross-Linker Concentration On Particle Formulations
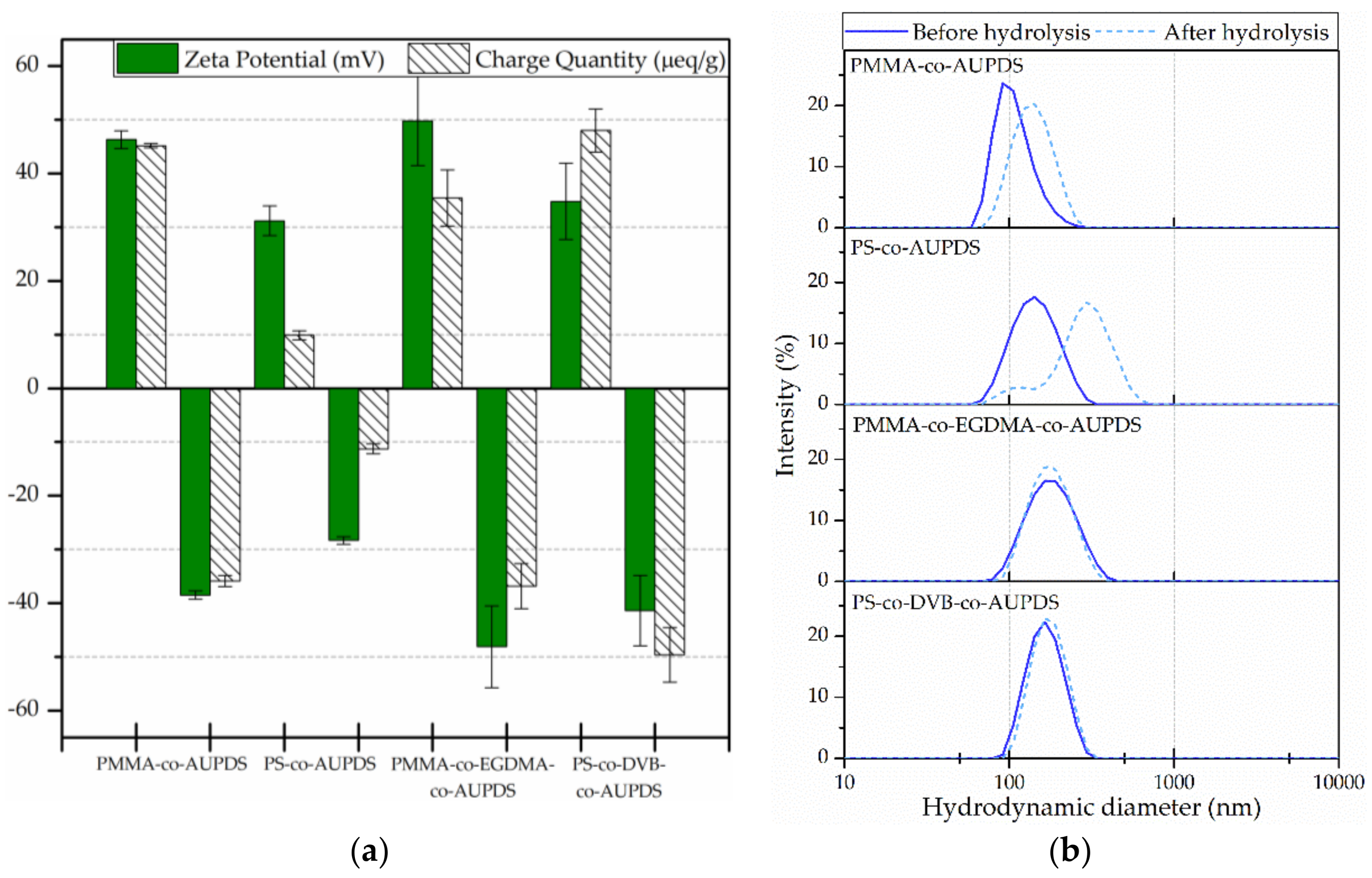
3.5. Characterization of the Surface Charge of Particles Produced Using AUPDS Surfmer
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Kawaguchi, H. Functional polymer microspheres. Prog. Polym. Sci. 2000, 25, 1171–1210. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Landfester, K. The generation of nanoparticles in miniemulsions. Adv. Mater. 2001, 13, 765–768. [Google Scholar] [CrossRef]
- Asua, J.M. Emulsion polymerization: From fundamental mechanisms to process developments. J. Polym. Sci. A 2004, 42, 1025–1041. [Google Scholar] [CrossRef]
- Schork, F.J.; Luo, Y.; Smulders, W.; Russum, J.P.; Butté, A.; Fontenot, K. Miniemulsion polymerization. Adv. Polym. Sci. 2005, 175, 129–255. [Google Scholar]
- Landfester, K.; Bechthold, N.; Tiarks, F.; Antonietti, M. Miniemulsion polymerization with cationic and nonionic surfactants: A very efficient use of surfactants for heterophase polymerization. Macromolecules 1999, 32, 2679–2683. [Google Scholar] [CrossRef]
- Froimowicz, P.; Munoz-Espi, R.; Landfester, K.; Musyanovych, A.; Crespy, D. Surface-functionalized particles: From their design and synthesis to materials science and bio-applications. Curr. Org. Chem. 2013, 17, 900–912. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. 2004, 43, 6042–6108. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Kalia, J.; Raines, R.T. Advances in bioconjugation. Curr. Org. Chem. 2010, 14, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Bajaj, A.; Duncan, B.; Rotello, V.M. Beauty is skin deep: A surface monolayer perspective on nanoparticle interactions with cells and bio-macromolecules. Small 2011, 7, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- De Crozals, G.; Bonnet, R.; Farre, C.; Chaix, C. Nanoparticles with multiple properties for biomedical applications: A strategic guide. Nano Today 2016, 11, 435–463. [Google Scholar] [CrossRef]
- Duchene, D.; Gref, R. Small is beautiful: Surprising nanoparticles. Int. J. Pharm. 2016, 502, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Neoh, K.G.; Kang, E.T. Functionalization of inorganic nanoparticles with polymers for stealth biomedical applications. Polym. Chem. 2011, 2, 747–759. [Google Scholar] [CrossRef]
- Smeets, N.M.B.; Imbrogno, S.; Bloembergen, S. Carbohydrate functionalized hybrid latex particles. Carbohydr. Polym. 2017, 173, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Borzenkov, M.; Hevus, O. Surface Active Monomers: Synthesis, Properties and Applications; Springer Briefs in Materials; Springer: Cham, Switzerland, 2014; ISBN 978-3-319-08445-9. [Google Scholar]
- Guyot, A. Polymerizable surfactants. Curr. Opin. Colloid Interface Sci. 1996, 1, 580–586. [Google Scholar] [CrossRef]
- Asua, J.M.; Schoonbrood, H.A.S. Reactive surfactans in heterophase polymerization. Acta Polym. 1998, 49, 671–686. [Google Scholar] [CrossRef]
- Tadros, T. Polymeric surfactants in disperse systems. Adv. Colloid Interface Sci. 2009, 147–148, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Guyot, A. Advances in reactive surfactants. Adv. Colloid Interface Sci 2004, 108–109, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Guyot, A.; Tauer, K.; Asua, J.M.; Van Es, S.; Gauthier, C.; Hellgrenp, A.C.; Sherringtonpp, D.C.; Montoya-Gonipp, A.; Sjobergp, M.; Sindt, O.; et al. Reactive surfactants in heterophase polymerization. Acta Polym. 1999, 50, 57–66. [Google Scholar] [CrossRef]
- Sauer, R.; Froimowicz, P.; Scholler, K.; Cramer, J.M.; Ritz, S.; Mailander, V.; Landfester, K. Design, synthesis, and miniemulsion polymerization of new phosphonate surfmers and application studies of the resulting nanoparticles as model systems for biomimetic mineralization and cellular uptake. Chem. Eur. J. 2012, 18, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Gao, Q.; Fang, M.; Tan, Y.; Wang, X.; Meng, F. Synthesis, characterization and aqueous properties of a new hybrid fluorocarbon cationic surfmer. J. Surfactants Deterg. 2014, 18, 233–244. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Liu, W.; Xu, L.; Li, H. Preparation of monodispersed core-shell microspheres with surface antibacterial property employing N-(4-vinylbenzyl)-N,N-diethylamine hydrochloride as surfmer. Int. J. Polym. Mater. Polym. Biomater. 2015, 65, 143–150. [Google Scholar] [CrossRef]
- Dominic, G.J.; Yashoda, M.P.; Ajith Kumar, M.P.; Prasannakumar, S. Process of making antimicrobial polymers from quaternary ammonium maleic di-ester surfmers and methyl methacrylate by emulsion polymerization. Macromol. Symp. 2016, 362, 119–128. [Google Scholar] [CrossRef]
- Fischer, V.; Landfester, K.; Muñoz-Espí, R. Molecularly controlled coagulation of carboxyl-functionalized nanoparticles prepared by surfactant-free miniemulsion polymerization. ACS Macro Lett. 2012, 1, 1371–1374. [Google Scholar] [CrossRef]
- Suresh, K.I.; Bartsch, E. Effect of sulfonated 3-pentadecyl phenyl acrylate as surfmer in the emulsion polymerization of styrene: Synthesis and polymer properties. Colloid Polym. Sci. 2013, 291, 1843–1853. [Google Scholar] [CrossRef]
- Mekki, S.; Saïdi-Besbes, S.; Elaïssari, A.; Valour, J.-P.; Derdour, A. Novel polymerizable surfactants: Synthesis and application in the emulsion polymerization of styrene. Polym. J. 2010, 42, 401–405. [Google Scholar] [CrossRef]
- Dufour, M.; Guyot, A. Nonionic reactive surfactants. I. Synthesis and characterization. Colloid Polym. Sci. 2003, 281, 97–104. [Google Scholar] [CrossRef]
- Wald, S.; Simon, J.; Dietz, J.P.; Wurm, F.R.; Landfester, K. Polyglycerol surfmers and surfactants for direct and inverse miniemulsion. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Morizur, J.-F.; Irvine, D.J.; Rawlins, J.J.; Mathias, L.J. Synthesis of new acrylate-based nonionic surfmers and their use in heterophase polymerization. Macromolecules 2007, 40, 8938–8946. [Google Scholar] [CrossRef]
- Friedrich, T.; Tieke, B.; Stadler, F.J.; Bailly, C. Improvement of elasticity and strength of poly(N-isopropylacrylamide) hydrogels upon copolymerization with cationic surfmers. Soft Matter 2011, 7, 6590–6597. [Google Scholar] [CrossRef]
- Kohri, M.; Kobayashi, A.; Fukushima, H.; Kojima, T.; Taniguchi, T.; Saito, K.; Nakahira, T. Enzymatic miniemulsion polymerization of styrene with a polymerizable surfactant. Polym. Chem. 2012, 3, 900–906. [Google Scholar] [CrossRef]
- Rooney, T.R.; Chovancová, A.; Lacík, I.; Hutchinson, R.A. Pulsed laser studies of cationic reactive surfactant radical propagation kinetics. Polymer 2017, 130, 39–49. [Google Scholar] [CrossRef]
- Sauer, R.; Turshatov, A.; Baluschev, S.; Landfester, K. One-pot production of fluorescent surface-labeled polymeric nanoparticles via miniemulsion polymerization with bodipy surfmers. Macromolecules 2012, 45, 3787–3796. [Google Scholar] [CrossRef]
- Herold, M.; Brunner, H.; Tovar, G.E.M. Polymer nanoparticles with activated ester surface by using functional surfmers. Macromol. Chem. Phys. 2003, 204, 770–778. [Google Scholar] [CrossRef]
- Herold, M.; Håkanson, M.; Brunner, H.; Tovar, G.E.M. Copolymer nanoparticles with activated ester surface for the facile immobilization of enzymes. Polym. Prepr. 2005, 46, 1125–1126. [Google Scholar]
- Herold, M.; Håkanson, M.; Brunner, H.; Tovar, G.E.M. Modular surfmers with activated ester function—A colloidal tool for the preparation of bioconjugative nanoparticles. Prog. Colloid Polym. Sci. 2006, 133, 30–34. [Google Scholar]
- Putlitz, B.Z.; Hentze, H.-P.; Landfester, K.; Antonietti, M. New cationic surfactant with sulfonium headgroups. Langmuir 2000, 16, 3214–3220. [Google Scholar] [CrossRef]
- Nagai, K.; Ohashi, T.; Kaneko, R.; Taniguchi, T. Preparation and applications of polymeric microspheres having active ester groups. Colloids Surf A 1999, 153, 133–136. [Google Scholar] [CrossRef]
- Takahashi, K.; Nagai, K. Preparation of reactive polymeric microspheres by seeded copolymerization using a polymerizable surfactant bearing an active ester group. Polymer 1996, 37, 1257–1266. [Google Scholar] [CrossRef]
- ISO 22412: Particle Size Analysis: Dynamic Light Scattering (DLS); International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Herold, M. Herstellung und Charakterisierung von Polymernanopartikeln mit Aktivester-Oberfläche. Doctoral Thesis, University of Stuttgart, Stuttgart, Germany, 2004. [Google Scholar]
- Landfester, K. Miniemulsions for nanoparticle synthesis. Top. Curr. Chem. 2003, 227, 75–123. [Google Scholar]
- Landfester, K. Polyreactions in miniemulsions. Macromol. Rapid Commun. 2001, 22, 896–936. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Landfester, K. Synthesis of colloidal particles in miniemulsions. Annu. Rev. Mater. Res. 2006, 36, 231–279. [Google Scholar] [CrossRef]
- Landfester, K.; Bechthold, N.; Tiarks, F.; Antonietti, M. Formulation and stability of polymerizable emulsions. Macromolecules 1999, 32, 5222–5228. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Controll. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Schoonbrood, H.A.S.; Asua, J.M. Reactive surfactants in heterophase polymerization. 9. Optimum surfmer behavior in emulsion polymerization. Macromolecules 1997, 30, 6034–6041. [Google Scholar] [CrossRef]
- Bork, J.F.; Wyman, D.P.; Coleman, L.E. Nitrogen-containing monomers. III. Reactivity ratios of N-alkyl acrylamides with vinyl monomers. J. Appl. Polym. Sci. 1963, 7, 451–459. [Google Scholar] [CrossRef]
- Chiang, T.-C.; Yu, K.-H. Copolymerization of N-tert-butyl acrylamide and with methyl methacrylate. Polym. Bull. 1981, 4, 425–427. [Google Scholar] [CrossRef]
- Jordan, E.F.; Bennett, R.; Shuman, A.C.; Wrigley, A.N. Reactivity ratios and copolymerization parameters for copolymers incorporating n-octaceyl acrylate and N-n-octadecylacrylamide. J. Polym. Sci. Part A Polym. Chem. 1970, 8, 3113–3121. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Sugi, Y.; Ohtsuka, Y. Copolymerization of styrene with acrylamide derivatives in an emulsifier-free aqueous medium. J. Appl. Polym. Sci. 1981, 26, 1649–1657. [Google Scholar] [CrossRef]
- Choi, J.; Kwak, S.Y.; Kang, S.; Lee, S.S.; Park, M.; Lim, S.; Kim, J.; Choe, C.R.; Hong, S.I. Synthesis of highly crosslinked monodisperse polymer particles: Effect of reaction parameters on the size and size distribution. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4368–4377. [Google Scholar] [CrossRef]
- Kim, D.; Lee, D.Y.; Lee, K.; Choe, S. Effect of crosslinking agents on the morphology of polymer particles produced by one-step seeded polymerization. Macromol. Res. 2009, 17, 250–258. [Google Scholar] [CrossRef]
- Böckenhoff, K.; Fischer, W. Determination of electrokinetic charge with a particle-charge detector, and its relationship to the total charge. Fresenius J. Anal. Chem. 2001, 371, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Speyerer, C.; Borchers, K.; Hirth, T.; Tovar, G.E.; Weber, A. Surface etching of methacrylic microparticles via basic hydrolysis and introduction of functional groups for click chemistry. J. Colloid Interface Sci. 2013, 397, 185–191. [Google Scholar] [CrossRef] [PubMed]


| AUPDS (mol%) | Z-Average (nm) | PI | ZP (mV) a |
|---|---|---|---|
| PMMA-co-AUPDS particles | |||
| 0.25 | - | - | - |
| 0.5 | - | - | - |
| 1 | - | - | - |
| 2 | 118.5 ± 0.3 | 0.05 ± 0.00 | 40.1 ± 0.8 |
| 3 | 134.0 ± 0.2 | 0.10 ± 0.00 | 42.8 ± 1.2 |
| 4 | 124.5 ± 0.1 | 0.07 ± 0.02 | 45.9 ± 1.1 |
| 6 | 129.0 ± 1.1 | 0.08 ± 0.02 | 42.9 ± 0.9 |
| 8 | 150.0 ± 1.1 | 0.13 ± 0.01 | 44.0 ± 0.2 |
| PS-co-AUPDS particles | |||
| 0.25 | - | - | - |
| 0.5 | 488.3 ± 19.4 | 0.16 ± 0.04 | 8.7 ± 0.5 |
| 1 | 331.2 ± 29.5 | 0.37 ± 0.02 | −4.5 ± 0.2 |
| 2 | 90.5 ± 1.8 | 0.13 ± 0.01 | 35.7 ± 0.4 |
| 4 | 82.5 ± 2.9 | 0.42 ± 0.02 | 26.1 ± 0.2 |
| 6 | 61.3 ± 0.3 | 0.23 ± 0.01 | 35.4 ± 1.7 |
| 8 | 55.5 ± 0.5 | 0.24 ± 0.00 | 43.3 ± 1.3 |
| Cross-Linker (mol%) | Z-Average (nm) | PI | ZP (mV) a |
|---|---|---|---|
| PMMA-co-EGDMA-co-AUPDS particles | |||
| 2.5 | 168.7 ± 0.6 | 0.07 ± 0.03 | 38.5 ± 8.6 |
| 5 | 162.7 ± 1.0 | 0.07 ± 0.00 | 52.2 ± 15.3 |
| 10 | 159.6 ± 1.9 | 0.10 ± 0.01 | 30.9 ± 3.5 |
| 20 | 225.3 ± 5.4 | 0.29 ± 0.04 | 14.9 ± 4.4 |
| 40 | 297.6 ± 3.0 | 0.30 ± 0.03 | 17.5 ± 6.0 |
| 60 | 268.8 ± 3.6 | 0.23 ± 0.04 | 8.7 ± 3.2 |
| 80 | 587.6 ± 8.3 | 0.40 ± 0.07 | −24.7 ± 3.9 |
| PS-co-DVB-co-AUPDS particles | |||
| 2.5 | 120.3 ± 1.4 | 0.04 ± 0.02 | 26.6 ± 0.6 |
| 5 | 98.6 ± 0.5 | 0.03 ± 0.02 | 26.4 ± 0.7 |
| 10 | 160.4 ± 1.2 | 0.03 ± 0.03 | 26.7 ± 1.0 |
| 20 | 161.7 ± 0.1 | 0.09 ± 0.01 | 17.0 ± 3.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albernaz, V.L.; Bach, M.; Weber, A.; Southan, A.; Tovar, G.E.M. Active Ester Containing Surfmer for One-Stage Polymer Nanoparticle Surface Functionalization in Mini-Emulsion Polymerization. Polymers 2018, 10, 408. https://doi.org/10.3390/polym10040408
Albernaz VL, Bach M, Weber A, Southan A, Tovar GEM. Active Ester Containing Surfmer for One-Stage Polymer Nanoparticle Surface Functionalization in Mini-Emulsion Polymerization. Polymers. 2018; 10(4):408. https://doi.org/10.3390/polym10040408
Chicago/Turabian StyleAlbernaz, Vanessa L., Monika Bach, Achim Weber, Alexander Southan, and Günter E. M. Tovar. 2018. "Active Ester Containing Surfmer for One-Stage Polymer Nanoparticle Surface Functionalization in Mini-Emulsion Polymerization" Polymers 10, no. 4: 408. https://doi.org/10.3390/polym10040408