Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups
Abstract
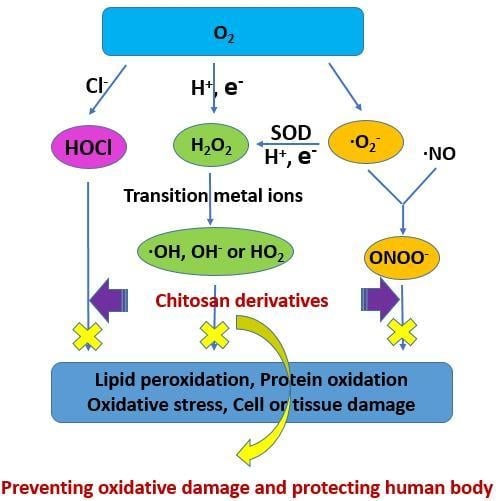
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
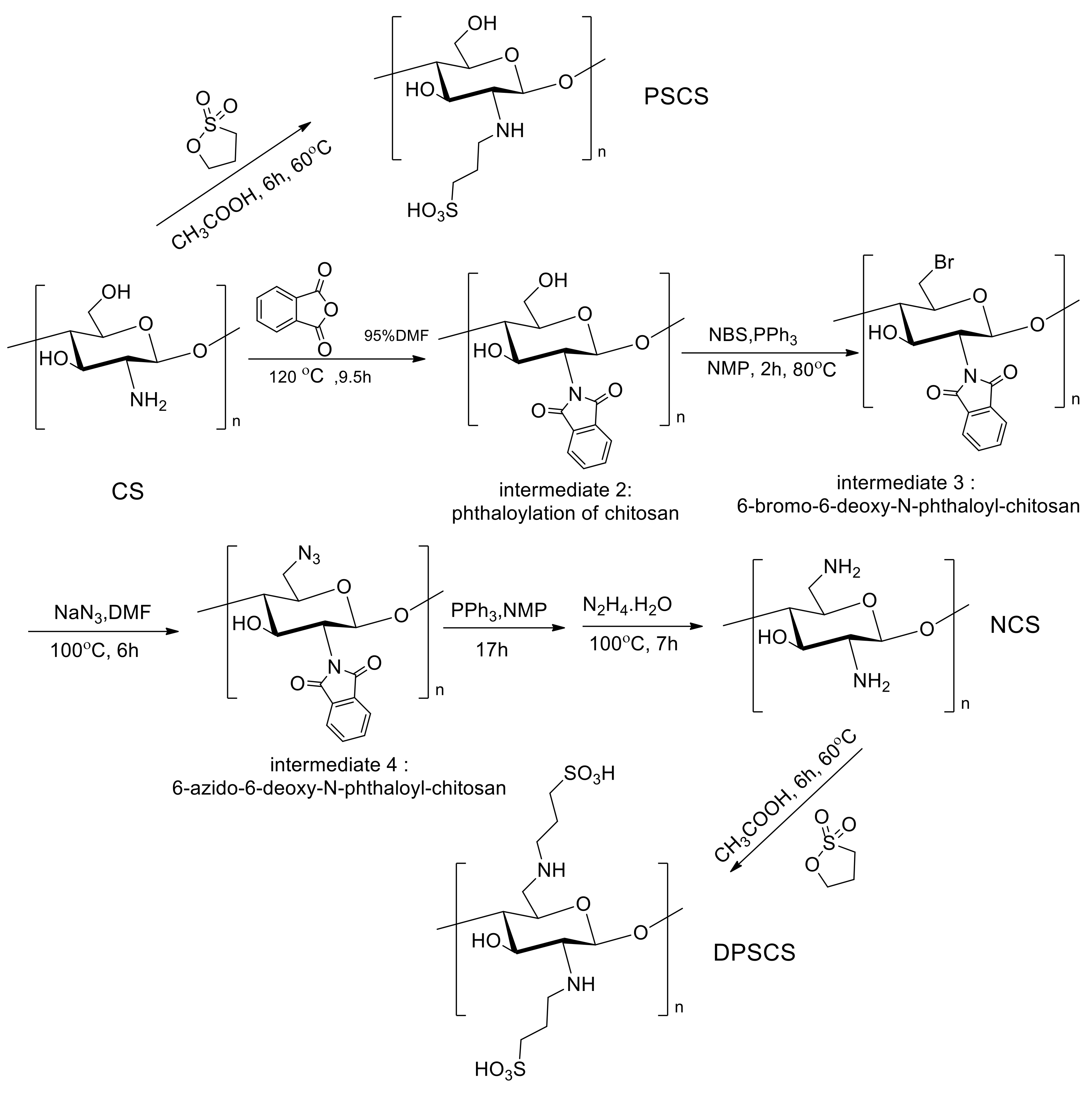
2.3. Synthesis
2.3.1. 6-Deoxy-N-phthaloyl-chitosan (Intermediate 2)
2.3.2. Preparation of 6-Bromo-6-deoxy-N-phthaloyl-chitosan (Intermediate 3)
2.3.3. Preparation of 6-Azido-6-deoxy-N-phthaloyl-chitosan (Intermediate 4)
2.3.4. Preparation of NCS
2.3.5. Preparation of Dipropane Sulfonated Chitosan (DPSCS)
2.3.6. Preparation of Propane Sulfonated Chitosan (PSCS)
2.4. Investigation of the Antioxidant Ability
2.4.1. Hydroxyl-Radical Scavenging Ability Assay
2.4.2. Superoxide-Radical Scavenging Ability Assay
2.4.3. DPPH-Radical Scavenging Ability Assay
2.4.4. Reducing Power Assay
2.5. Evaluation of Antifungal Activity In Vitro
3. Results and Discussion
3.1. Chitosan Derivatives Preparation and Characterization
3.1.1. Synthesis of Chitosan Derivatives
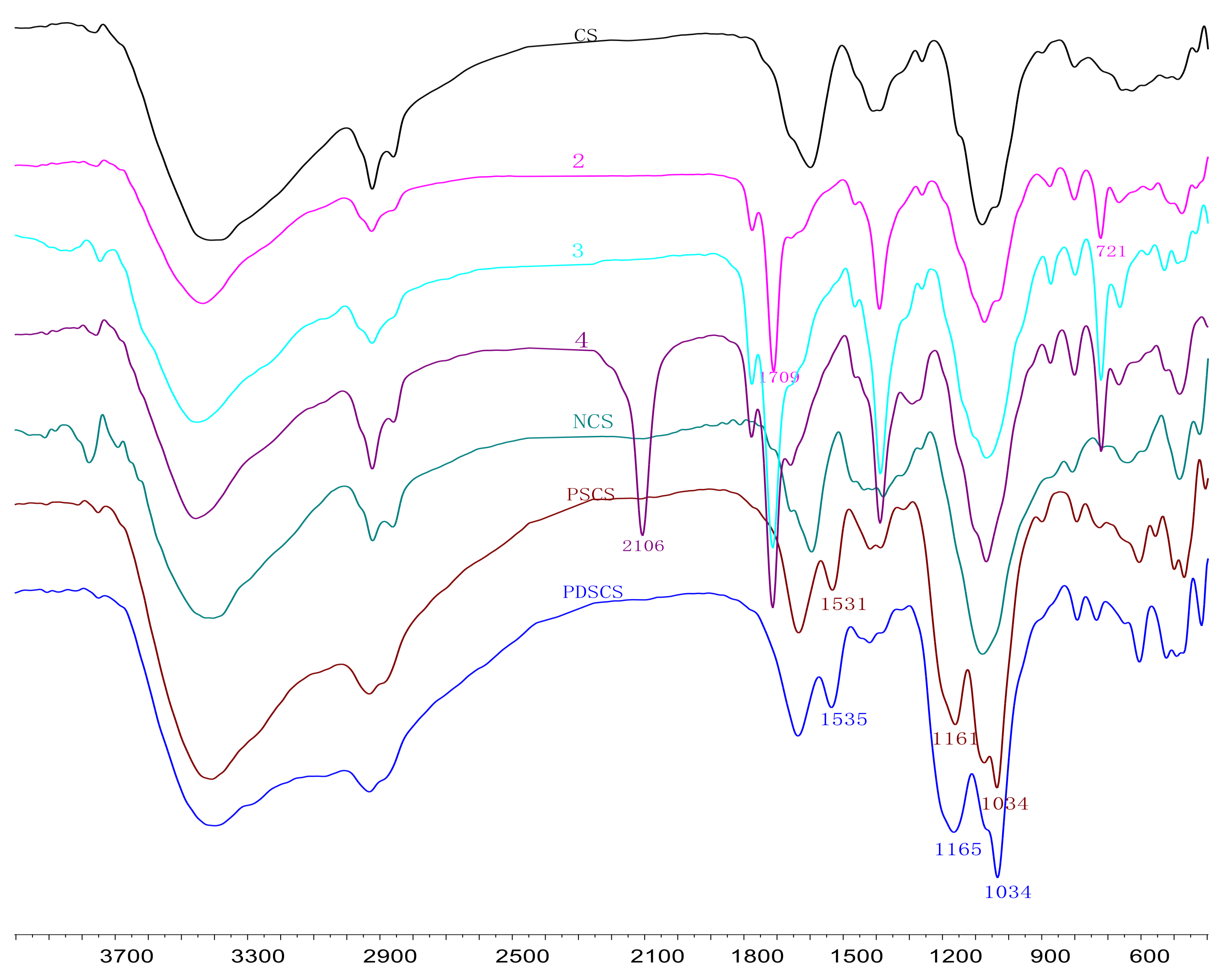
3.1.2. FT-IR Analysis
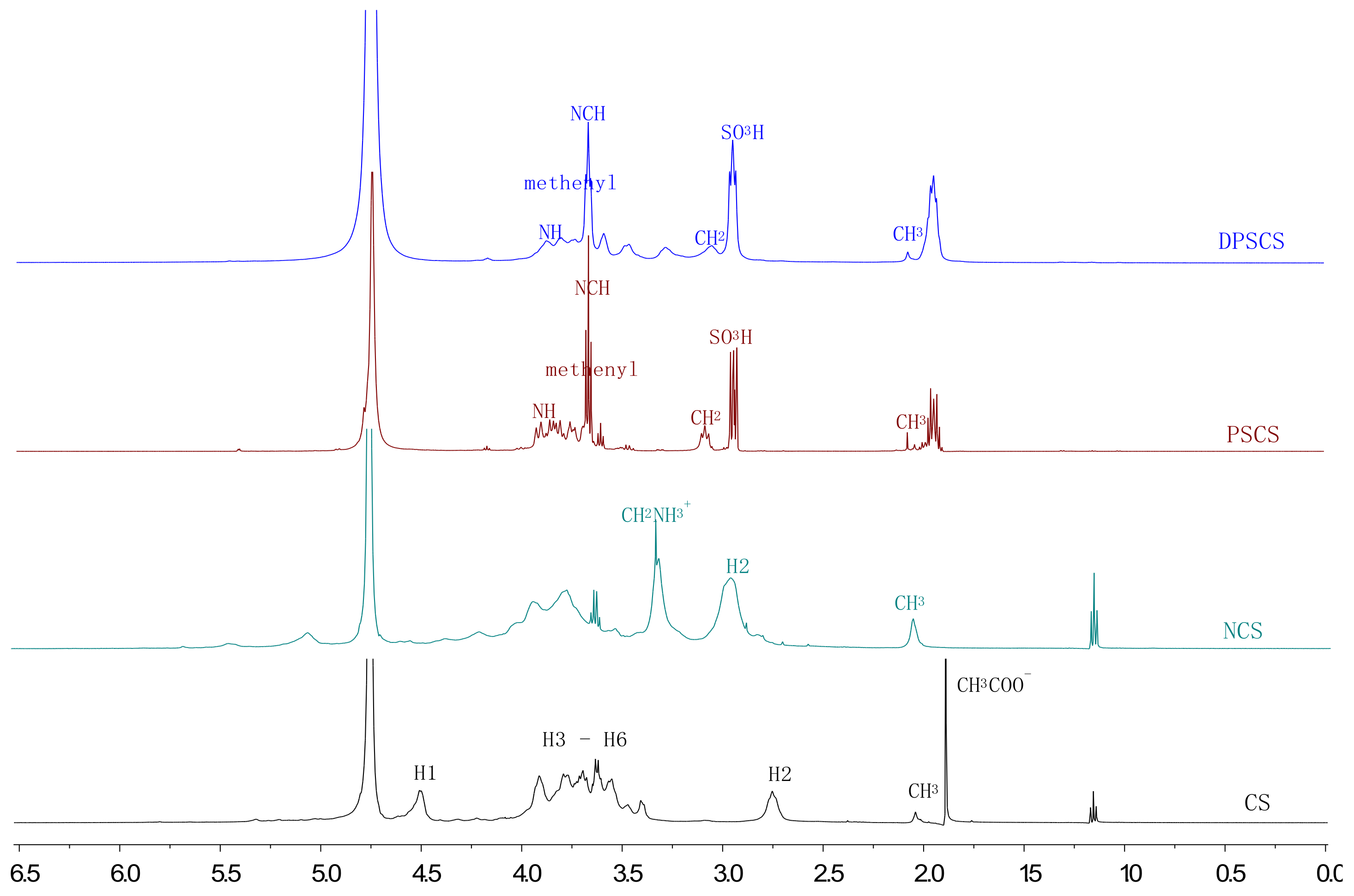
3.1.3. NMR Analysis
3.2. Antioxidant Activities
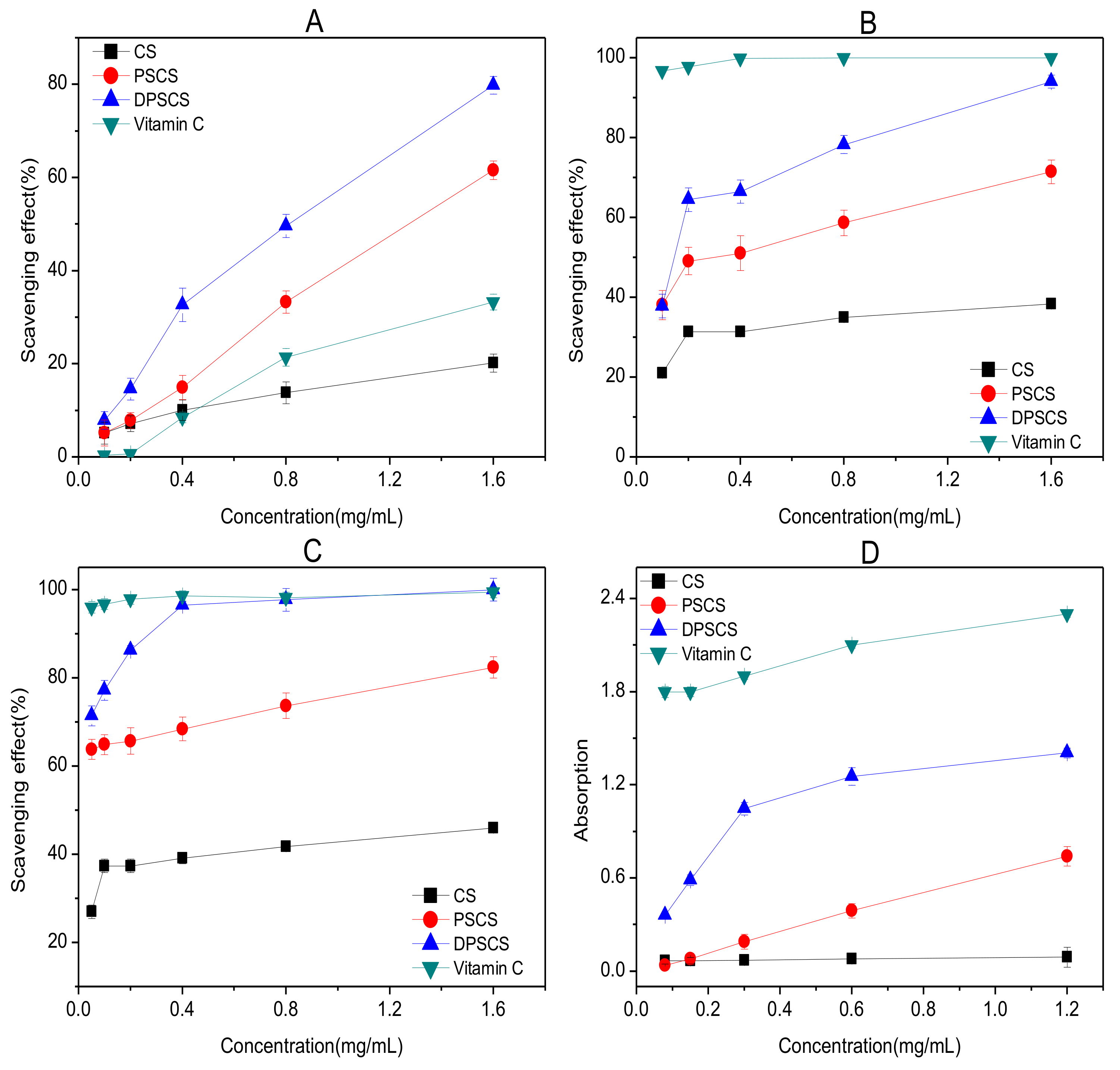
3.2.1. Hydroxyl-Radical Scavenging Ability Assay
3.2.2. Superoxide-Radical Scavenging Ability Assay
3.2.3. DPPH-Radical Scavenging Ability Assay
3.2.4. Reducing Power Assay
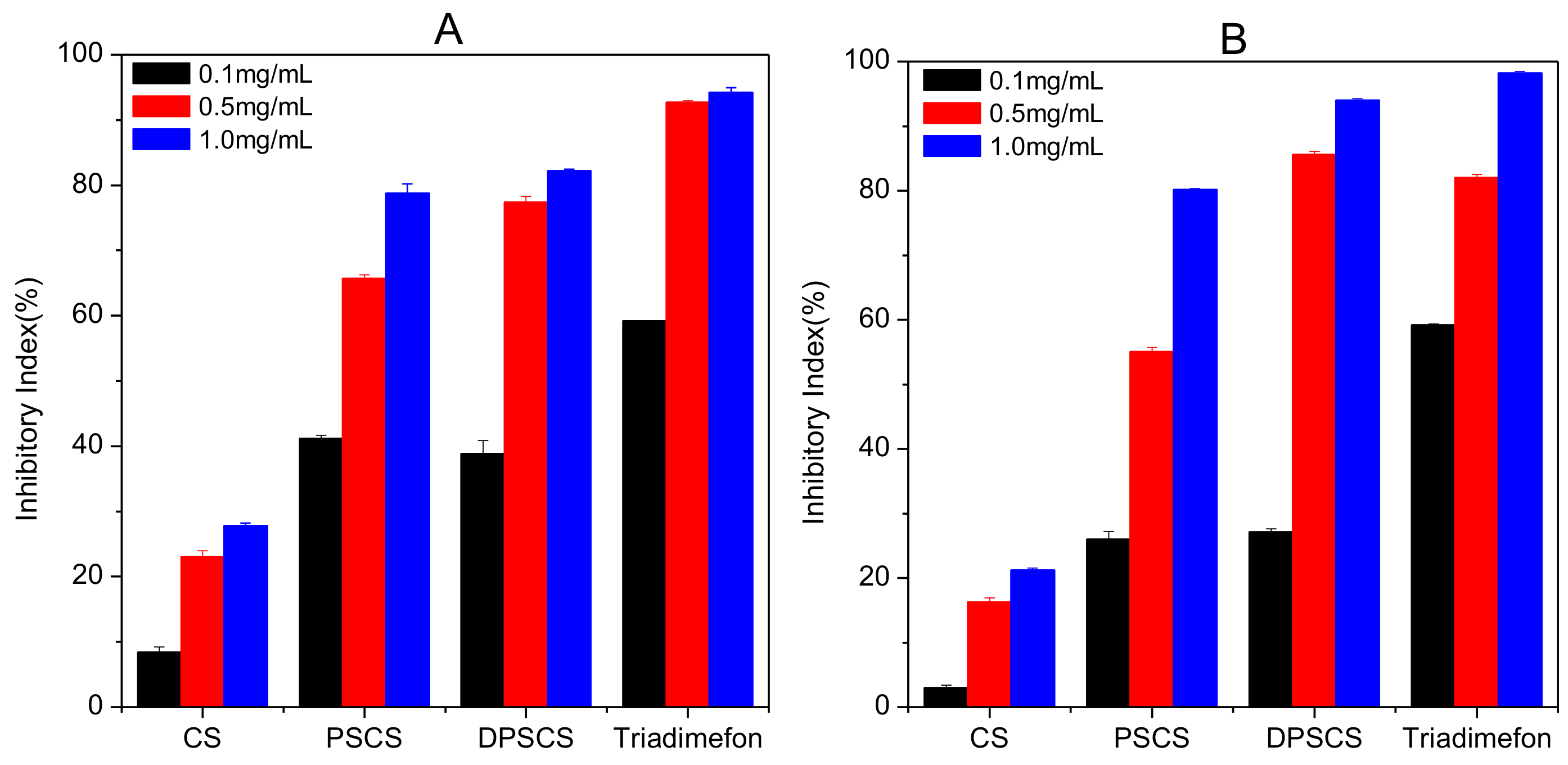
3.3. Antifungal Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2014, 48, 40–50. [Google Scholar] [CrossRef]
- Kerch, G. The potential of chitosan and its derivatives in prevention and treatment of age-related diseases. Mar. Drugs 2015, 13, 2158–2182. [Google Scholar] [CrossRef] [PubMed]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Siafaka, P.I.; Lambropoulou, D.A.; Lazaridis, N.K.; Bikiaris, D.N. Poly(itaconic acid)-Grafted Chitosan Adsorbents with Different Cross-Linking for Pb(II) and Cd(II) Uptake. Langmuir 2014, 30, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Koutroumanis, K.P.; Avgoustakis, K.; Bikiaris, D. Synthesis of cross-linked N-(2-carboxybenzyl)chitosan pH sensitive polyelectrolyte and its use for drug controlled delivery. Carbohydr. Polym. 2010, 82, 181–188. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Titopoulou, A.; Koukaras, E.N.; Kostoglou, M.; Koutris, E.; Karavas, E.; Bikiaris, D.N. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int. J. Pharm. 2015, 495, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.B.; Nasario, F.D.; Silva-Goncalves, L.C.; Oliveira Tiera, V.A.; Arcisio-Miranda, M.; Tiera, M.J.; Dos Santos Cabrera, M.P. Chitosan derivatives targeting lipid bilayers: Synthesis, biological activity and interaction with model membranes. Carbohydr. Polym. 2018, 181, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Masson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Han, J.; Zhang, Y.; Wei, L.; Yu, S.; Wang, X.; Jin, Z.; Wang, Y. Enhancing Mucosal Immune Response of Newcastle Disease Virus DNA Vaccine Using N-2-Hydroxypropyl Trimethylammonium Chloride Chitosan and N,O-Carboxymethyl Chitosan Nanoparticles as Delivery Carrier. Mol. Pharm. 2018, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Hashimoto-Hill, S.; Kim, M.; Tsifansky, M.D.; Kim, C.H.; Yeo, Y. Succinylated Chitosan Derivative Has Local Protective Effects on Intestinal Inflammation. ACS Biomater. Sci. Eng. 2017, 3, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.S.; Wang, Y.Z.; Lin, J.J.; Lien, W.F. Preparation and properties of sulfopropyl chitosan derivatives with various sulfonation degree. Appl. Polym. Sci. 2010, 116, 1686–1693. [Google Scholar] [CrossRef]
- Zhang, K.; Helm, J.; Peschel, D.; Gruner, M.; Groth, T.; Fischer, S. NMR and FT Raman characterisation of regioselectively sulfated chitosan regarding the distribution of sulfate groups and the degree of substitution. Polymer 2010, 51, 4698–4705. [Google Scholar] [CrossRef]
- Xing, R.; He, X.; Liu, S.; Yu, H.; Qin, Y.; Chen, X.; Li, K.; Li, R.; Li, P. Antidiabetic activity of differently regioselective chitosan sulfates in alloxan-induced diabetic rats. Mar. Drugs 2015, 13, 3072–3090. [Google Scholar] [CrossRef] [PubMed]
- Seedevi, P.; Moovendhan, M.; Vairamani, S.; Shanmugam, A. Evaluation of antioxidant activities and chemical analysis of sulfated chitosan from Sepia prashadi. Int. J. Biol. Macromol. 2017, 99, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, L.; Tan, W.; Gu, G.; Guo, Z. Novel 1,2,3-triazolium-functionalized inulin derivatives: Synthesis, free radical-scavenging activity, and antifungal activity. RSC Adv. 2017, 7, 42225–42232. [Google Scholar] [CrossRef]
- Tan, M.; Wang, H.; Wang, Y.; Chen, G.; Yuan, L.; Chen, H. Recyclable antibacterial material: Silicon grafted with 3,6-O-sulfated chitosan and specifically bound by lysozyme. J. Mater. Chem. B 2014, 2, 569–576. [Google Scholar] [CrossRef]
- Pires, N.R.; Cunha, P.L.; Maciel, J.S.; Angelim, A.L.; Melo, V.M.; De Paula, R.C.; Feitosa, J.P. Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydr. Polym. 2013, 91, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, K.; Li, D.; Yu, S.; Cai, J.; Chen, L.; Du, Y. Preparation, characterization and in vitro anticoagulant activity of highly sulfated chitosan. Int. J. Biol. Macromol. 2013, 52, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A New Horizon in Modifications of Chitosan: Syntheses and Applications. Critical Reviews in Therapeutic Drug Carrier Systems. Ther. Drug Carrier Syst. 2013, 30, 91–181. [Google Scholar]
- Prem, V.K.; Sharma, P. Synthesis of Highly Stable and High Water Retentive Functionalized Biopolymer-Graphene Oxide Modified Cation Exchange Membranes. RSC Adv. 2015, 5, 56498–56506. [Google Scholar]
- Jeon, J.H.; Cheedarala, R.K.; Kee, C.D.; Oh, I.K. Dry-Type Artificial Muscles Based on Pendent Sulfonated Chitosan and Functionalized Graphene Oxide for Greatly Enhanced Ionic Interactions and Mechanical Stiffnes. Adv. Funct. Mater. 2013, 23, 6007–6018. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Li, Q.; Tan, W.; Wei, L.; Zhang, J.; Dong, F.; Gu, G.; Guo, Z. The evaluation of antioxidant and antifungal properties of 6-amino-6-deoxychitosan in vitro. Int. J. Biol. Macromol. 2017, 107, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Guo, Z. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. Int. J. Biol. Macromol. 2014, 70, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Q.; Tan, W.; Dong, F.; Luan, F.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Double Quaternized Chitosan Derivatives. Molecules 2017, 22, 501. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Li, W.; Dong, F.; Guo, Z. Synthesis and antioxidant property of novel 1,2,3-triazole-linked starch derivatives via ‘click chemistry’. Int. J. Biol. Macromol. 2016, 82, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Zhong, Z.; Xing, R.; Li, P.; Mo, G. The preparation and antioxidant activity of 2-[phenylhydrazine (or hydrazine)-thiosemicarbazone]-chitosan. Int. J. Biol. Macromol. 2010, 47, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Slaga, T.J.; Klein-Szanto, A.J.; Triplett, L.L.; Yotti, L.P.; Trosko, J.E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science 1981, 213, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Dong, F.; Qiu, S.; Zhang, J.; Guo, Z. Novel 1,2,3-triazolium-functionalized starch derivatives: Synthesis, characterization, and evaluation of antifungal property. Carbohydr. Polym. 2017, 160, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, Y.; Wu, H.; Xiao, L. Relationship between molecular structure and moisture-retention ability of carboxymethyl chitin and chitosan. J. Appl. Polym. Sci. 2002, 83, 1233–1241. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Wang, W.D. Optimization of ultrasonic-assisted extraction and in vitro antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Shon, M.; Kim, T.; Sung, N. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar] [CrossRef]
- Chung, Y.; Chang, C.; Chao, W.; Lin, C.; Chou, S. Antioxidative Activity and Safety of the 50% Ethanolic Extract from Red Bean Fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.; Du, P.; Yen, G. Action of Methanolic Extract of Mung Bean Hulls as Inhibitors of Lipid Peroxidation and Non-lipid Oxidative Damage. Food Chem. Toxicol. 1999, 37, 1055–1061. [Google Scholar] [CrossRef]

| Name | N% | C% | H% | S% | DS | Yield |
|---|---|---|---|---|---|---|
| CS | 8.49 | 44.72 | 6.88 | - | - | - |
| intermediate 2 | 4.68 | 52.92 | 4.45 | - | DSphthaloyl: 0.92 | 83% |
| intermediate 3 | 4.24 | 47.78 | 3.91 | - | DSbromo: 0.89 | 47% |
| intermediate 4 | 15.99 | 51.66 | 4.15 | - | DSazido: 0.88 | 98% |
| NCS | 15.01 | 42.54 | 7.83 | - | DSamino: 0.81 | 41% |
| PSCS | 4.76 | 35.70 | 6.38 | 7.14 | DSsulfonate: 0.66 | 45% |
| DPSCS | 3.79 | 32.61 | 5.81 | 10.4 | DSsulfonate: 1.2 | 57% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, F.; Wei, L.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups. Polymers 2018, 10, 395. https://doi.org/10.3390/polym10040395
Luan F, Wei L, Zhang J, Mi Y, Dong F, Li Q, Guo Z. Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups. Polymers. 2018; 10(4):395. https://doi.org/10.3390/polym10040395
Chicago/Turabian StyleLuan, Fang, Lijie Wei, Jingjing Zhang, Yingqi Mi, Fang Dong, Qing Li, and Zhanyong Guo. 2018. "Antioxidant Activity and Antifungal Activity of Chitosan Derivatives with Propane Sulfonate Groups" Polymers 10, no. 4: 395. https://doi.org/10.3390/polym10040395