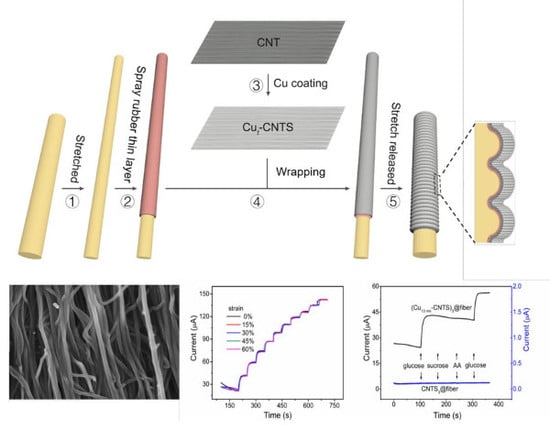
Fabrication of Stretchable Copper Coated Carbon Nanotube Conductor for Non-Enzymatic Glucose Detection Electrode with Low Detection Limit and Selectivity
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
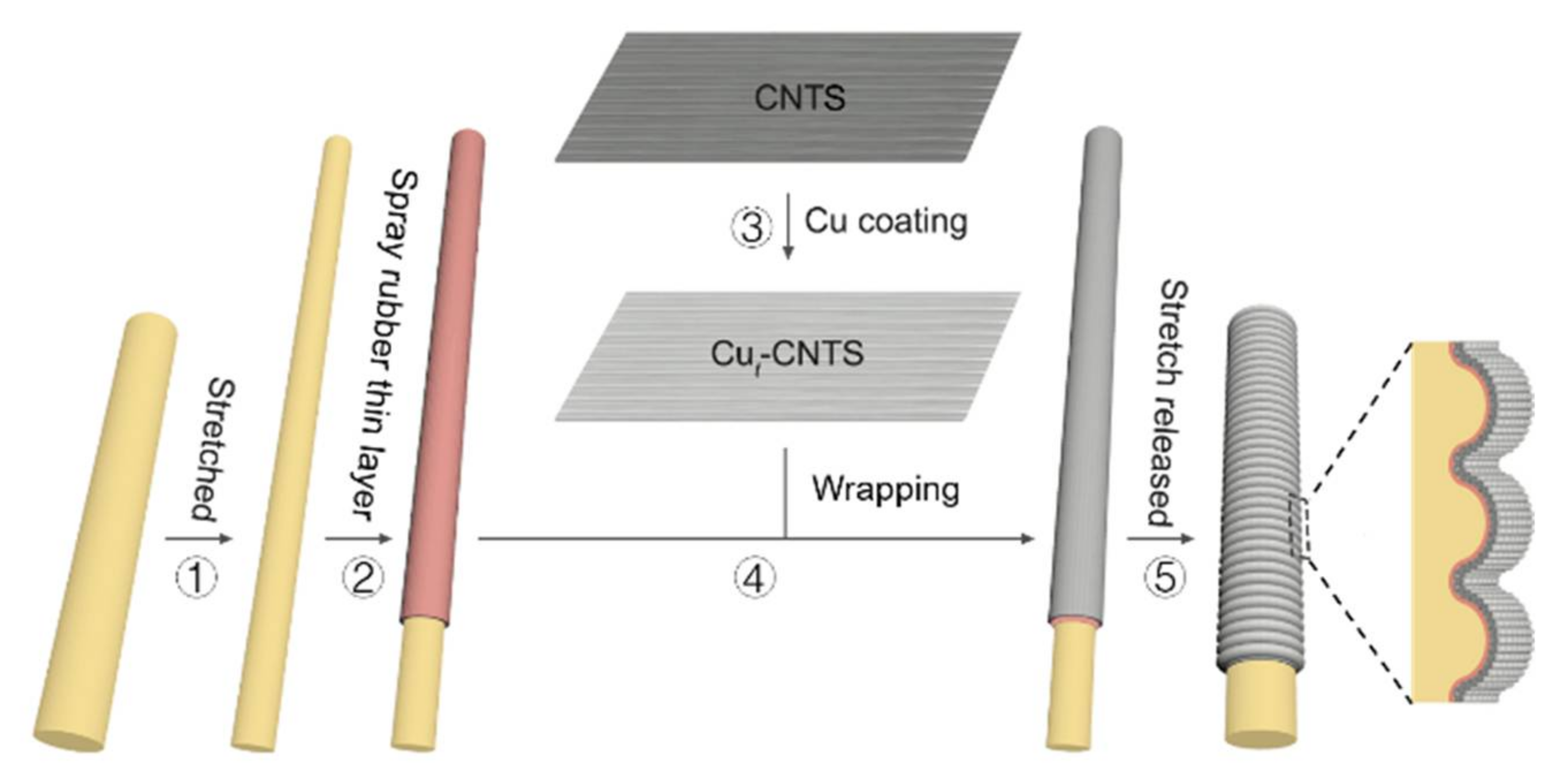
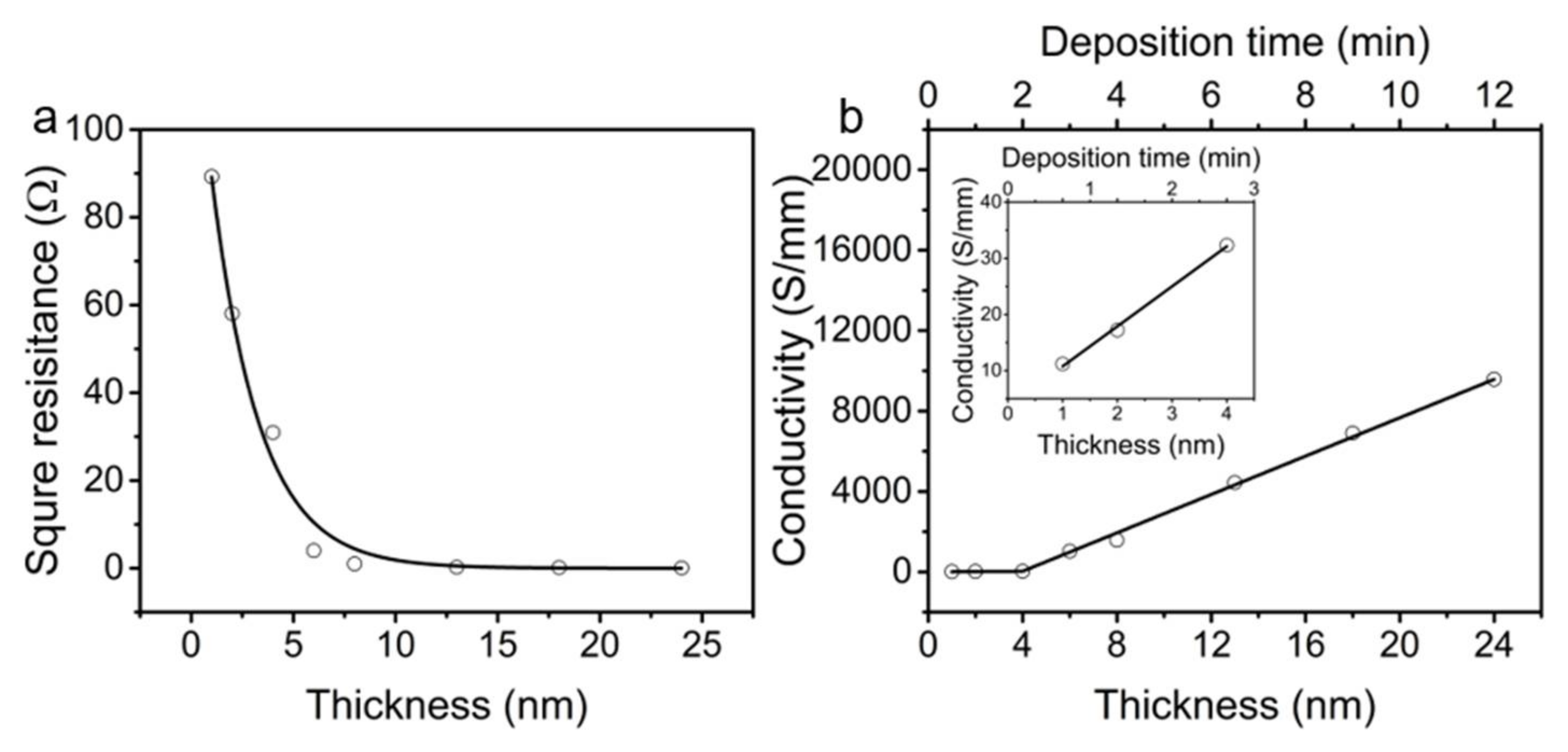
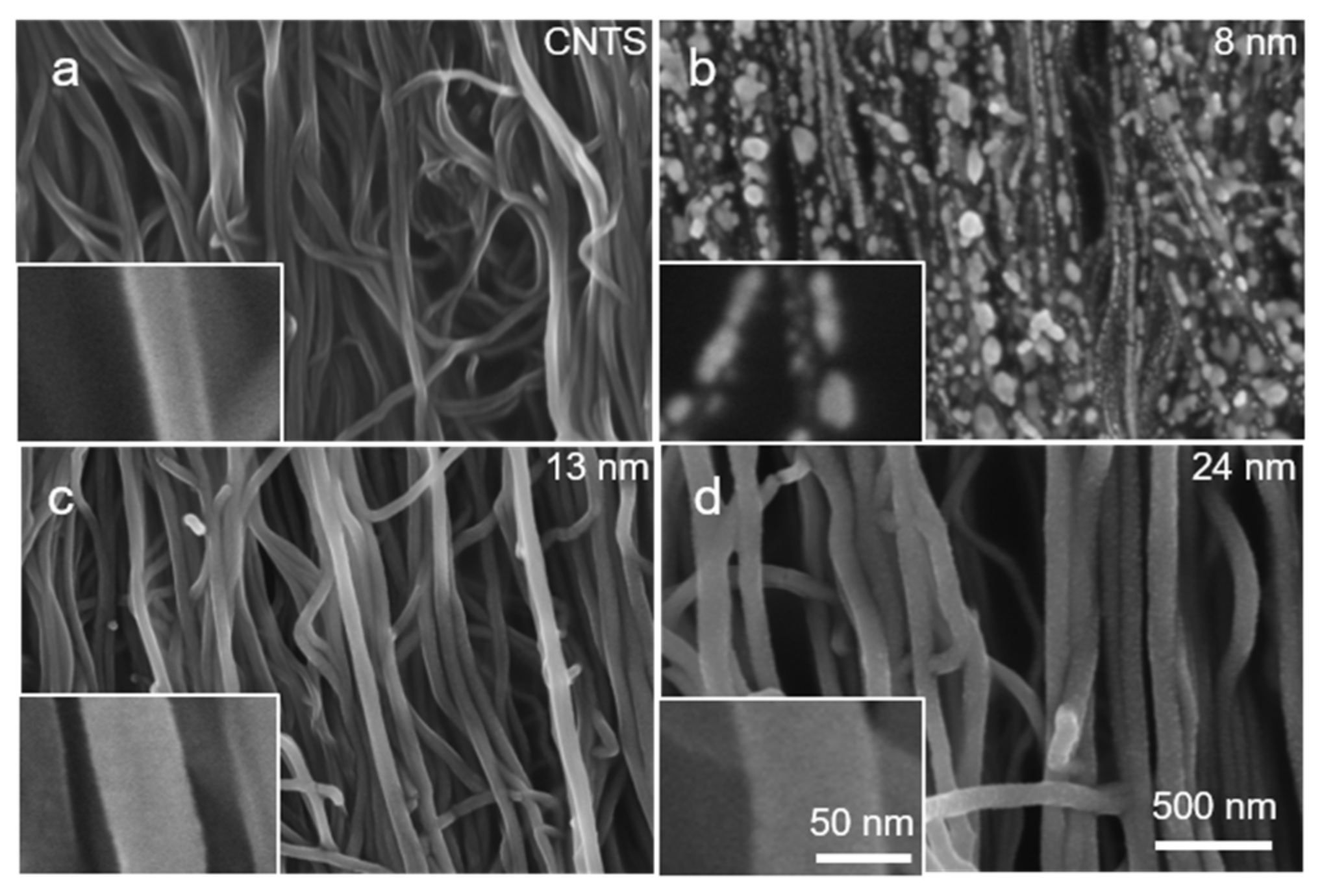
3.1. Electrical Properties of (Cut-CNTS)m@fibers
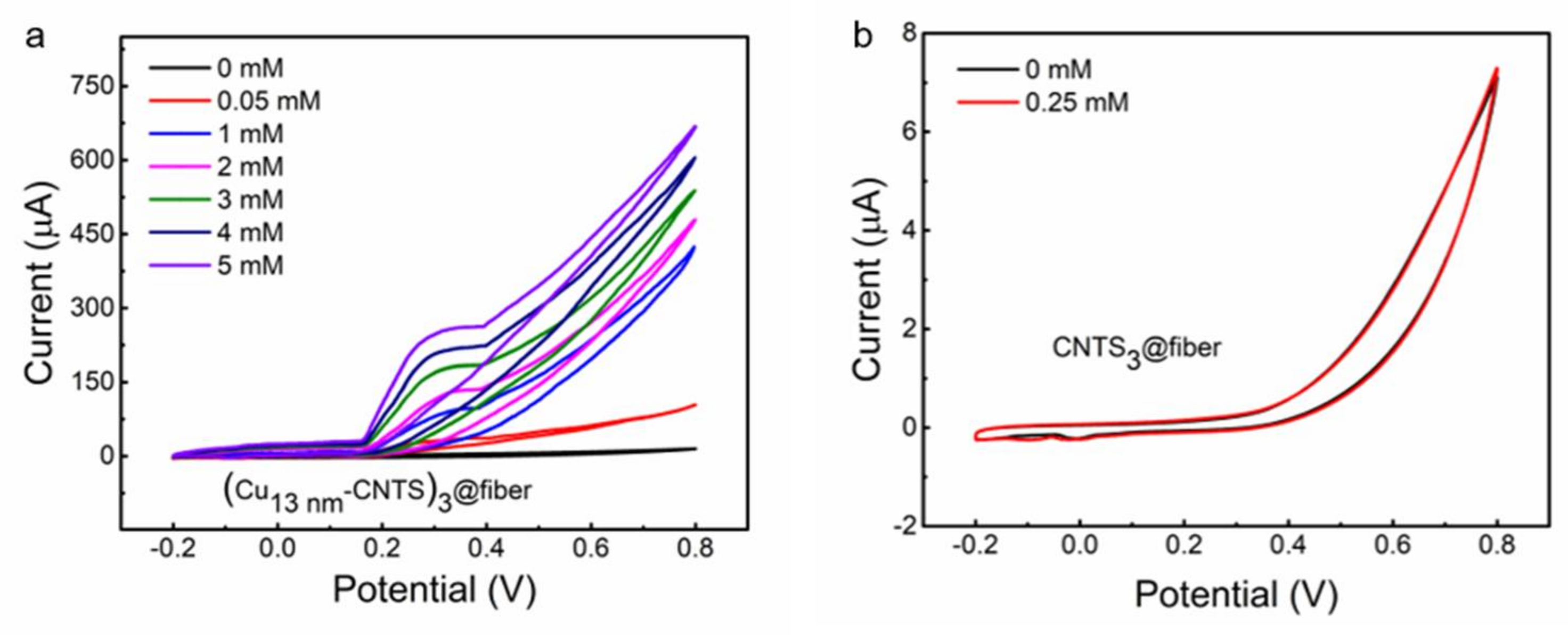
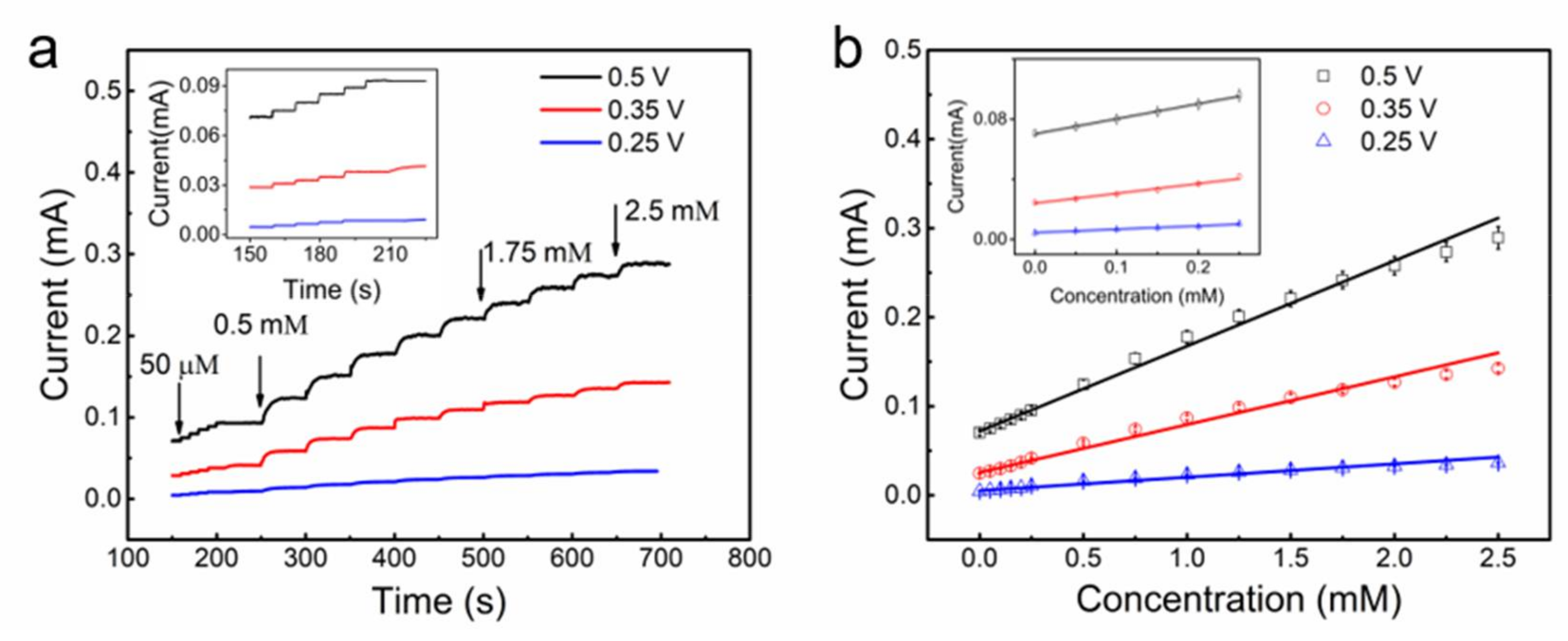
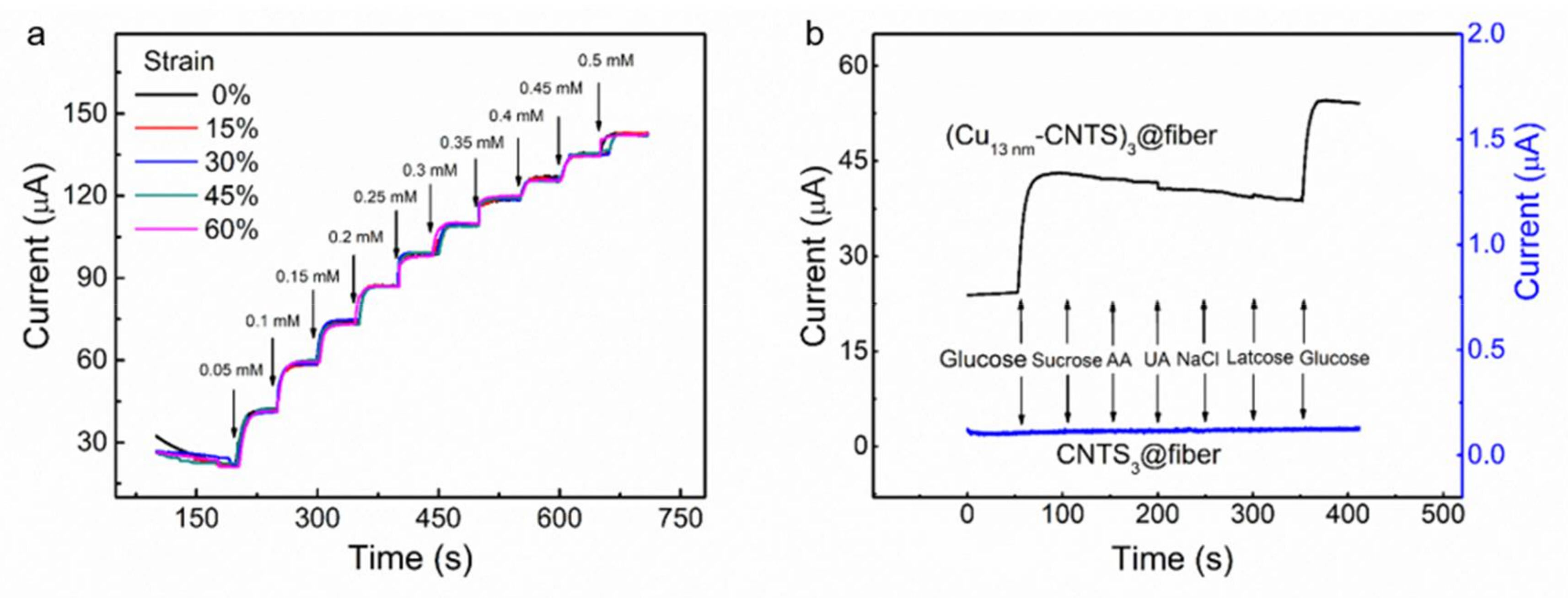
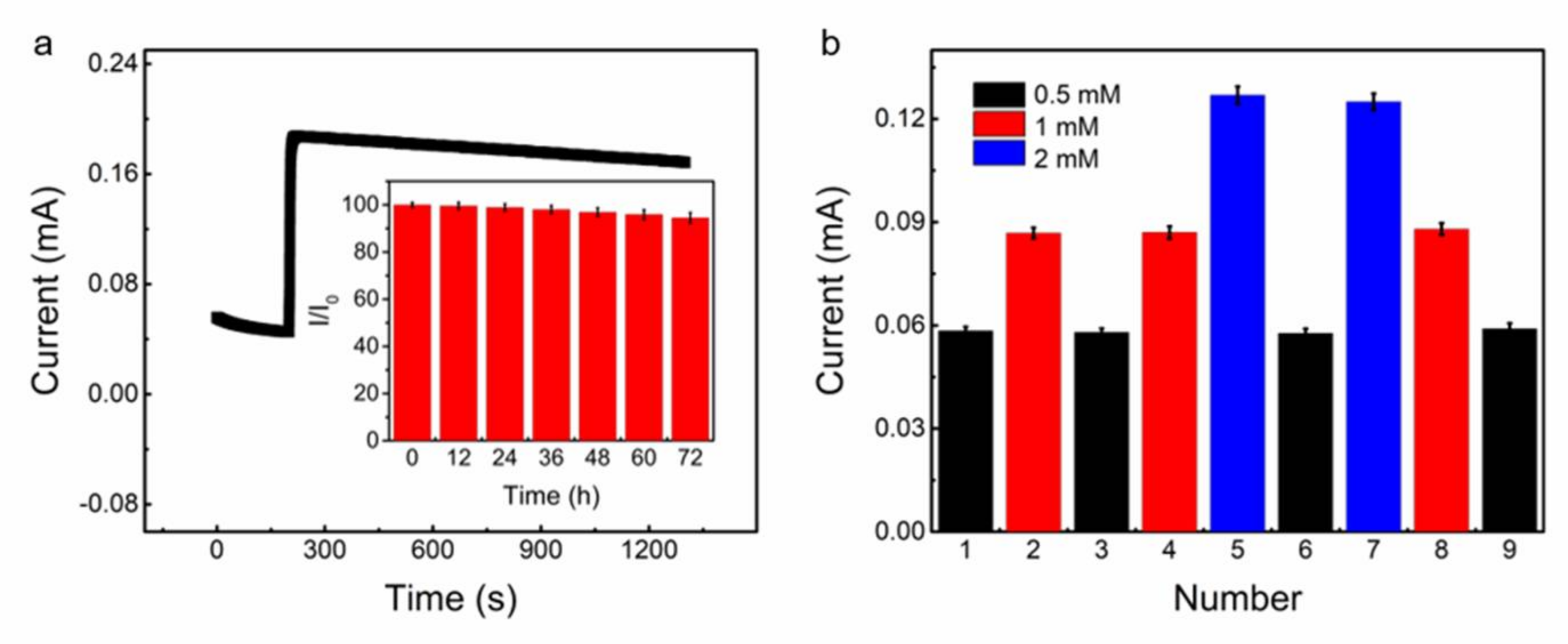
3.2. Stretchable Non-Enzymatic Electrode for Glucose Sensing Based on the (Cut-CNTS)m@fibers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.; Ma, X.H.; Hao, Y. Electronic devices for human-machine interfaces. Adv. Mater. Interfaces 2017, 4, 1600709. [Google Scholar] [CrossRef]
- Edwards, J. Wireless sensors relay medical insight to patients and caregivers. IEEE Signal Proc. Mag. 2012, 29, 8–12. [Google Scholar] [CrossRef]
- Haidar, Z.S. Bio-inspired/-functional colloidal core-shell polymeric-based nanosystems: Technology promise in tissue engineering, bioimaging and nanomedicine. Polymers 2010, 2, 323–352. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.C. Wearable sensors for human activity monitoring: A review. IEEE Sens. J. 2014, 15, 1321–1330. [Google Scholar] [CrossRef]
- Wang, X.W.; Liu, Z.; Zhang, T. Flexible sensing electronics for wearable/attachable health monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Weng, Z.Y.; Li, X.; Liu, X.M.; Wu, S.L.; Yeung, K.W.K.; Wang, X.B.; Cui, Z.D.; Yang, X.J.; Chu, P.K. Biomedical applications of functionalized ZnO nanomaterials: From biosensors to bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. Stretchable carbon nanotube strain sensor for human motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Song, L.; Luan, P.; Zhang, Q.; Zhang, N.; Gao, Q.; Zhao, D.; Zhang, X.; Tu, M.; Yang, F. Super-stretchable, transparent carbon nanotube-based capacitive strain sensors for human motion detection. Sci. Rep. 2013, 3, 3402. [Google Scholar] [CrossRef]
- Ji, T.K.; Pyo, J.; Rho, J.; Ahn, J.H.; Je, J.H.; Margaritondo, G. Three-dimensional writing of highly stretchable organic nanowires. ACS Macro Lett. 2012, 1, 375–379. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, W.; Yue, Z.; Too, C.O.; Wallace, G.G. Buckled, stretchable polypyrrole electrodes for battery applications. Adv. Mater. 2011, 23, 3580–3584. [Google Scholar] [CrossRef] [PubMed]
- Ata, S.; Kobashi, K.; Yumura, M.; Hata, K. Mechanically durable and highly conductive elastomeric composites from long single-walled carbon nanotubes mimicking the chain structure of polymers. Nano Lett. 2012, 12, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Wakuda, D.; Suganuma, K. Stretchable fine fiber with high conductivity fabricated by injection forming. Appl. Phys. Lett. 2011, 98, 073304. [Google Scholar] [CrossRef]
- Bowden, N.; Brittain, S.; Evans, A.G.; Hutchinson, J.W.; Whitesides, G.M. Spontaneous formation of ordered structures in thin films of metals supported on an elastomeric polymer. Nature 1998, 393, 146–149. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, Y. Highly conductive and stretchable silver nanowire conductors. Adv. Mater. 2012, 24, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Masarapu, C.; Rong, J.; Wei, B.; Jiang, H. Stretchable supercapacitors based on buckled single-walled carbon-nanotube macrofilms. Adv. Mater. 2009, 21, 4793–4797. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, H.; Chen, P.; Guan, G.; Deng, J.; Peng, H. Smart, stretchable supercapacitors. Adv. Mater. 2014, 26, 4444–4449. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Shang, Y.Y.; He, X.D.; Li, Y.B.; Zhang, L.H.; Li, Z.; Ji, C.Y.; Shi, E.Z.; Li, P.X.; Zhu, K.; Peng, Q.Y.; et al. Carbon Nanotubes: Super-Stretchable Spring-Like Carbon Nanotube Ropes. Adv. Mater. 2012, 24, 2935. [Google Scholar] [CrossRef]
- Zang, J.F.; Ryu, S.; Pugno, N.; Wang, Q.M.; Tu, Q.; Buehler, M.J.; Zhao, X.H. Multifunctionality and control of the crumpling and unfolding of large-area graphene. Nat. Mater. 2013, 12, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Hou, C.; Wang, G.; Wang, X.; Zhang, Q.; Li, Y.; Wang, H.; Zhu, M. An elastic transparent conductor based on hierarchically wrinkled reduced graphene oxide for artificial muscles and sensors. Adv. Mater. 2016, 28, 9491–9497. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 978 92 4 156525 7. [Google Scholar]
- Dawish, M.A.A.; Robert, A.A.; Braham, R.; Hayek, A.A.A.; Saeed, A.A.; Ahmed, R.A.A.; Sabaan, F.S.A. Diabetes mellitus in Saudi Arabia: A review of the recent literature. Curr. Diabetes Rev. 2016, 12, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, F.B. The global implications of diabetes and cancer. Lancet 2014, 383, 1947–1948. [Google Scholar] [CrossRef]
- Beckles, G.L.A.; Engelgau, M.M.; Narayan, K.M.V.; Herman, W.H.; Aubert, R.E.; Williamson, D.F. Population-based assessment of the level of care among adults with diabetes in the U.S. Diabetes Care 1998, 21, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Liu, Z.F.; Ding, J.N.; Lepró, X.; Fang, S.L.; Jiang, N.; Yuan, N.Y.; Wang, R.; Yin, Q.; Lv, W.; et al. Downsized sheath–core conducting fibers for weavable superelastic wires, biosensors, supercapacitors, and strain sensors. Adv. Mater. 2016, 28, 4998–5007. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.B.; Liu, C.B.; Chen, H.P.; Shi, H.X.; Wang, L.H.; Chen, P.; Huang, W.; Dong, X.C. Free-standing electrochemical electrode based on Ni(OH)2/3D graphene foam for nonenzymatic glucose detection. Nanoscale 2014, 6, 7424–7429. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; You, J.M.; Nuñez-Flores, R.; Wang, J. Highly stretchable fully-printed CNT-based electrochemical sensors and biofuel cells: Combining intrinsic and design-induced stretchability. Nano Lett. 2015, 16, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Sappia, L.D.; Piccinini, E.; Marmisollé, W.; Santilli, N.; Maza, E.; Moya, S.; Battaglini, F.; Madrid, R.E.; Azzaroni, O. Integration of biorecognition elements on PEDOT platforms through supramolecular interactions. Adv. Mater. Interfaces 2017, 4, 1700502. [Google Scholar] [CrossRef]
- Zubkovs, V.; Schuergers, N.; Lambert, B.; Ahunbay, E.; Boghossian, A.A. Mediatorless, reversible optical nanosensor enabled through enzymatic pocket doping. Small 2017, 13, 1701654. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.S.; Toumazou, C.; Cass, A.E.G.; Johnston, D.G. Glucose sensors: A review of current and emerging technology. Diabet. Med. 2009, 26, 197–210. [Google Scholar] [CrossRef]
- Li, Y.; Hodak, M.; Lu, W.; Bernholc, J. Selective sensing of ethylene and glucose using carbon-nanotube-based sensors: An ab initio investigation. Nanoscale 2017, 9, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.D. Materials science: Flexible electronics. Science 2009, 323, 1566–1567. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Honda, W.; Arie, T.; Akita, S.; Takei, K. Fully printed, highly sensitive multifunctional artificial electronic whisker arrays integrated with strain and temperature sensors. ACS Nano 2014, 8, 3921–3927. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, J.; Lee, M.S.; Kim, J.; Hyun, B.G.; Kang, D.J.; Lee, C.Y.; Bien, F.; Park, J.U. In-situ synthesis of carbon nanotube-graphite electronic devices and their integrations onto surfaces of live plants and insects. Nano Lett. 2014, 14, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, C.; Kim, J.; Ren, F.; Pearton, S.J. Flexible graphene-based chemical sensors on paper substrates. Phys. Chem. Chem. Phys. 2013, 15, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, W.Q.; Zhang, T.; Jing, Q.; Chen, J.; Zhou, Y.S.; Bai, P.; Wang, Z.L. Self-powered, ultrasensitive, flexible tactile sensors based on contact electrification. Nano Lett. 2014, 14, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Elouarzaki, K.; Bourourou, M.; Holzinger, M.; Goff, A.L.; Marks, R.S.; Cosnier, S. Freestanding HRP–GOx redox buckypaper as an oxygen-reducing biocathode for biofuel cell applications. Energy Environ. Sci. 2015, 8, 2069–2074. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, F.; Di, C.; Zhu, D. Advances of flexible pressure sensors toward artificial intelligence and health care applications. Mater. Horiz. 2015, 2, 140–156. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Xi, J.; Abdurhman, A.A.; Ren, J.; Duan, H. Printing graphene-carbon nanotube-ionic liquid gel on graphene paper: Towards flexible electrodes with efficient loading of PtAu alloy nanoparticles for electrochemical sensing of blood glucose. Anal. Chim. Acta 2015, 903, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C Mater. 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.W.; Cheng, Y.H.; Ho, K.C. Single layer of nickel hydroxide nanoparticles covered on a porous Ni foam and its application for highly sensitive non-enzymatic glucose sensor. Sens. Actuators B Chem. 2014, 204, 159–166. [Google Scholar] [CrossRef]
- Iwu, K.O.; Lombardo, A.; Sanza, R.; Scirè, S.; Mirabella, S. Facile synthesis of Ni nanofoam for flexible and low-cost non-enzymatic glucose sensing. Sens. Actuators B Chem. 2015, 224, 764–771. [Google Scholar] [CrossRef]
- Soomro, R.A.; Ibupoto, Z.H.; Sirajuddina; Abro, M.I.; Willander, M. Electrochemical sensing of glucose based on novel hedgehog-like NiO nanostructures. Sens. Actuators B Chem. 2015, 209, 966–974. [Google Scholar] [CrossRef]
- Guo, C.Y.; Wang, Y.M.; Zhao, Y.Q.; Xu, C.L. Non-enzymatic glucose sensor based on three dimensional nickel oxide for enhanced sensitivity. Anal. Methods 2013, 5, 1644–1647. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Beni, V.; Liu, X.J.; Willander, M. Synthesis of novel CuO nanosheets and their non-enzymatic glucose sensing applications. Sensors 2013, 13, 7926–7938. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Wang, J.H.; Wang, Q.G.; Qian, X.; Qian, Y.; Yang, M.; Li, F.Y.; Lu, H.; Wang, H. Chemical fractionation of arsenic and heavy metals in fine particle matter and its implications for risk assessment: A case study in Nanjing, China. Atmos. Environ. 2015, 103, 339–346. [Google Scholar] [CrossRef]
- Li, M.; Bo, X.J.; Zhang, Y.F.; Han, C.; Guo, L.P. One-pot ionic liquid-assisted synthesis of highly dispersed PtPd nanoparticles/reduced graphene oxide composites for nonenzymatic glucose detection. Biosens. Bioelectron. 2014, 56, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Zhu, Y.H.; Chen, W.; Zhou, Y.; Li, C.Z. Flexible 3D porous CuO nanowire arrays for enzymeless glucose sensing: In situ engineered versus ex situ piled. Nanoscale 2015, 7, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Y.Q.; Gao, H.C.; Ge, S.B.; Duan, H.W. Growth of coral-like PtAu–MnO2 binary nanocomposites on free-standing graphene paper for flexible nonenzymatic glucose sensors. Biosens. Bioelectron. 2013, 41, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Lee, G.J.; Ryu, J.H.; Park, K.C.; Park, H.K. A flexible, nonenzymatic glucose biosensor based on Ni-coordinated, vertically aligned carbon nanotube arrays. RSC Adv. 2014, 4, 48310–48316. [Google Scholar] [CrossRef]
- Ruan, J.L.; Chen, C.; Shen, J.H.; Zhao, X.L.; Qian, S.H.; Zhu, Z.G. A gelated colloidal crystal attached lens for noninvasive continuous monitoring of tear glucose. Polymers 2017, 9, 125. [Google Scholar] [CrossRef]
- Lee, J.; Ko, S.; Kwon, C.H.; Lima, M.D.; Baughman, R.H.; Kim, S.J. Artificial muscle: Carbon nanotube yarn-based glucose sensing artificial muscle. Small 2016, 12, 2100. [Google Scholar] [CrossRef]
- Liu, Z.F.; Fang, S.L.; Moura, F.A.; Ding, J.N.; Jiang, N.; Di, J.T.; Zhang, M.; Lepró, X.; Galvão, D.S.; Haines, C.S.; et al. Hierarchically buckled sheath-core fibers for superelastic electronics, sensors, and muscles. Science 2015, 349, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Liu, J.; Chen, H.B.; Li, H.M. Copper/nickel nanoparticle decorated carbon nanotubes for nonenzymatic glucose biosensor. J. Solid State Electron. 2015, 19, 1511–1521. [Google Scholar] [CrossRef]
- Qin, X.Y.; Lu, W.B.; Luo, Y.L.; Chang, G.H.; Asiri, A.M.; Al-Youbi, A.; Sun, X.P. Synthesis of Ag nanoparticle-decorated 2,4,6-tris(2-pyridyl)-1,3,5-triazine nanobelts and their application for H2O2 and glucose detection. Analyst 2012, 137, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, S.S.; Zhang, H.Y.; Jiang, J.; Liu, X.Y. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.F.; Xu, Y.H.; Zhang, Z.X.; Song, W.B. Dendritic bimetallic nanostructures supported on self-assembled titanate films for sensor application. J. Phys. Chem. C 2010, 114, 20925–20931. [Google Scholar] [CrossRef]
- Lv, W.; Jiang, N.; Ding, J.N.; Liu, Z.F.; Yuan, N.Y.; Ovalle-Robles, R.; Inoue, K.; Lepró, X.; Zhang, M. Three-dimensional conducting elastomeric composites based on buckling carbon nanotube sheets for interconnects and temperature sensor. J. Nanosci. Nanotechnol. 2017, 17, 1934–1941. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, S.L.; Zakhidov, A.; Lee, S.B.; Aliev, A.E.; Williams, C.D.; Atkinson, K.R.; Baughman, R.H. Strong, transparent, multifunctional, carbon nanotube sheets. Science 2005, 309, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Arvinte, A.; Sesay, A.M.; Virtanen, V. Carbohydrates electrocatalytic oxidation using CNT–NiCo-oxide modified electrodes. Talanta 2011, 84, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiang, N.; Su, J.; Yin, Q.; Zhang, Y.; Liu, Z.S.; Lin, H.B.; Moura, F.A.; Yuan, N.Y.; Roth, S.; et al. A Bi-sheath fiber sensor for giant tensile and torsional displacements. Adv. Funct. Mater. 2017, 27, 1702134. [Google Scholar] [CrossRef]
- Pierson, J.; Wiederkehr, D.; Billard, A. Reactive magnetron sputtering of copper, silver, and gold. Thin Solid Films 2005, 478, 196–205. [Google Scholar] [CrossRef]
- Li, S.; Su, Y.W.; Li, R. Splitting of the neutral mechanical plane depends on the length of the multi-layer structure of flexible electronics. Proc. R. Soc. Lond. A 2016, 472, 20160087. [Google Scholar] [CrossRef] [PubMed]
- Lacour, S.; Wagner, S.; Huang, Z.Y.; Suo, Z.G. Stretchable gold conductors on elastomeric substrates. Appl. Phys. Lett. 2003, 82, 2404–2406. [Google Scholar] [CrossRef]
- Efimenko, K.; Rackaitis, M.; Manias, E.; Vaziri, A.; Mahadevan, L.; Genzer, J. Nested self-similar wrinkling patterns in skins. Nat. Mater. 2005, 4, 293–297. [Google Scholar] [CrossRef] [PubMed]

| Electrode | VApp. 1 (V) | LOD 2 (µM) | Strain (%) | Selectivity Test | Reference |
|---|---|---|---|---|---|
| Graphene/CNT 3/Ionic Liquid | +0.2 | 100 | Flexible | DA 4/AA 5/UA 6/NaCl | [36] |
| 3D CuO Nanowire Arrays | +0.35 | 20 | Flexible | AA/Lactose/Maltose | [45] |
| PtAu-MnO2/Graphene Paper | 0 | 20 | Flexible | DA/AA/UA | [46] |
| Ni/VCNTs 7/Graphene | +0.5 | 30 | Flexible | AA/GA 8/XY 9/UA | [47] |
| NiO QDs 10-ZnO NRs 11/Polyimide | n/a | 26 | Flexible | DA/AA/UA/cholesterol | [48] |
| Au Nanoparticles/Polyaniline/Carbon Cloth | 0 | 12.6 | Flexible | AA/UA/AMP 12/d-galactose/fructose | [49] |
| Cu Nanoparticles/RGO 13 | +0.6 | 1000 | Flexible | AA/UA/DA | [50] |
| Pt/CNTS 14/Rubber | +0.3 | 660 | 45% | n/a | [51] |
| Pt-Graphite | +0.3 | 4800 | 75% | AA/UA/DP 15/EPI 16 | [52] |
| Cu/CNTS/Rubber | +0.35 | 50 | 60% | AA/Sucrose/Lactose/UA/NaCl | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Liu, Z.; Wu, K.; Mou, L.; Ovalle-Robles, R.; Inoue, K.; Zhang, Y.; Yuan, N.; Ding, J.; Qiu, J.; et al. Fabrication of Stretchable Copper Coated Carbon Nanotube Conductor for Non-Enzymatic Glucose Detection Electrode with Low Detection Limit and Selectivity. Polymers 2018, 10, 375. https://doi.org/10.3390/polym10040375
Jiang D, Liu Z, Wu K, Mou L, Ovalle-Robles R, Inoue K, Zhang Y, Yuan N, Ding J, Qiu J, et al. Fabrication of Stretchable Copper Coated Carbon Nanotube Conductor for Non-Enzymatic Glucose Detection Electrode with Low Detection Limit and Selectivity. Polymers. 2018; 10(4):375. https://doi.org/10.3390/polym10040375
Chicago/Turabian StyleJiang, Dawei, Zhongsheng Liu, Kunkun Wu, Linlin Mou, Raquel Ovalle-Robles, Kanzan Inoue, Yu Zhang, Ningyi Yuan, Jianning Ding, Jianhua Qiu, and et al. 2018. "Fabrication of Stretchable Copper Coated Carbon Nanotube Conductor for Non-Enzymatic Glucose Detection Electrode with Low Detection Limit and Selectivity" Polymers 10, no. 4: 375. https://doi.org/10.3390/polym10040375