Thermal, Structural, and Rheological Characterization of Waxy Starch as a Cryogel for Its Application in Food Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Thermal Analysis by Modulated Differential Scanning Calorimetry (MDSC)
2.3. Structural Ordering Degree
2.4. Scanning Electron Microscopy (SEM)
2.5. Rheological Analysis
2.6. Fourier Transform Infrared-Diffuse Reflectance (FTIR-DR) Spectroscopy
2.7. Statistical Analysis
3. Results and Discussion
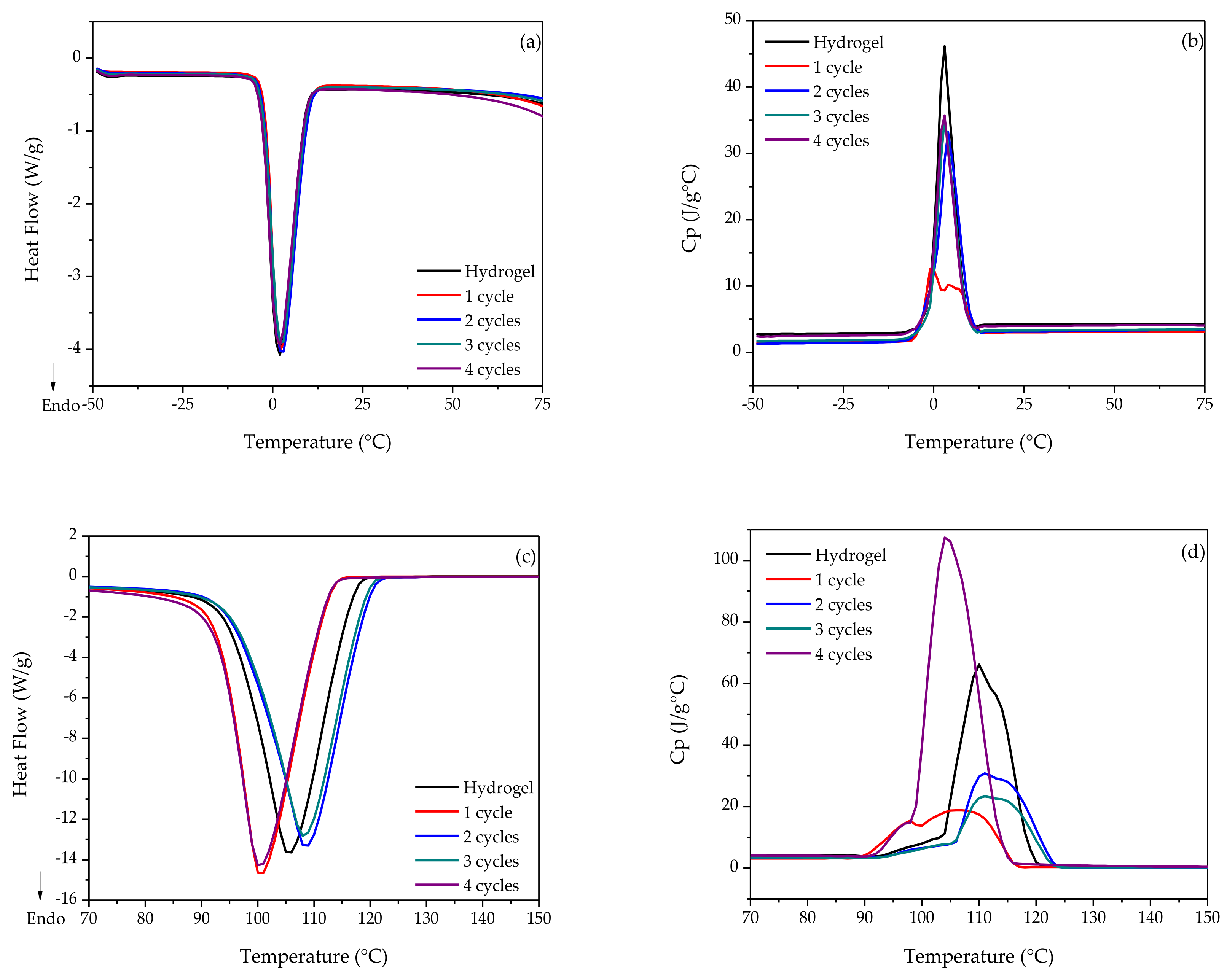
3.1. MDSC Analysis
3.2. Structural Ordering Degree
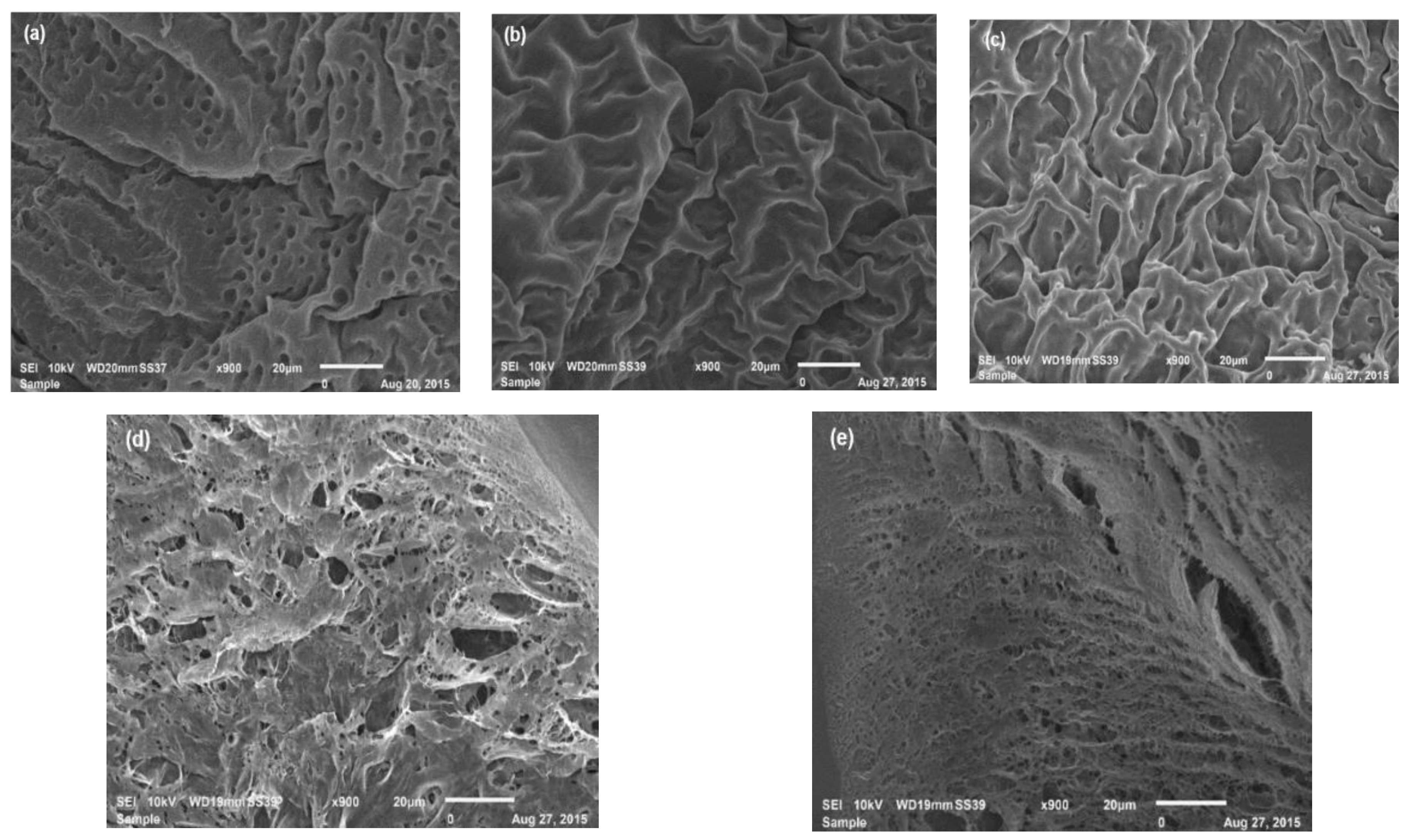
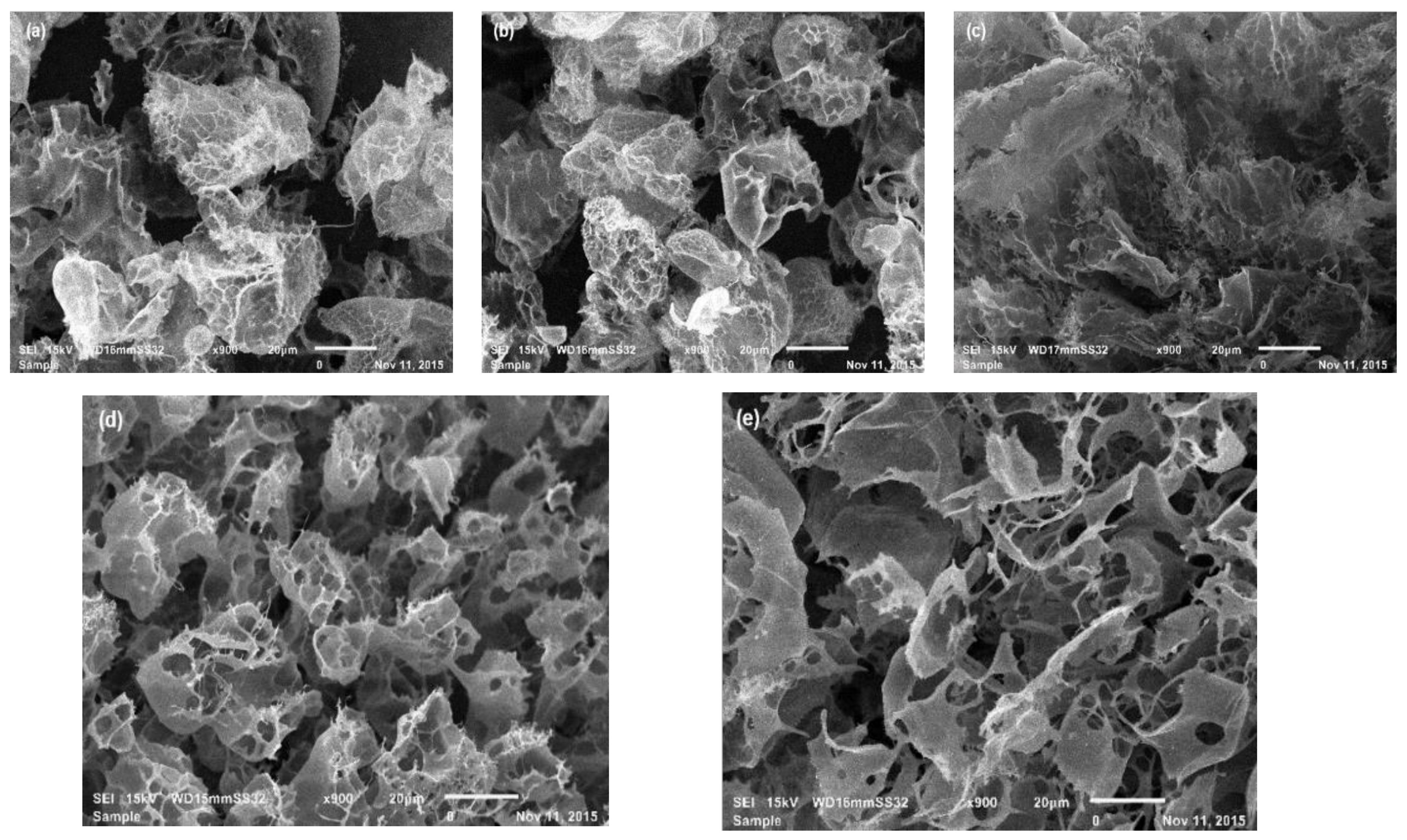
3.3. SEM Analysis
3.4. Rheological Analysis
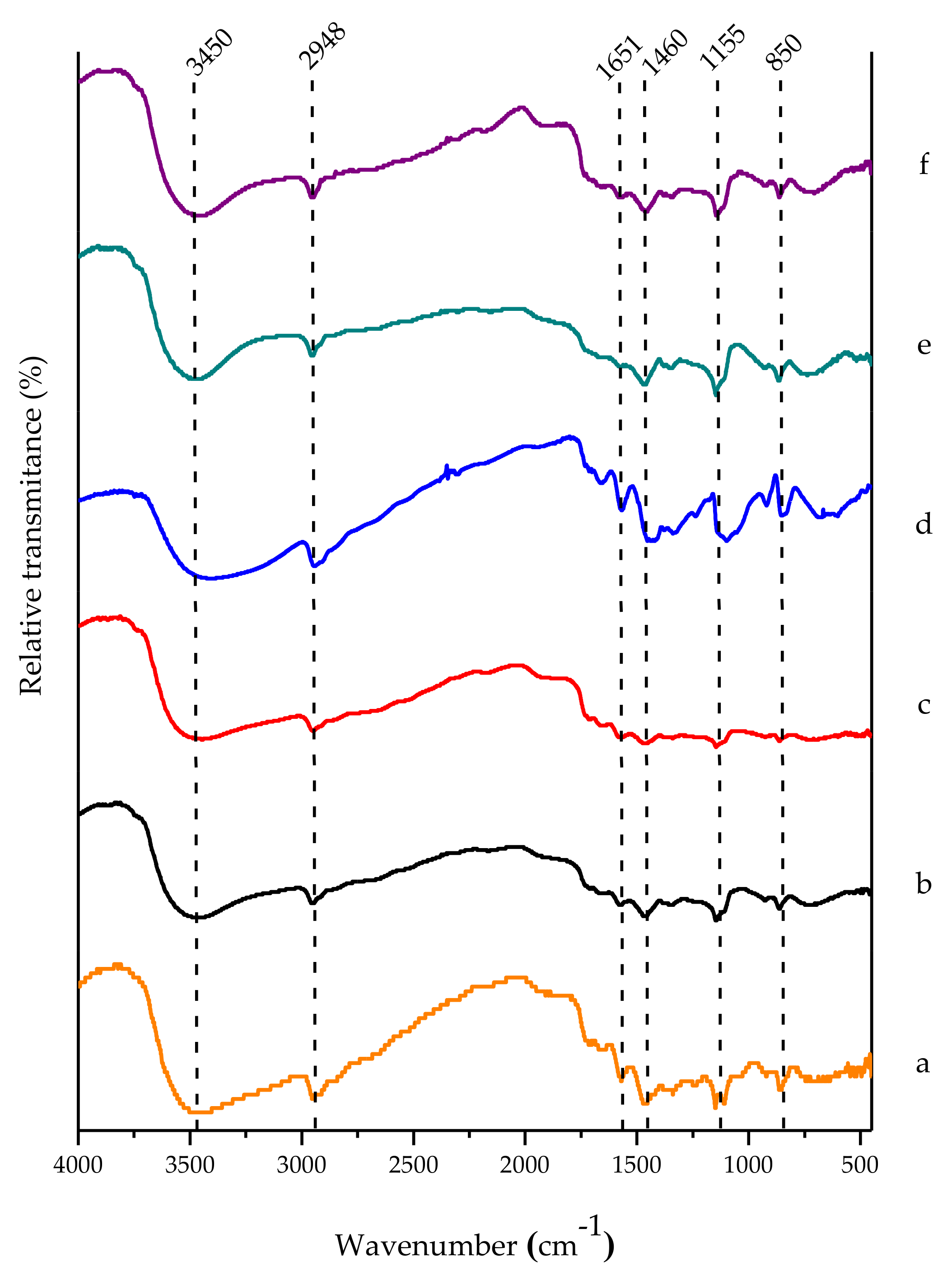
3.5. FTIR Spectroscopy Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Phisically and Chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regand, A.; Goff, H.D. Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocoll. 2003, 17, 95–102. [Google Scholar] [CrossRef]
- Morris, G.J.; Acton, E. Controlled ice nucleation in cryopreservation—A review. Cryobiology 2013, 66, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.M.; Kenawy, E.R.S. Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arabian J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of poly (vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Patachia, S.; Dobritoiu, R.; Coviello, T. Determination of the sorption efficiency of poly (vinyl alcohol)/scleroglucan cryogels, against Cu+2 ions. Env. Eng. Manag. J. 2011, 10, 193–195. [Google Scholar]
- Smith, T.J.; Kennedy, J.E.; Higginbotham, C.L. The rheological and thermal characteristics of freeze-thawed hydrogels containing hydrogen peroxide for potential wound healing applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, F.; Maffezzoli, A.; Ottenhof, M.-A.; Farhat, I.A.; Mitchell, J.R. Ultrasonic investigation of wheat starch retrogradation. J. Food Eng. 2006, 75, 258–266. [Google Scholar] [CrossRef]
- Zobel, H. Starch crystal transformations and their industrial importance. Starch-Stärke 1988, 40, 1–7. [Google Scholar] [CrossRef]
- Van Hung, P.; Maeda, T.; Morita, N. Waxy and high-amylose wheat starches and flours—characteristics, functionality and application. Trends Food Sci. Technol. 2006, 17, 448–456. [Google Scholar] [CrossRef]
- Seetapan, N.; Limparyoon, N.; Fuongfuchat, A.; Gamonpilas, C.; Methacanon, P. Effect of freezing rate and starch granular morphology on ice formation and non-freezable water content of flour and starch gels. Int. J. Food Prop. 2016, 19, 1616–1630. [Google Scholar] [CrossRef]
- Yu, X.; Yu, H.; Zhang, J.; Shao, S.; Xiong, F.; Wang, Z. Endosperm structure and physicochemical properties of starches from normal, waxy, and super-sweet maize. Int. J. Food Prop. 2015, 18, 2825–2839. [Google Scholar] [CrossRef]
- Giannouli, P.; Morris, E.R. Cryogelation of xanthan. Food Hydrocoll. 2003, 17, 495–501. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: preparation, properties and application. Russ. Chem. Rev. 2002, 71, 489. [Google Scholar] [CrossRef]
- Meléndez, P.R.; Arjona, R.J.L.; Velázquez, C.R.R.; Méndez, A.A.; Vázquez, D.A. On the thermal properties of frozen, refrozen and freeze drying porcine Longissimus dorsi. J. Anim. Vet. Adv. 2011, 10, 2956–2960. [Google Scholar]
- Arjona, R.J.L.; Hernández, G.R.P.; Navarro, L.I.; Coria, H.J.; Rosas, M.M.E.; Meléndez, P.R. Heat capacity prediction during pork meat thawing: Application of artificial neural network. J. Food Process Eng. 2016, 40, 1–8. [Google Scholar]
- Patel, A.K.; Bajpai, R.; Keller, J.M. On the crystallinity of PVA/palm leaf biocomposite using DSC and XDR techniques. Microsyst. Technol. 2014, 20, 41–49. [Google Scholar] [CrossRef]
- El-Sayed, S.; Mahmoud, K.H.; Fatah, A.A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose/polyvinyl alcohol blends. Physica B 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of salecan/poly(vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015, 118, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sudhamani, S.R.; Prasad, M.S.; Udaya-Sankar, K. DSC and FTIR studies on gellan and polyvinyl alcohol (PVA) blend films. Food Hydrocoll. 2003, 17, 245–250. [Google Scholar] [CrossRef]
- Gómez, I.; Otazo, E.M.; Hernández, H.; Rubio, E.; Varela, J.; Ramírez, M.; Barajas, I.; Gordillo, A.J. Thermal degradation study of PVA derivative with pendant phenylthionecarbamate groups by DSC/TGA and GC/MS. Polym. Degrad. Stab. 2015, 112, 132–136. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Elliot, S.R. Physics of Amorphous Materials, 2nd ed.; Longman Scientific & Technical: New York, NY, USA, 1993. [Google Scholar]
- Ball, S.; Guan, H.P.; James, M.; Myers, A.; Keeling, P.; Mouille, G. From glycogen to amylopectin: A model for the biogenesis of the plant starch granule. Cell 1996, 86, 349–352. [Google Scholar] [CrossRef]
- Hulleman, S.H.D.; Janssen, F.H.P.; Feil, H. The role of water during plasticization of native starches. Polymer 1998, 39, 2043–2048. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Donald, A.M. The influence of amylose on starch granule structure. Int. J. Biol. Macromol. 1995, 17, 315–321. [Google Scholar] [CrossRef]
- Lourdin, D.; Dellavalle, G.; Colonna, P. Influence of amylose content on starch films and foams. Carbohydr. Polym. 1995, 27, 261–270. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Hulleman, S.H.D.; De Wit, D.; Vliegenthart, J.F.G. Crystallinity in starch bioplastics. Ind. Crop. Prod. 1996, 5, 11–22. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bajpai, A.K.; Bajpai, J.; Singh, S.K. Evaluation of poly (vinyl alcohol) based cryogel-zinc oxide nanocomposites for possible applications as wound dressing materials. Mater. Sci. Eng. 2016, 65, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Aouada, F.A.; De-Moura, M.R.; Fernandes, P.R.G.; Rubira, A.F.; Muniz, E.C. Optical and morphological characterization of polyacrylamide hydrogel and liquid crystal systems. Eur. Polym. J. 2005, 41, 2134–2141. [Google Scholar] [CrossRef]
- Huijbrechts, A.M.L.; Desse, M.; Budtova, T.; Franssen, M.C.R.; Visser, G.M.; Boeriu, C.G.; Sudhölter, E.J.R. Physicochemical properties of etherified maize starches. Carbohydr. Polym. 2008, 74, 170–184. [Google Scholar] [CrossRef]
- Shi, M.; Chen, Y.; Yu, S.; Gao, Q. Preparation and properties of RS III from waxy maize starch with pullulanase. Food Hydrocoll. 2013, 33, 19–25. [Google Scholar] [CrossRef]
- Yoon, H.S.; Lee, J.H.; Lim, S.T. Utilization for retrograded waxy maize starch gels as tablet matrix for controlled release of theophylline. Carbohydr. Polym. 2009, 76, 449–453. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Chen, X.; Yu, F.; Zhu, Z. Investigation of PVA/WS-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr. Polym. 2008, 73, 401–408. [Google Scholar] [CrossRef]
- Wang, Y.; Lue, A.; Zhang, L. Rheological behavior of waterbone polyurethane/starch aqueous dispersions during cure. Polymer 2009, 50, 5474–5481. [Google Scholar] [CrossRef]
- Bhunia, T.; Giri, A.; Nasim, T.; Chattopadhyay, D.; Bandyopadhyay, A. Uniquely different PVA-xanthan gum irradiated membranes as transdermal diltiazem delivery device. Carbohydr. Polym. 2013, 95, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.W.; Zhang, L.M.; Wang, C.; Chen, R.F. Preparation and properties of new micellar drug carriers based on hydrophobically modified amylopectin. Carbohydr. Polym. 2011, 83, 1499–1506. [Google Scholar] [CrossRef]
- Shalviri, A.; Liu, Q.; Abdekhodaie, M.J.; Wu, X.Y. Novel modified starch-xanthan gum hydrogels for controlled drug delivery: Synthesis and characterization. Carbohydr. Polym. 2010, 79, 898–907. [Google Scholar] [CrossRef]
- Zou, W.; Yu, L.; Liu, X.; Chen, L.; Zhang, X.; Qiao, D.; Zhang, R. Effects of amylose/amylopectin ratio on starch-based superabsorbent polymers. Carbohydr. Polym. 2012, 87, 1583–1588. [Google Scholar] [CrossRef]




| Sample | PVA | WS |
|---|---|---|
| Hydrogel | 84.47 ± 0.52 | 84.12 ± 0.33 |
| 1 cycle | 85.90 ± 0.97 | 90.80 ± 0.18 |
| 2 cycle | 81.70 ± 0.92 | 94.72 ± 0.14 |
| 3 cycles | 85.53 ± 1.17 | 89.05 ± 0.87 |
| 4 cycles | 93.30 ± 1.11 | 89.41 ± 0.56 |
| Sample | PVA | WS | ||
|---|---|---|---|---|
| n | k | n | k | |
| Hydrogel | 0.9767 ± 0.0008 | 0.0183 ± 0.0007 | 1.4999 ± 0.0015 | 4 × 10−4 ± 0.0002 |
| 1 cycle | 1.0425 ± 0.0011 | 0.0113 ± 0.0014 | 1.5855 ± 0.0009 | 2 × 10−4 ± 0.0001 |
| 2 cycles | 1.0723 ± 0.0002 | 0.0091 ± 0.0003 | 1.6198 ± 0.0006 | 1 × 10−4 ± 0.0001 |
| 3 cycles | 1.0973 ± 0.0010 | 0.0074 ± 0.0004 | 1.6696 ± 0.0012 | 1 × 10−4 ± 0.0001 |
| 4 cycles | 1.1940 ± 0.0009 | 0.0036 ± 0.0002 | 1.7510 ± 0.0018 | 1 × 10−5 ± 0.00001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coria-Hernández, J.; Méndez-Albores, A.; Meléndez-Pérez, R.; Rosas-Mendoza, M.E.; Arjona-Román, J.L. Thermal, Structural, and Rheological Characterization of Waxy Starch as a Cryogel for Its Application in Food Processing. Polymers 2018, 10, 359. https://doi.org/10.3390/polym10040359
Coria-Hernández J, Méndez-Albores A, Meléndez-Pérez R, Rosas-Mendoza ME, Arjona-Román JL. Thermal, Structural, and Rheological Characterization of Waxy Starch as a Cryogel for Its Application in Food Processing. Polymers. 2018; 10(4):359. https://doi.org/10.3390/polym10040359
Chicago/Turabian StyleCoria-Hernández, Jonathan, Abraham Méndez-Albores, Rosalía Meléndez-Pérez, Marta Elvia Rosas-Mendoza, and José Luis Arjona-Román. 2018. "Thermal, Structural, and Rheological Characterization of Waxy Starch as a Cryogel for Its Application in Food Processing" Polymers 10, no. 4: 359. https://doi.org/10.3390/polym10040359