Synthesis of Waterborne Polyurethane by the Telechelic α,ω-Di(hydroxy)poly(n-butyl acrylate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Diiodoxylene
2.4. Degenerative Iodine Transfer Polymerization of n-BA
2.5. Kinetics Study of Poly(n-butyl acrylate) by Degenerative Iodine Transfer Polymerization
2.6. Synthesis of Polyacrylate-Based Polyurethane Dispersions (PUDs)
3. Results and Discussion
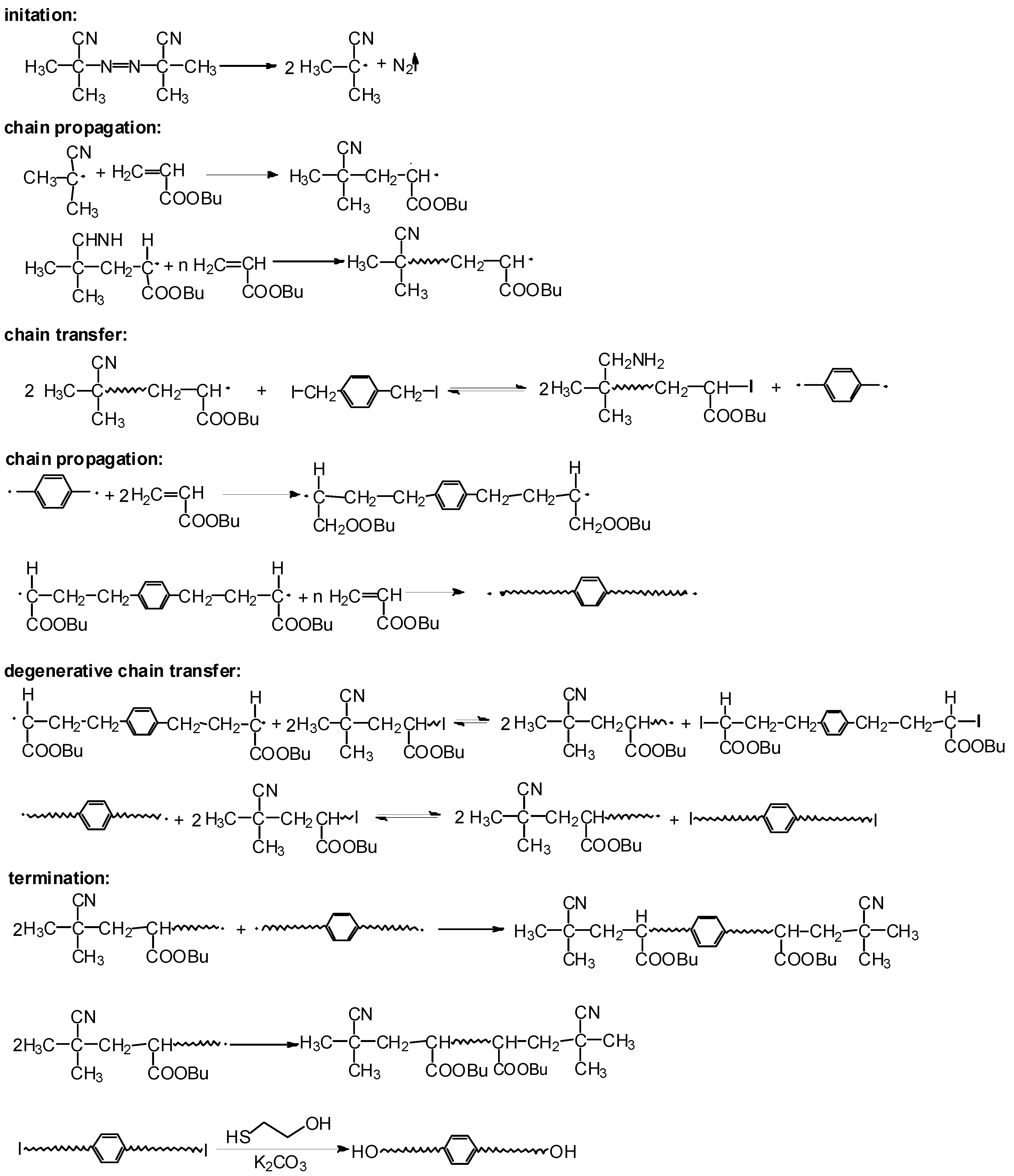
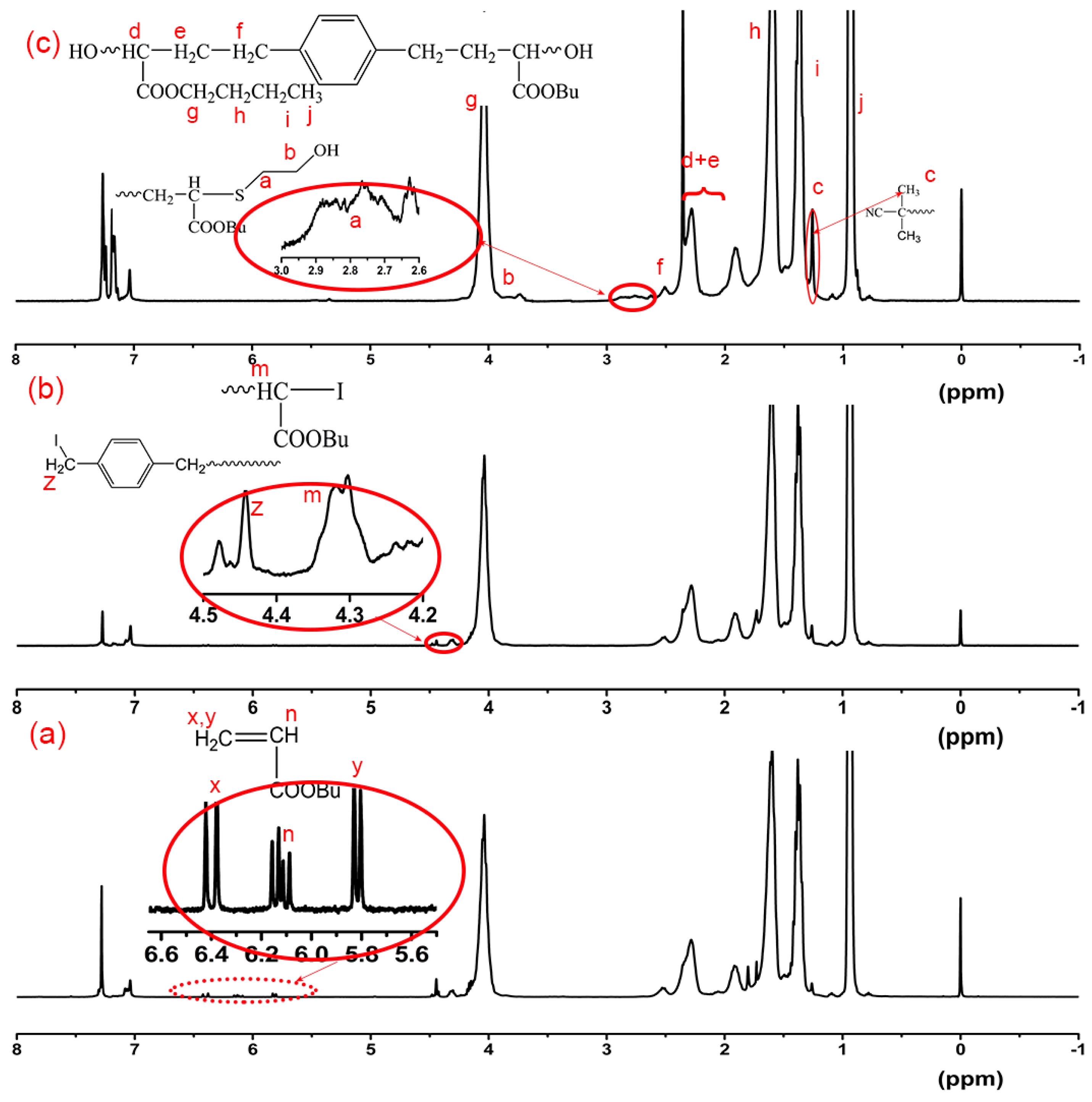
3.1. Design of DITP System for Preparation of Hydroxyl-Terminated Poly(butyl acrylate)
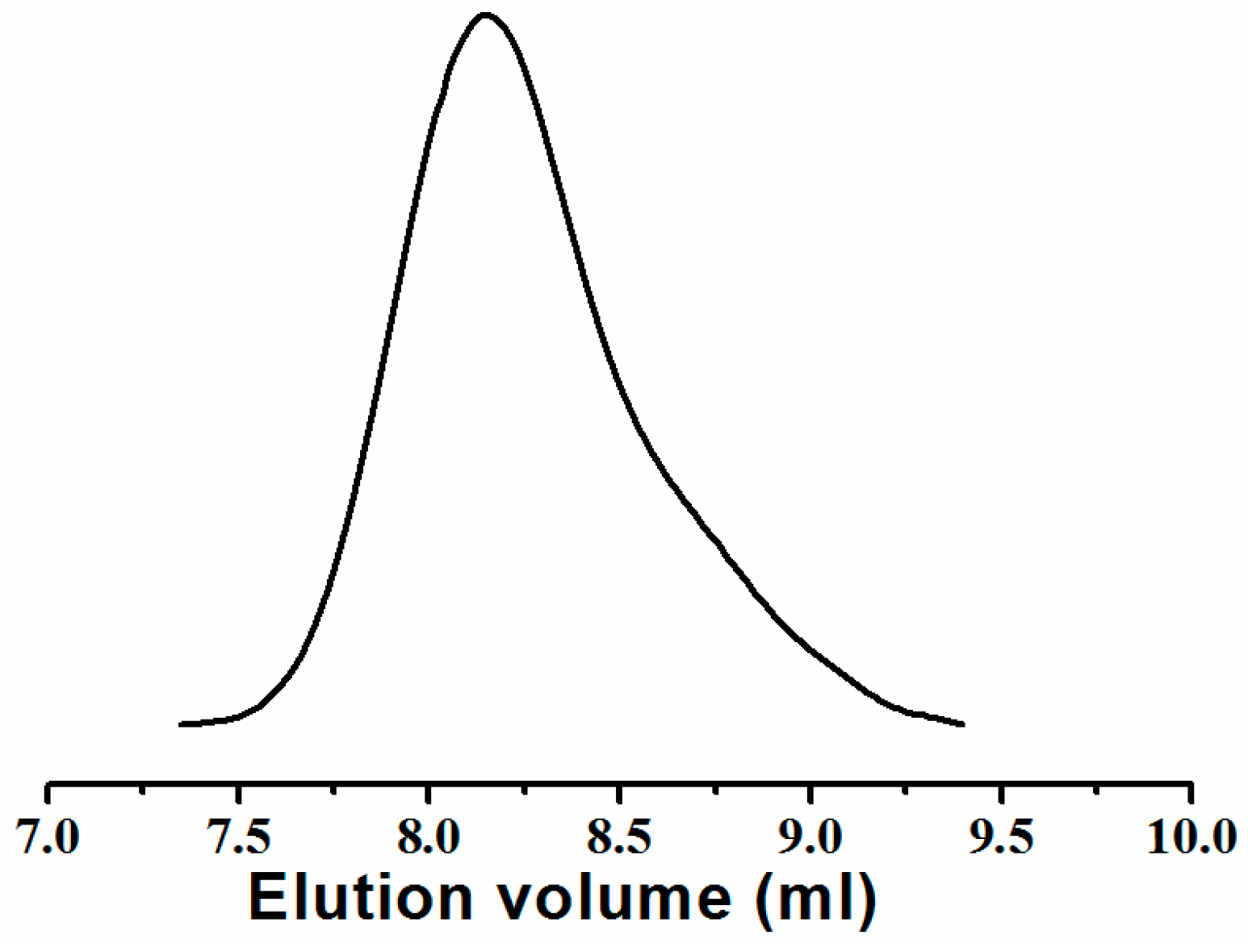
3.2. DITP of n-BA Using DIX and ACPO as Transfer Agent and Initiator
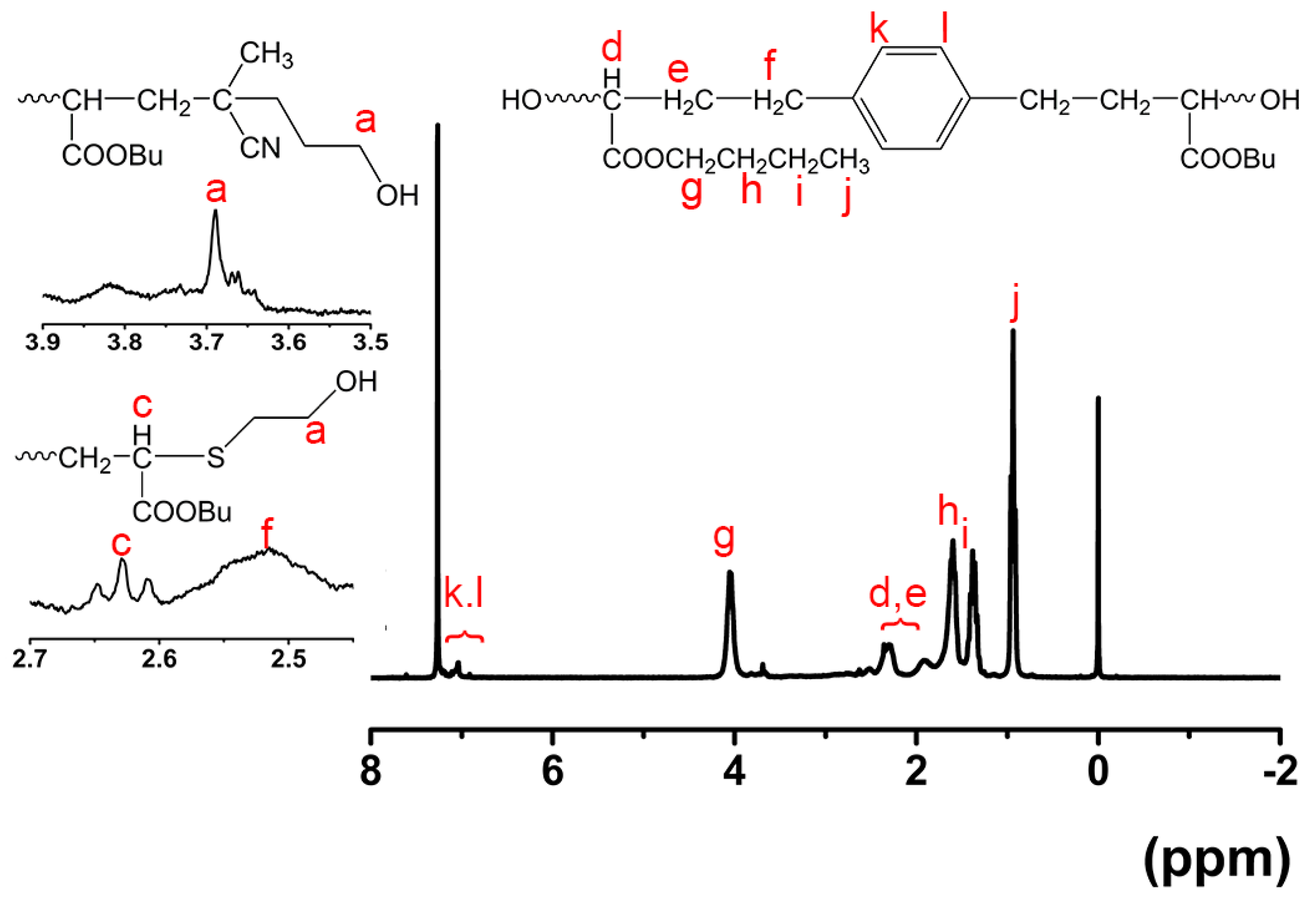
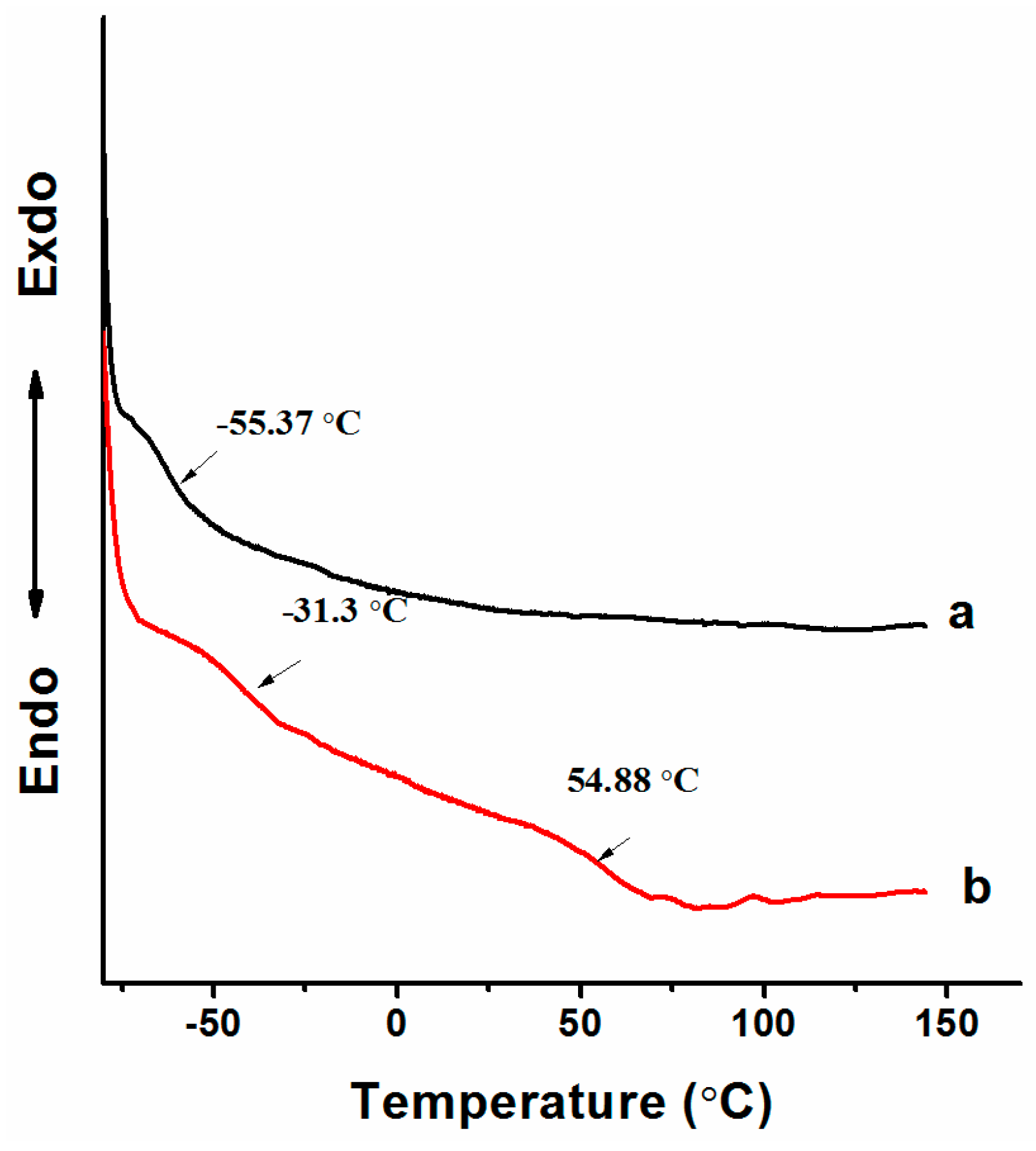
3.3. Synthesizing Aqueous Polyurethane Dispersions with α,ω-Di(hy-droxy)poly(n-butyl acrylate)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; Benhardt, H.A., Lister, L., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 135–139. ISBN 978-1-4398-6313-8. [Google Scholar]
- Zhang, K.; Fu, H.Q.; Huang, H.; Chen, H.Q. Waterborne Epoxy-Acrylic Dispersions Modified by Siloxane. J. Dispers. Sci. Technol. 2007, 28, 1209–1217. [Google Scholar] [CrossRef]
- Chai, G.; Sun, D. Preparation and Properties of Reactive Polyurethane Polyacrylate Blends based on Carbonyl-Hydrazide reaction. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Chen, J.F.; Xiao, L.I.; Zhang, W.Y.; Cai, J.X. Research Progress of Waterborne Polyurethane Modified by Polyacrylate. China Adhes. 2009, 18, 54–58. [Google Scholar]
- Tennebroek, R.; Geurts, J.; Overbeek, A.; Harmsen, A. Self Crosslinkable Urethanes and Urethane-Acrylics. Surf. Coat. Int. 2000, 83, 13–19. [Google Scholar] [CrossRef]
- Wu, L.; Yu, H.; Yan, J.; You, B. Structure and composition of the surface of urethane/acrylic composite latex films. Polym. Int. 2001, 50, 1288–1293. [Google Scholar] [CrossRef]
- Peruzzo, P.J.; Anbinder, P.S.; Pardini, O.R.; Vega, J.; Costa, C.A.; Galembeck, F.; Amalvy, J.I. Waterborne Polyurethane/Acrylate: Comparison of Hybrid and Blend Systems. Prog. Org. Coat. 2011, 72, 429–437. [Google Scholar] [CrossRef]
- Hegedus, C.R.; Kloiber, K.A. Aqueous Acrylic-Polyurethane Hybrid Dispersions and Their Use in Industrial Coatings. J. Coat. Technol. 1996, 68, 39–48. [Google Scholar]
- Xu, P.L.; Zhang, S.Q. Handbook of Polyurethane Materials, 2nd ed.; Lu, J.H., Zheng, J., Eds.; Chemical Industry Press: Beijing, China, 2011; pp. 106–107. ISBN 978-7-122-10845-6. [Google Scholar]
- Shi, R. Synthesis of TDI-Polyurethane/Polyacrylate Composite Emulsion by Solvent-free Method and Performances of the Latex Film. J. Macromol. Sci. A Pure Appl. Chem. 2013, 50, 350–357. [Google Scholar] [CrossRef]
- Fierens, S.K.; Telitel, S.; Van Steenberge, P.H.M.; Reyniers, M.; Marin, G.B.; Lutz, J.; D’Hooge, D.R. Model-Based Design to Push the Boundaries of Sequence Control. Macromolecules 2016, 49, 9336–9344. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Dai, J.; Bai, C. Synthesis and Properties of PDMS Modified Waterborne Polyurethane-Acrylic Hybrid Emulsion by Solvent-Free Method. Prog. Org. Coat. 2008, 63, 238–244. [Google Scholar] [CrossRef]
- Chai, S.L.; Jin, M.M.; Tan, H.M. Comparative Study between Core-Shell and Interpenetrating Network Structure Polyurethane/Polyacrylate Composite Emulsions. Eur. Polm. J. 2008, 44, 3306–3313. [Google Scholar] [CrossRef]
- Athawale, V.D.; Kulkarni, M.A. Core-Shell and Interpenetrating-Network PU/AC Hybrids: Synthesis and Comparison-Waterborne Polyurethane/Polyacrylate Hybrid Dispersions. Pigment Resin Technol. 2010, 39, 141–148. [Google Scholar] [CrossRef]
- Chai, S.L.; Jin, M.M. Morphology and Particle Size of Nanograde Polyurethane/Polyacrylate Hybrid Emulsions. J. Appl. Polym. Sci. 2010, 114, 2030–2035. [Google Scholar] [CrossRef]
- Cai, W.S.; Cao, D.L. Synthesis of Hydroxyl Acrylate Resin with Lower Molecular Weight. Mod. Chem. Ind. 2010, 30, 46–48. [Google Scholar]
- Li, H.; Chen, J.H.; Zeng, X.R.; Zou, F.Z.; Zheng, Y.M. Progress in Synthesis of Hydroxyl Acrylate Resin. Chem. Adhes. 2013, 35, 45–48. [Google Scholar]
- Lee, J.; Nicholas, P.P.; Pourahmady, N.; Puts, R.D. End-Functionalized Polymers by Controlled Free-Radical Polymerization Process and Polymers Made Therefrom. U.S. Patent 6,784,256, 7 November 2000. [Google Scholar]
- Goto, A.; Fukuda, T. Kinetics of Living Radical Polymerization. Prog. Polym. Sci. 2004, 29, 329–385. [Google Scholar] [CrossRef]
- Lacroix-Desmazes, P.; Severac, R.; Boutevin, B. Reverse Iodine Transfer Polymerization of Methyl Acrylate and n-Butyl Acrylate. Macromolecules 2005, 38, 6299–6309. [Google Scholar] [CrossRef]
- David, G.; Boyer, C.; Tonnar, J.; Ameduri, B.; Lacroix-Desmazes, P.; Boutevin, B. Use of Iodocompounds in Radical Polymerization. Chem. Rev. 2006, 106, 3936–3962. [Google Scholar] [CrossRef] [PubMed]
- Tonnar, J.; Lacroix-Desmazes, P.; Boutevin, B. Living Radical Ab Initio Emulsion Polymerization of n-Butyl Acrylate by Reverse Iodine Transfer Polymerization. ACS Symp. 2006, 944, 281–282. [Google Scholar]
- Pouget, E.; Tonnar, J.; Eloy, C.; Lacroix-Desmazes, P.; Boutevin, B. Synthesis of Poly(styrene)-b-Poly(dimethylsiloxane)-b-Poly(styrene) Triblock Copolymers by Iodine Transfer Polymerization in Miniemulsion. Macromolecules 2006, 39, 6009–6016. [Google Scholar] [CrossRef]
- Boyer, C.; Lacroix-Desmazes, P.; Robin, J.; Boutevin, B. Reverse Iodine Transfer Polymerization (RITP) of Methyl Methacrylate. Macromolecules 2006, 39, 4044–4053. [Google Scholar] [CrossRef]
- Rocha, N.; Coelho, J.F.J.; Gois, J.R.; Gil, M.H.; Gonçalves, P.M.O.F.; Guthrie, J.T. Poly(vinyl chloride)-b-Poly(hydroxypropyl acrylate)-b-Poly(vinyl chloride): Understanding the Synthesis of an Amphiphilic PVC block Copolymer on a Pilot Scale. J. Vinyl Addit. Technol. 2013, 19, 94–104. [Google Scholar] [CrossRef]
- Banerjee, S.; Patil, Y.; Ono, T.; Ameduri, B. Synthesis of ω-Iodo and Telechelic Diiodo Vinylidene Fluoride-Based (Co.)polymers by Iodine Transfer Polymerization Initiated by an Innovative Persistent Radical. Macromolecules 2016, 50, 203–214. [Google Scholar] [CrossRef]
- Farcet, C.; Lansalot, M.; Pirri, R.; Vairon, J.P.; Charleux, B. Polystyrene-block-Poly(butyl acrylate) and Polystyrene-block-Poly[(butyl acrylate)-co-Styrene] block Copolymers Prepared via Controlled Free-Radical Miniemulsion Polymerization using Degenerative Iodine Transfer. Macromol. Rapid Commun. 2000, 21, 921–926. [Google Scholar] [CrossRef]
- Clerc, S.; Tonnar, J.; Lacroix-Desmazes, P. Controlled Radical Polymerization of 1,1,2,2-Tetrahydroperfluorodecyl Acrylate by Reverse Iodine Transfer Polymerization (RITP). Eur. Polym. J. 2013, 49, 682–692. [Google Scholar] [CrossRef]
- Nabuurs, T.; Overbeek, G.C.; Visser, D.; Vaes, F. Process for Obtaining Low Free Monomer Levels in a block Copolymer Emulsion Prepared with (Reverse) Iodine Transfer Polymerisation. U.S. Patent 9,096,756 B2, 4 August 2015. [Google Scholar]
- Percec, V.; Popov, A.V. Functionalization of the active chain ends of poly (vinyl chloride) obtained by single-electron-transfer/degenerative-chain-transfer mediated living radical polymerization: Synthesis of telechelic α,ω-di (hydroxy) poly (vinyl chloride). J. Polym. Sci. A Polym. Chem. 2005, 43, 1255–1260. [Google Scholar] [CrossRef]
- Finkelstein, H. Darstellung Organischer Jodide aus den Entsprechenden Bromiden und Chloriden. Eur. J. Inorg. Chem. 1910, 43, 1528–1532. [Google Scholar] [CrossRef]
- D’Hooge, D.R.; Steenberge, P.H.M.V.; Reyniers, M.F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Davis, T.P.; Stenzel, M.H. Probing Mechanistic Features of Conventional, Catalytic and Living Free Radical Polymerizations using Soft Ionization Mass Spectrometric Techniques. Polymer 2004, 45, 7791–7805. [Google Scholar] [CrossRef]
- Wright, T.G.; Pfukwa, H.; Pasch, H. Advanced Analytical Methods for the Structure Elucidation of Polystyrene-b-poly(n-butyl acrylate) block Copolymers Prepared by Reverse Iodine Transfer Polymerisation. Anal. Chim. Acta 2015, 892, 183–194. [Google Scholar] [CrossRef] [PubMed]
- De Rybel, N.; Van Steenberge, P.H.M.; Reyniers, M.R.; Barner Owollik, C.; D’Hooge, D.R.; Marin, G.B. An Update on the Pivotal Role of Kinetic Modeling for the Mechanistic Understanding and Design of Bulk and Solution RAFT Polymerization. Macromol. Theory Simul. 2017. [Google Scholar] [CrossRef]
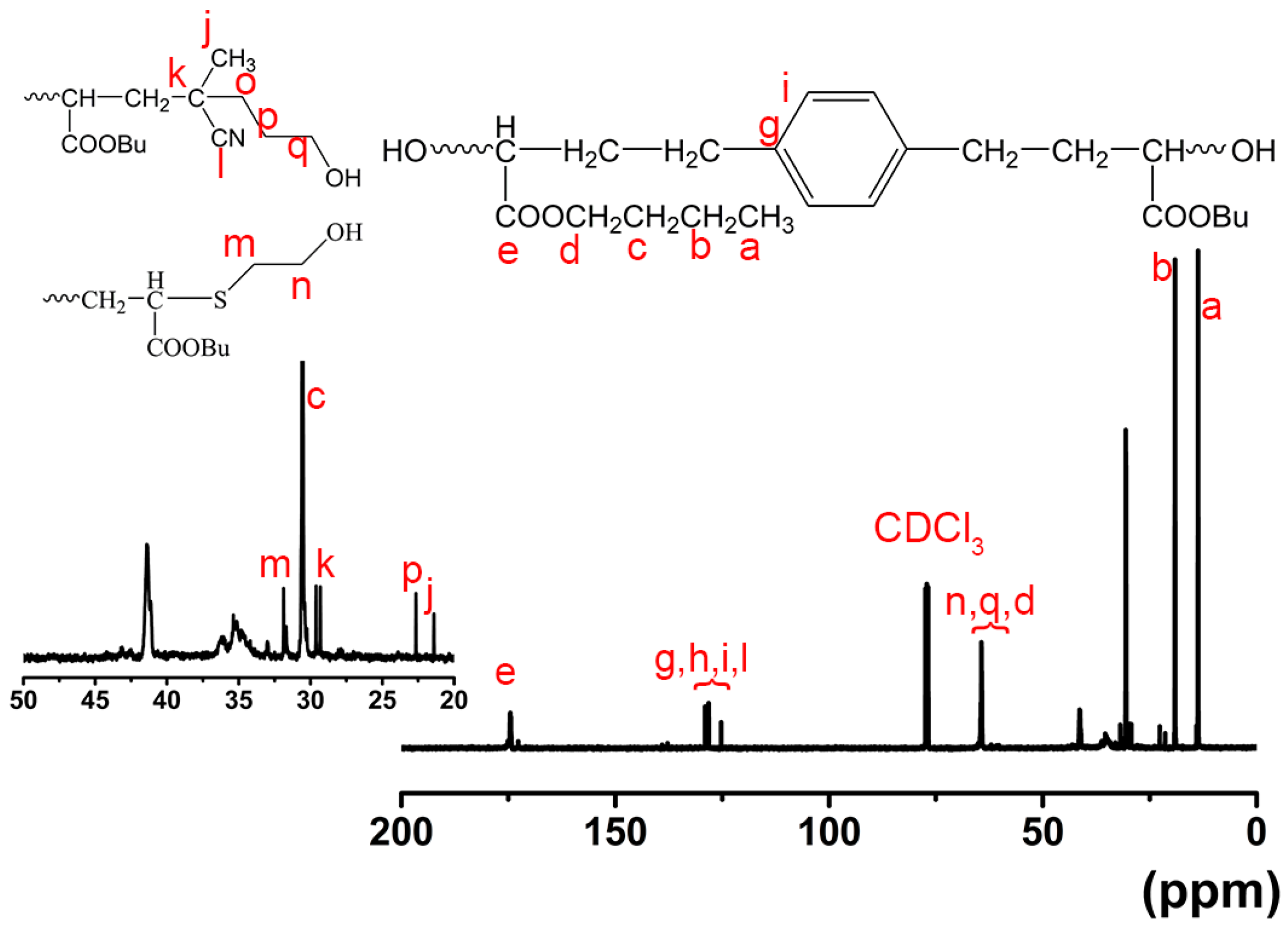
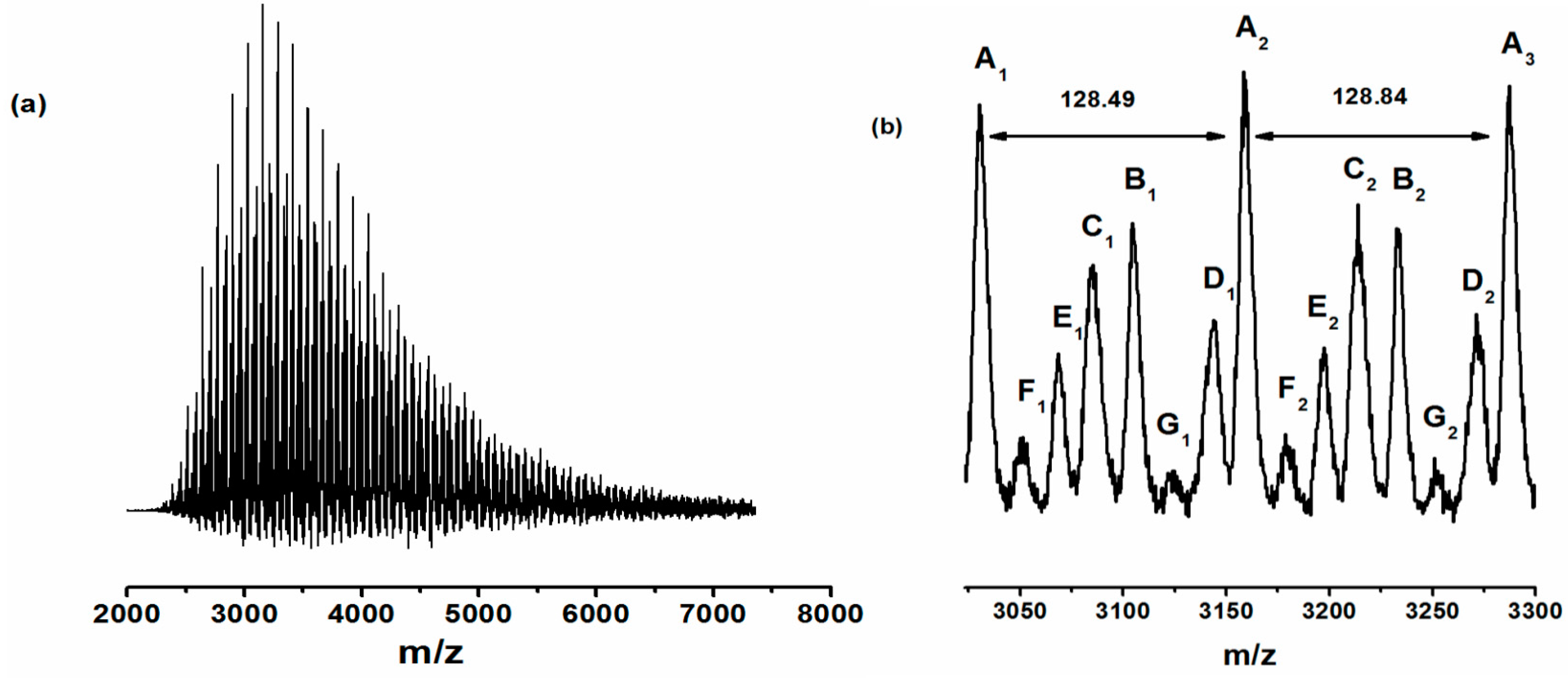
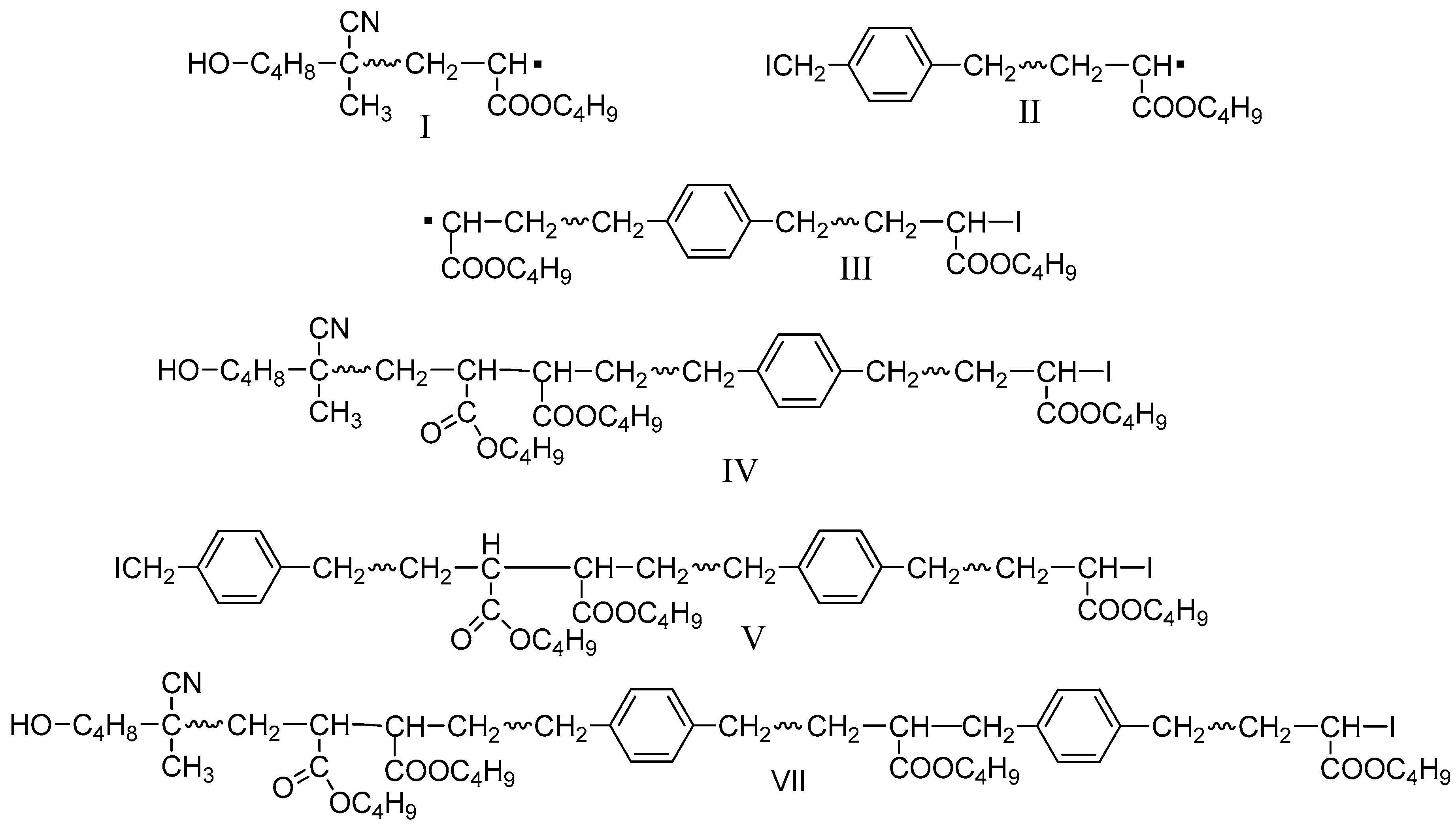
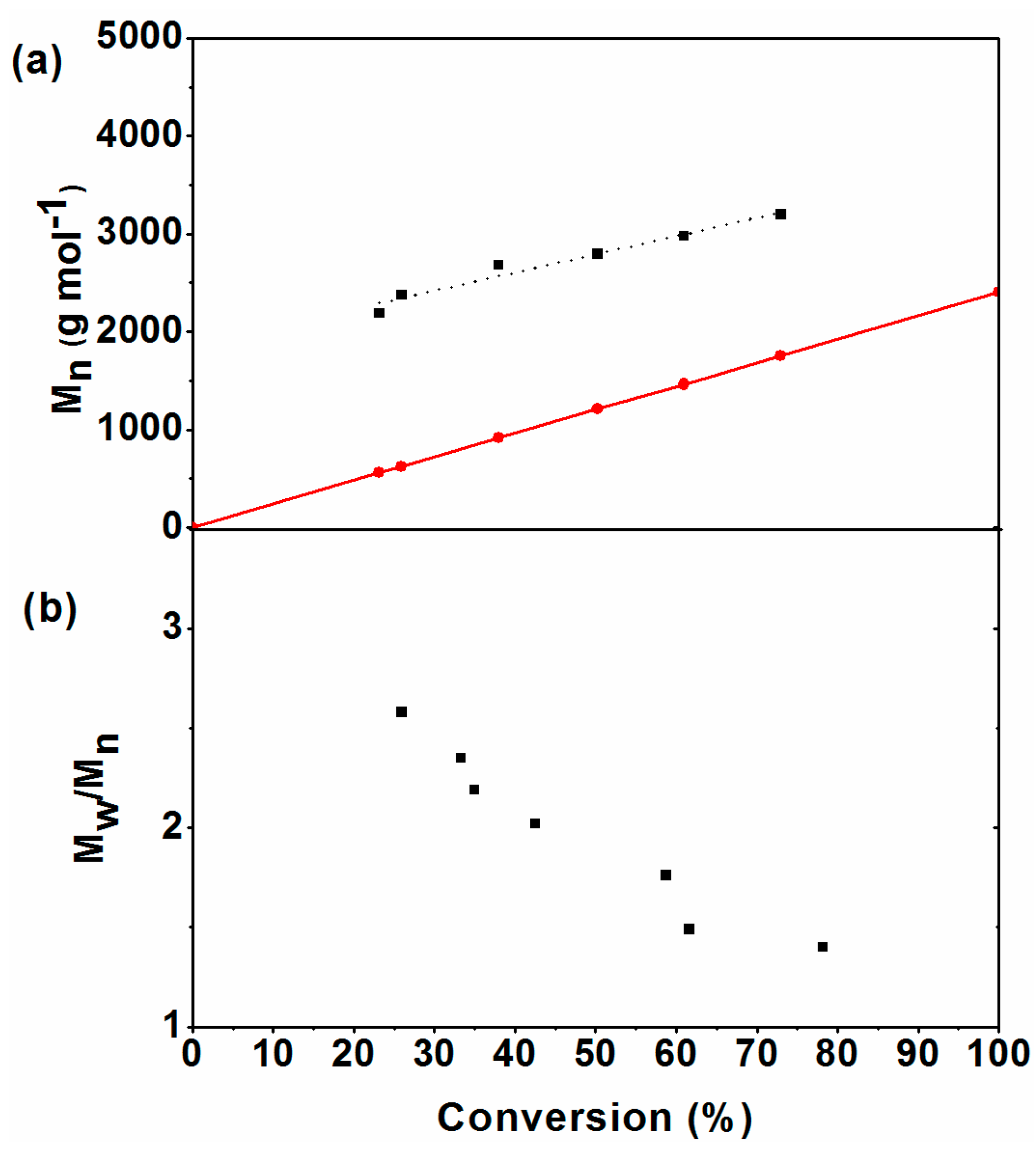
| Exp. * | [n-BA]:[AIBN]:[CTAs] | Conv. (%) | Mn,GPC (g mol−1) | OH value (mg KOH/g) | Functionality of end-hydroxyl (%) a |
|---|---|---|---|---|---|
| 1 | 10:1:0.5 | 89 | 2310 | 15 | 42 |
| 2 | 10:1:1 | 87 | 2020 | 20 | 40 |
| 3 | 10:0.5:1 | 83 | 1910 | 25 | 54 |
| 4 | 10:0.24:1 | 78 | 1680 | 26 | 49 |
| 5 | 10:0.16:1 | 76 | 1540 | 28 | 48 |
| No. | Structural formula a | (m/z)observation (g/mol) | (m/z)calc. (g/mol) |
|---|---|---|---|
| A | Na+HO–CPO–(n-BA)n–ME–OH | 3158.4 (n = 23) | 3159.2 (n = 23) |
| B | Na+HO–ME–(n-BA)n–xylene–(n-BA)m–ME–OH | 3232.5 (n2 = 23) | 3228.2 (n2 = 23) |
| C | Na+HO–CPO–(n-BA)n–xylene–(n-BA)m–xylene–(n-BA)p–xylene–(n-BA)q–ME–OH | 3214.3 (n4 = 21) | 3215.3 (n3 = 21) |
| D | Na+HO–CPO–(n-BA)n–xylene–(n-BA)m–xylene–(n-BA)p–CPO–OH | 3271.6 (n3 = 23) | 3274.4 (n3 = 23) |
| E | Na+HO–CPO–(n-BA)n–CPO–OH | 3196.2 (n = 23) | 3194.2 (n = 23) |
| F | Na+HO–ME–(n-BA)n–xylene–(n-BA)m–xylene–(n-BA)p–xylene–(n-BA)q–ME–OH | 3181.1 (n4 = 21) | 3180.3 (n4 = 21) |
| G | Na+HO–CPO–(n-BA)n–xylene–(n-BA)m–xylene–(n-BA)p–xylene–(n-BA)q–CPO–OH | 3122.1 (n4 = 20) | 3124.2 (n4 = 20) |
| Exp. | [n-BA]: [ACPO]:[DIX] a | Conv.b (%) | Mn,NMR c (g mol−1) | Mn,GPC d (g mol−1) | Mn,MS e (g mol−1) | Functionality of end-hydroxyl (%) f | OHtitr. value (mg KOH/g) | OHcalc. g value (mg KOH/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | 16:0.5:1 | 78 | 4409 | 4280 | 4090 | 76 | 29 | 27 |
| 2 | 16:0.25:1 | 75 | 3653 | 3480 | 3110 | 77 | 35 | 36 |
| 3 | 16:0.08:1 | 73 | 3545 | 3200 | 3230 | 72 | 31 | 35 |
| 4 | 16:0.04:1 | 71 | 3734 | 3160 | 3300 | 76 | 33 | 34 |
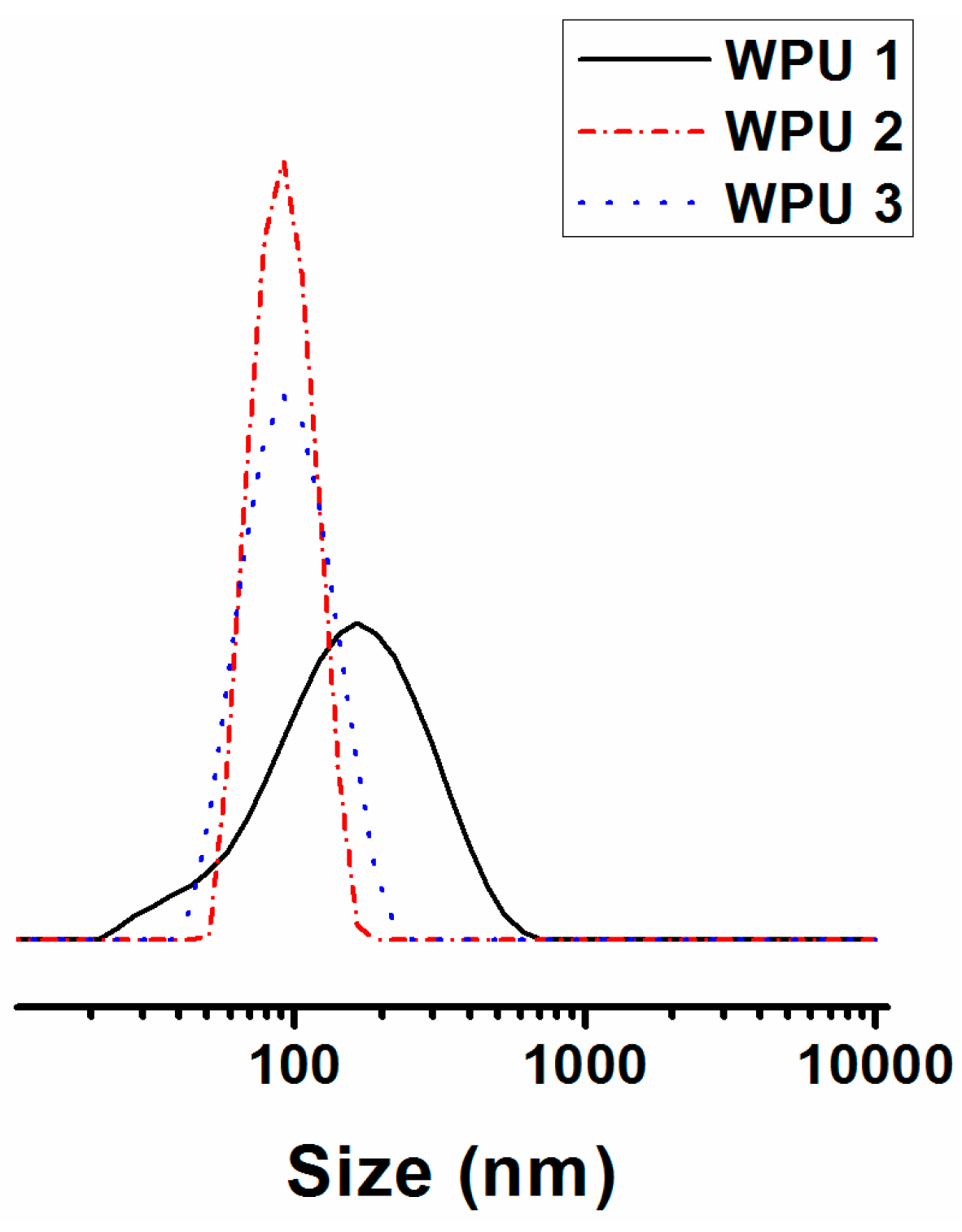
| No. | OH a value | Dispersion state b | Average particle size (nm) |
|---|---|---|---|
| 1 | 29 | Semi-transparent liquid | 134 |
| 2 | 31 | Semi-transparent liquid | 100 |
| 3 | 33 | Semi-transparent liquid | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, C.; Li, W.; Chen, L.; Wang, W. Synthesis of Waterborne Polyurethane by the Telechelic α,ω-Di(hydroxy)poly(n-butyl acrylate). Polymers 2018, 10, 219. https://doi.org/10.3390/polym10020219
Chen X, Zhang C, Li W, Chen L, Wang W. Synthesis of Waterborne Polyurethane by the Telechelic α,ω-Di(hydroxy)poly(n-butyl acrylate). Polymers. 2018; 10(2):219. https://doi.org/10.3390/polym10020219
Chicago/Turabian StyleChen, Xin, Chi Zhang, Weidong Li, Lei Chen, and Wusheng Wang. 2018. "Synthesis of Waterborne Polyurethane by the Telechelic α,ω-Di(hydroxy)poly(n-butyl acrylate)" Polymers 10, no. 2: 219. https://doi.org/10.3390/polym10020219





