Electrospun Gelatin–Chondroitin Sulfate Scaffolds Loaded with Platelet Lysate Promote Immature Cardiomyocyte Proliferation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polymeric Solutions
2.2.2. Rheological Measurements
2.2.3. Physical Properties of the Solutions
Surface Tension
Conductivity
Preparation of Patches (Electrospun Scaffolds)
2.2.4. Patches’ Characterizations
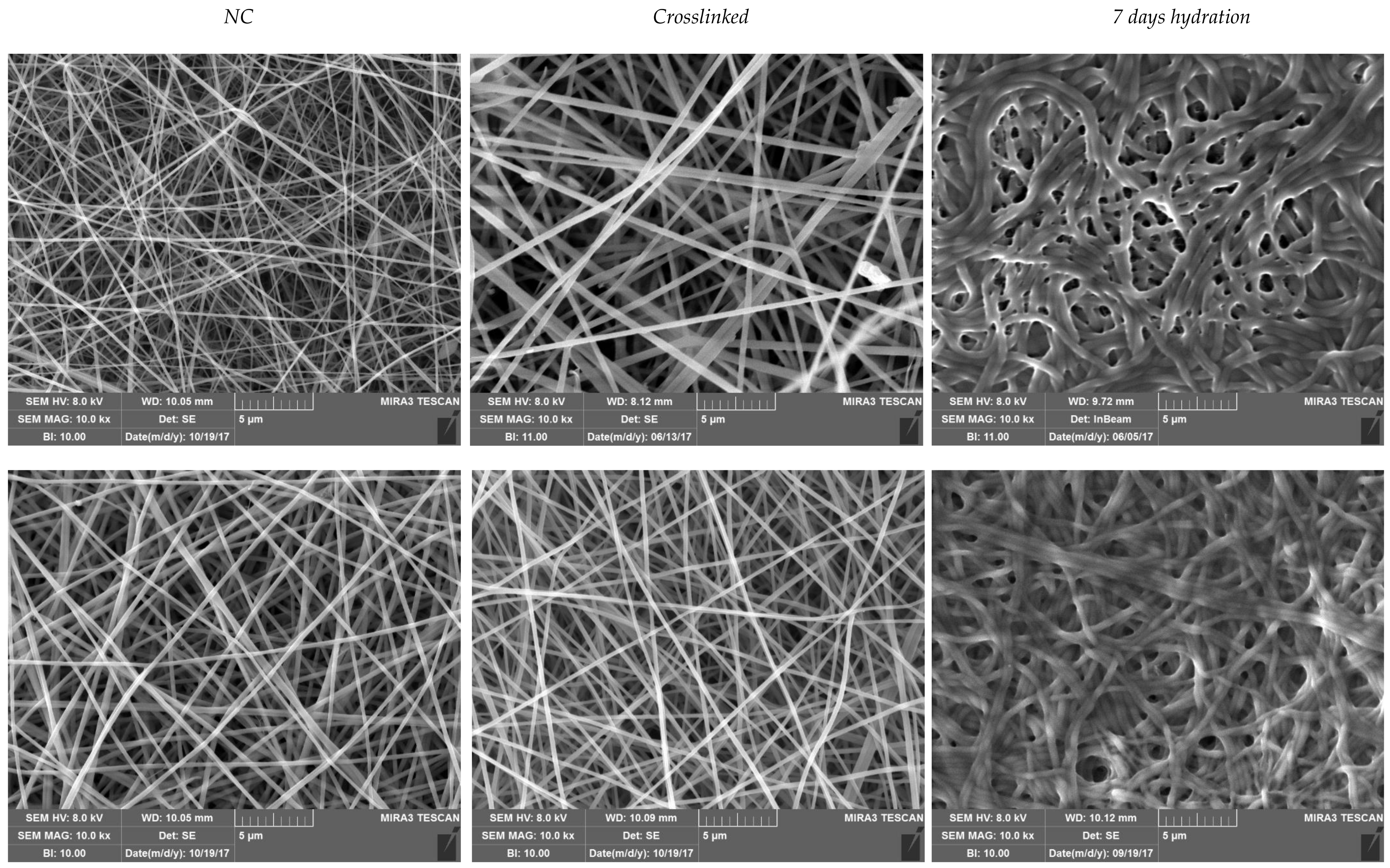
Scanning Electron Microscopy (SEM) Analysis
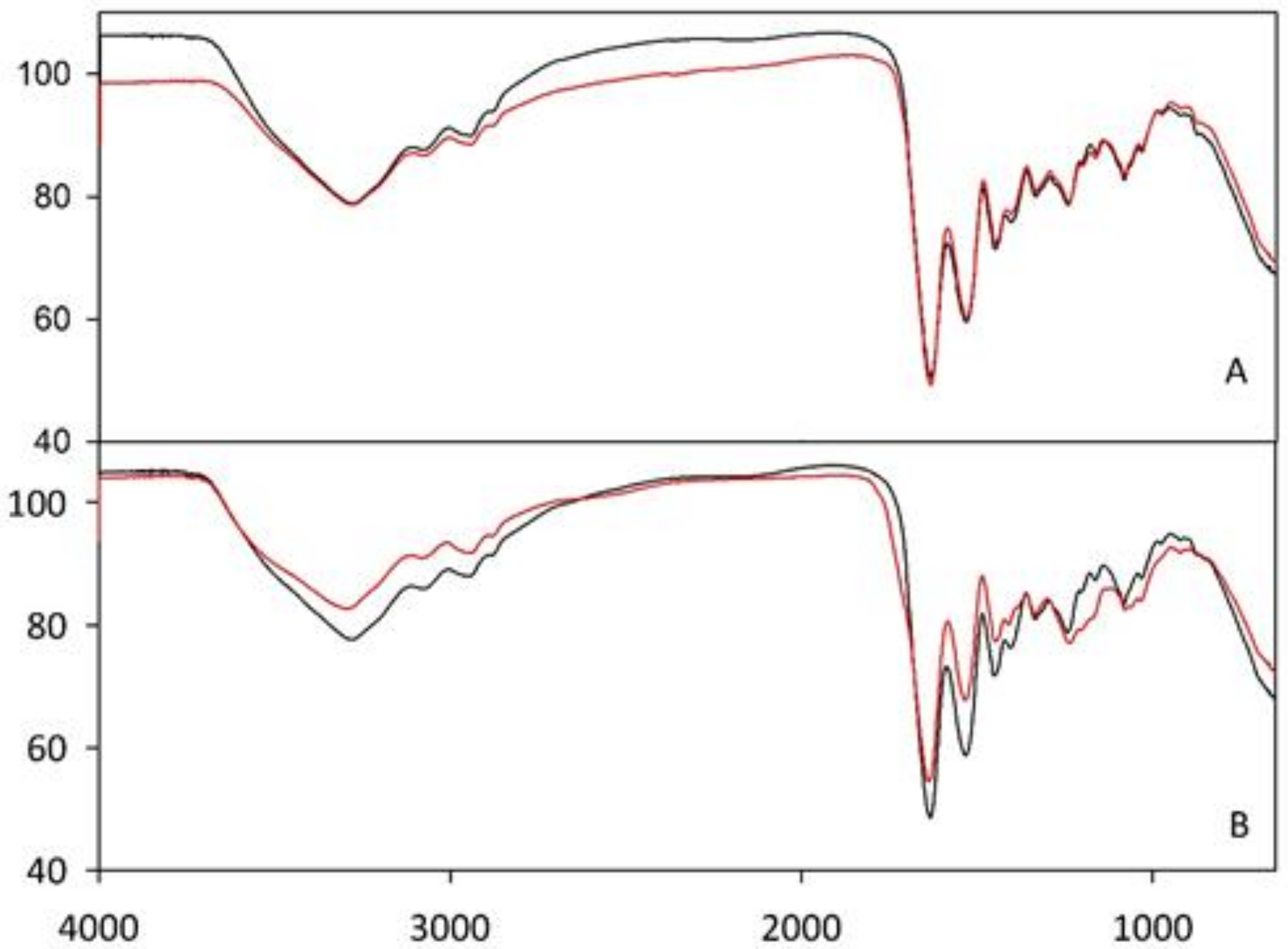
Fourier-Transform Infrared Spectroscopy (FT–IR) Analysis
Mechanical Properties
2.2.5. In Vitro Adhesion and Proliferation Assay
Normal Human Dermal Fibroblasts (NHDF)
Human Umbilical Vein Endothelial Cells (HUVEC)
Adhesion and Proliferation Tests
MTT Test
SEM Analysis
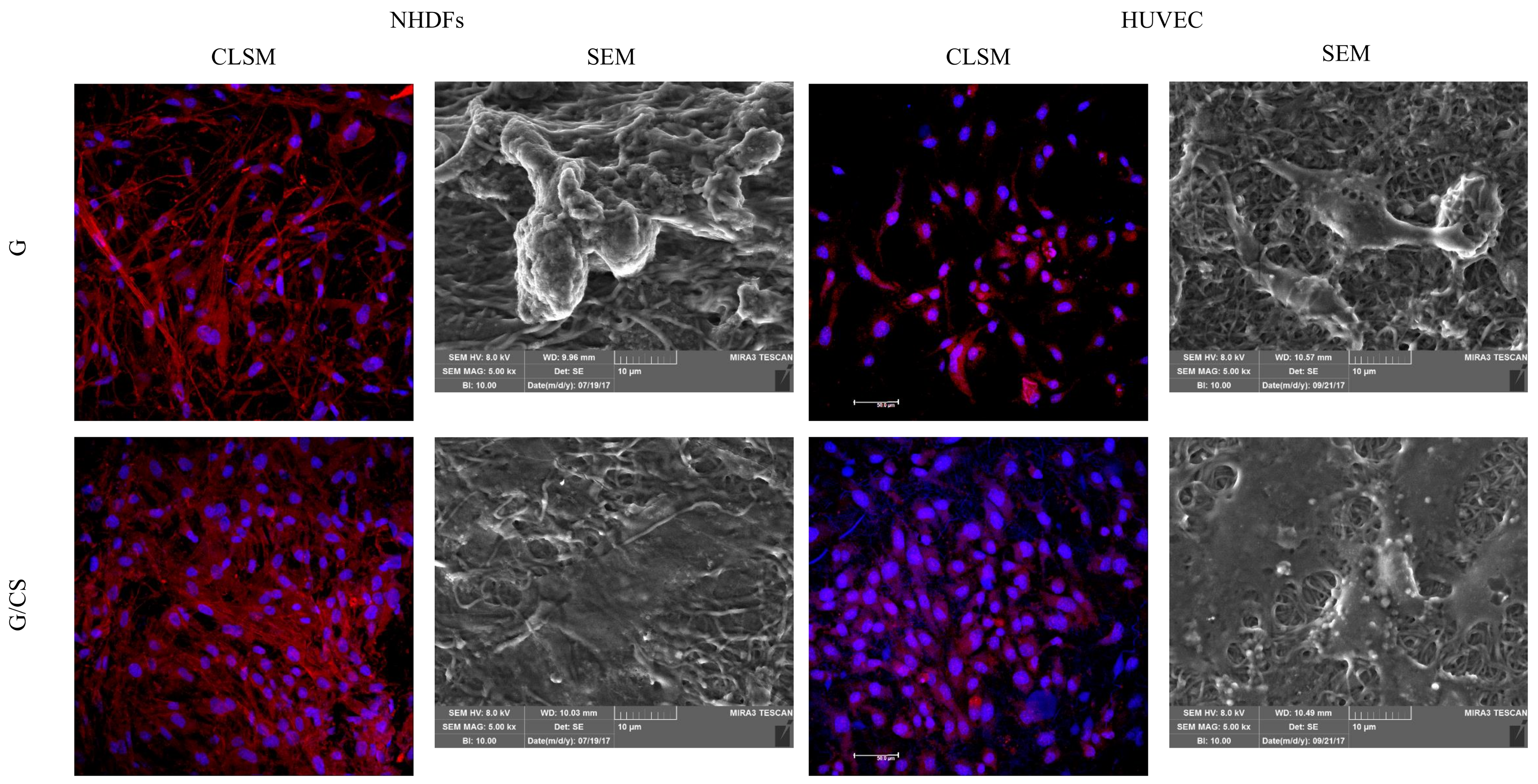
Confocal-Laser Scanning Microscopy (CLSM) Analysis
2.2.6. In Vitro Adhesion and Proliferation Assay: Cardiac Cells
CLSM Analysis
2.2.7. Statistical Analysis
3. Results
3.1. Characterization of Polymeric Solutions
3.1.1. Rheological Properties
3.1.2. Conductivity and Surface Tension
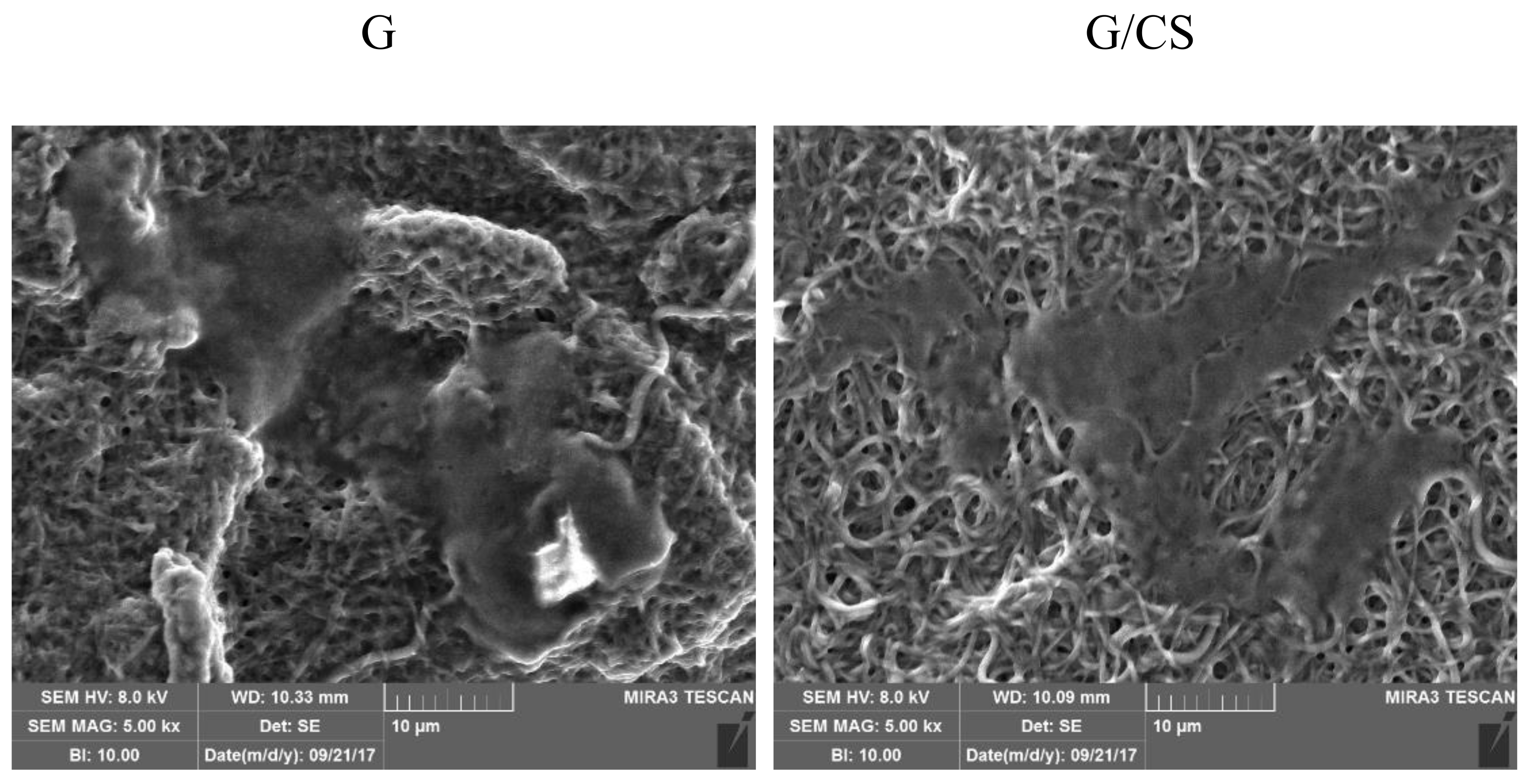
3.2. Characterization of Nanofibrous Scaffolds
3.3. In Vitro Adhesion and Proliferation Assay: Fibroblasts and Endothelial Cells
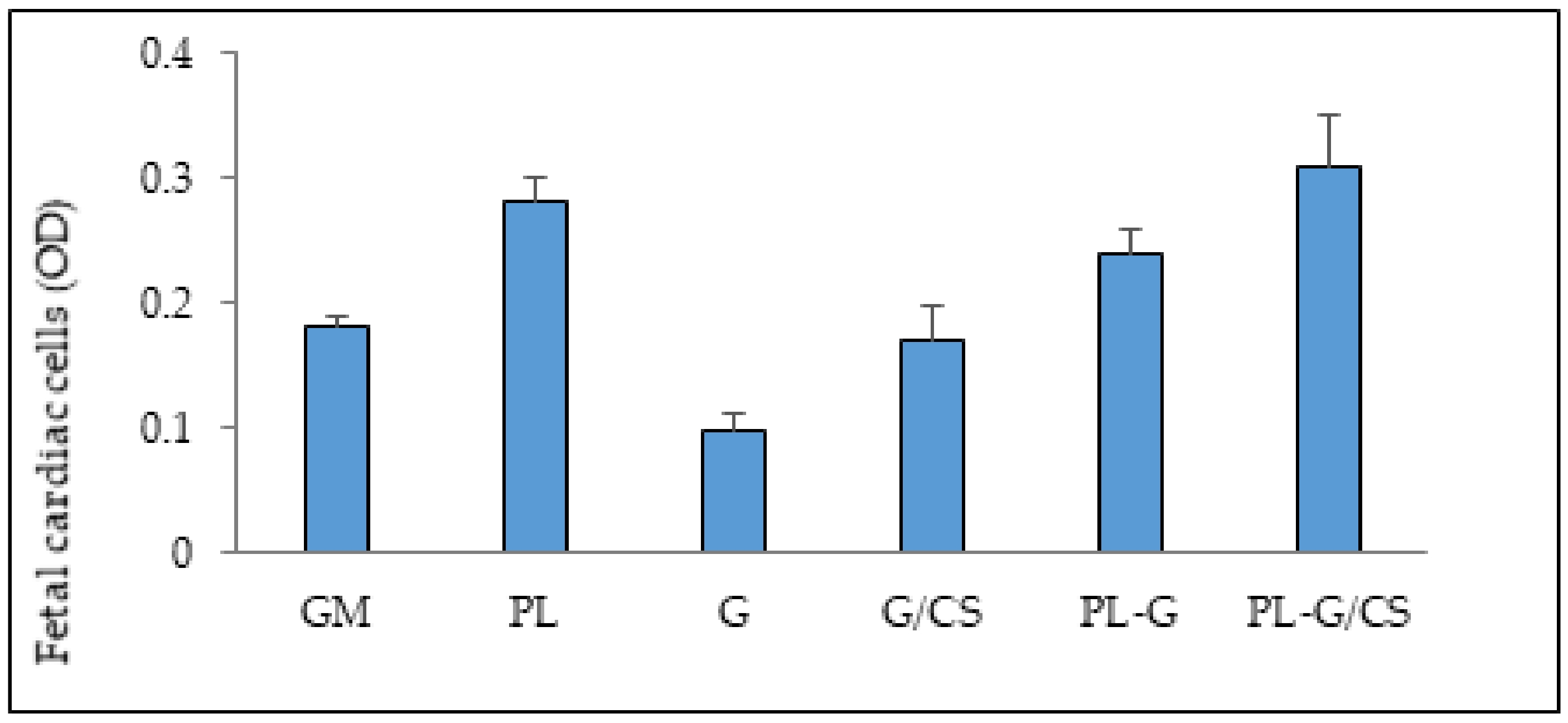
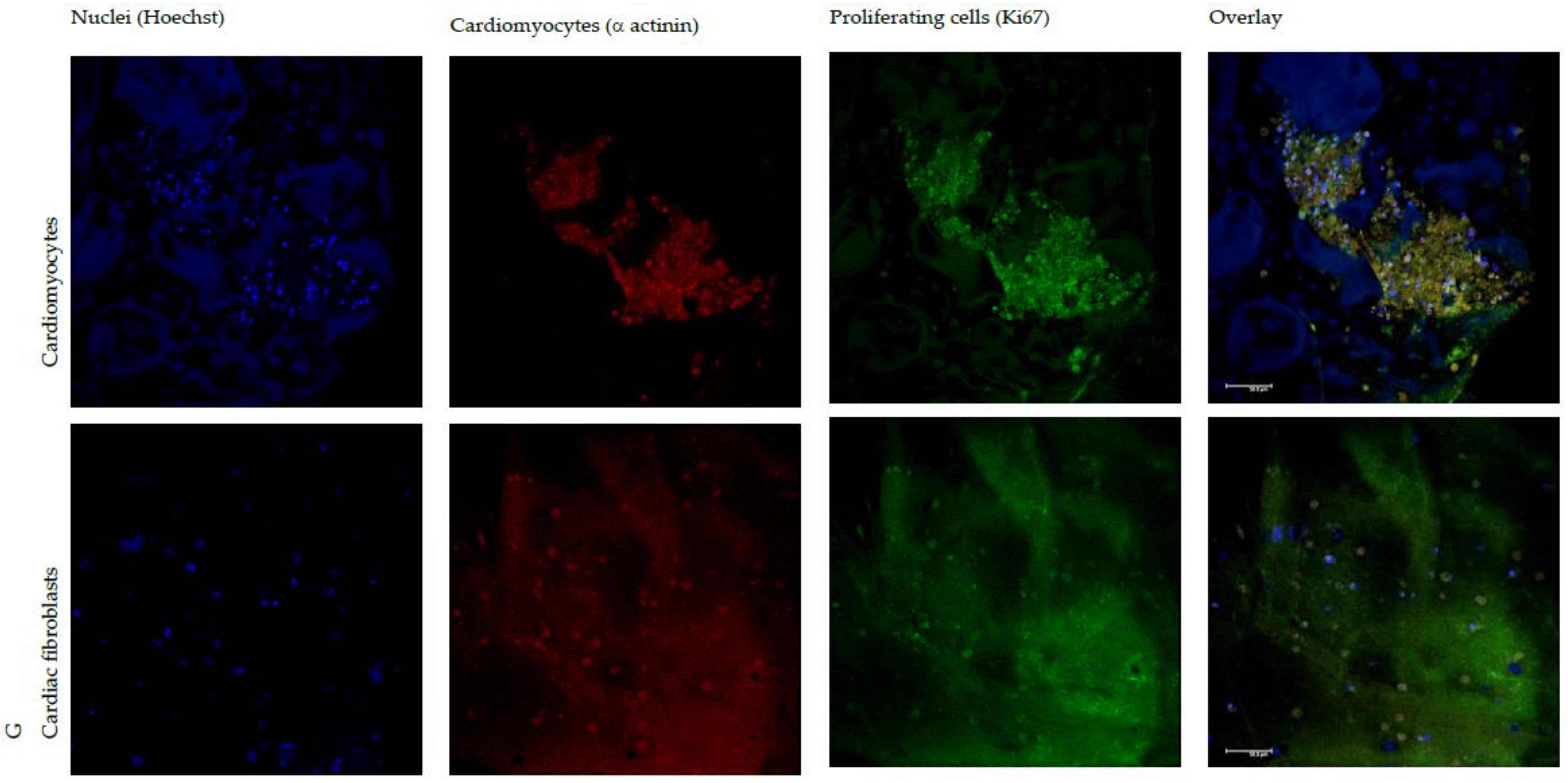
3.4. In Vitro Adhesion and Proliferation Assay: Cardiac Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oster, M.E.; Lee, K.A.; Honein, M.; Colarusso, T.; Shin, M.; Correa, A. Temporal trends in survival for infants with critical congenital heart defects. Pediatrics 2013, 131, e1502–e1508. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Rodriguez-Arabaolaza, I.; Spencer, H.L.; Riu, F.; Mangialardi, G.; Slater, S. C.; Rowlinson, J.; Alvino, V.V.; Idowo, O.O.; Soyombo, S.; et al. Expansion and characterization of neonatal cardiac pericytes provides a novel cellular option for tissue engineering in congenital heart disease. J. Am. Haert Assoc. 2015, 4, e002043. [Google Scholar] [CrossRef] [PubMed]
- Drews, J.D.; Miyachi, H.; Shinoka, T. Tissue-engineered vascular grafts for congenital cardiac disease: Clinical experience and current status. Trends Cardiovasc. Med. 2017, 27, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Hibino, N.; Breuer, C. K.; Shinoka, T. Tissue-engineered cardiac patch seeded with human induced pluripotent stem cell derived cardiomyocytes promoted the regeneration of host cardiomyocytes in a rat model. J. Cardiothorac. Surg. 2016, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Stupin, I.V.; Tsao, C.; Pautler, R.G.; Gao, Y.; Nieto, R.M.; Tao, Z.-W.; Fraser, C.D.; Annapragada, A.V.; Jacot, J.G. Full-thickness heart repair with an engineered multilayered myocardial patch in rat model. Adv. Healthc. Mater. 2017, 6, 1600549. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Budina, E.; Stoppel, W.L.; Sullivan, K.E.; Emani, S.; Emani, S.M.; Black, L.D. Cardiac extracellular matrix-fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomater. 2015, 14, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Sullivan, K.; Black, L. D. Partially digested adult cardiac extracellular matrix promotes cardiomyocyte proliferation in vitro. Adv. Healthc. Mater. 2015, 4, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Yester, J. W.; Kühn, B. Mechanisms of cardiomyocyte proliferation and differentiation in development and regeneration. Curr. Cardiol. Rep. 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Shah, G.; Wu, Y.; Torre-Amione, G.; King, N.M.P.; Lahmers, S.; Witt, C.C.; Becker, K.; Labeit, S.; Granzier, H.L. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation 2004, 110, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Elamparithi, A.; Punnoose, A.M.; Kuruvilla, S.F.D.; Kuruvilla, S. Gelatin electrospun nanofibrous matrices for cardiac tissue engineering applications. Int. J. Polym. Mater. 2017, 66, 20–27. [Google Scholar] [CrossRef]
- Pushp, P.; Castelo Ferreira, F.; Sampaio Cabral, J.M.; Gupta, M.K. Improved survival of cardiac cells on surface modified electrospun nanofibers. Polym. Sci. A 2017, 59, 515–523. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine. 2017, 12, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Deddens, J.C.; Hossein Sadeghi, A.; Hjortnaes, J.; Van Laake, L.W.; Buijsrogge, M.; Doevendans, P.A.; Khademhosseini, A.; Sluijter, J.P.G. Modeling the human scarred heart in vitro: Toward new tissue engineered models. Adv. Healthc. Mater. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V. Collagen vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: London, UK, 1996; pp. 17–52. [Google Scholar]
- Lynn, A.K.; Yannas, L.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Olsen, D.; Yang, C.; Bodo, M.; Chang, R.; Leigh, S.; Baez, J.; Carmichael, D.; Perala, M.; Hamalainen, E.R.; Jarvinen, M.; et al. Recombinant collagen and gelatin for drug delivery. Adv. Drug Deliv. Rev. 2003, 55, 1547–1567. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; Ursini, O.; Lilla, E.; Angelini, G. Radiation-induced crosslinking of collagen gelatin into a stable hydrogel. J. Radioanal. Nucl. Chem. 2008, 275, 125–131. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug Disc. Technol. 2008, 5, 289–301. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Mori, M.; Del Fante, C.; Perotti, C.; Caramella, C. Thermosensitive eyedrops containing platelet lysate for the treatment of corneal ulcers. Int. J. Pharm. 2012, 426, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Mori, M.; Cervio, M.; Riva, F.; Liakos, I.; Athanassiou, A.; Saporito, F.; et al. Platelet lysate embedded scaffolds for skin regeneration. Expert Opin. Drug Del. 2015, 12, 525–554. [Google Scholar]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Delfino, A.; Riva, F.; Icaro Cornaglia, A.; Marrubini, G.; Musitelli, G.; Del Fante, C.; Perotti, C.; et al. Platelet lysate and chondroitin sulfate loaded contact lenses to heal corneal lesions. Int. J. Pharm. 2016, 509, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Mildner, M.; Gyöngyösi, M.; Ankersmit, H.J. Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis 2016, 21, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanas, J.; Carrillo, F. Elctrospinning of gelatin fiber using solutions with low acetic acid concentration: effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. J. Appl. Polym. Sci. 2015, 132, 42115. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Chen, G.; Li, Y.; Li, T. Viscosity properties of gelatin in solutions of monovalent and divalent salts. Korea-Aust. Rheol. 2013, 25, 227–231. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Avd. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. Modification of hydroxyapatite/gelatin nanocomposite with the addition of chondroitin sulfate. J. Korean Ceram. Soc. 2008, 45, 573–578. [Google Scholar] [CrossRef]
- Wang, J.; Windbergs, M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299. [Google Scholar]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar] [PubMed]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and gel properties of gelatin from goat skin as influenced by alkaline-pretreatment conditions. Asian-Aust. J. Anim. Sci. 2016, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomed. 2012, 7, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008, 29, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Carag-Krieger, C.; Johnson, C.P.; Raab, M.; Tang, H.Y.; Speicher, D.W.; Sanger, J.W.; Sanger, J.M.; Discher, D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 2008, 121, 3794–3802. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, K.L.K.; Kaiser, N.J. Physiologically inspired cardiac scaffolds for tailored in vivo function and heart regeneration. Biomed. Mater. 2015, 10, 034003. [Google Scholar]
- Martella, D.; Paoli, P.; Pioner, J.M.; Sacconi, L.; Coppini, R.; Santini, L.; Lulli, M.; Cerbai, E.; Wiersma, D.S.; Poggesi, C.; et al. Liquid crystalline networks toward regenerative medicine and tissue repair. Small 2017, 13, 1702677. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Faccendini, A.; Bonferoni, M.C.; Ferrari, F.; Sandri, G.; Del Fante, C.; Perotti, C.; Caramella, C. “Sponge-like” dressings based on biopolymers for the delivery of platelet lysate to skin chronic wounds. Int. J. Pharm. 2013, 20, 207–215. [Google Scholar]
- Bianconi, V.; Sahebkar, A.; Kovanen, P.; Bagaglia, F.; Ricciuti, B.; Calabrò, P.; Patti, G.; Pirro, M. Endothelial and cardiac progenitor cells for cardiovascular repair: A controversial paradigm in cell therapy. Pharmacol. Ther. 2018, 181, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Parsa, H.; Ronaldson, K.; Vunjak-Novakovic, G. Bioengineering methods for myocardial regeneration. Adv. Drug Deliv. Rev. 2016, 96, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.; Zhang, B.; Radisic, M. Cardiac tissue vascularization. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Del Fante, C.; Perotti, C.; Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Scudeller, L.; Caramella, C. Platelet lysate mucohadesive formulation to treat oral mucositis in graft versus host disease patients: a new therapeutic approach. AAPS Pharm. Sci. Tech. 2011, 12, 893–899. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Malavasi, L.; Fante, C.D.; Vigani, B.; Black, L.; Ferrari, F. Electrospun Gelatin–Chondroitin Sulfate Scaffolds Loaded with Platelet Lysate Promote Immature Cardiomyocyte Proliferation. Polymers 2018, 10, 208. https://doi.org/10.3390/polym10020208
Saporito F, Sandri G, Bonferoni MC, Rossi S, Malavasi L, Fante CD, Vigani B, Black L, Ferrari F. Electrospun Gelatin–Chondroitin Sulfate Scaffolds Loaded with Platelet Lysate Promote Immature Cardiomyocyte Proliferation. Polymers. 2018; 10(2):208. https://doi.org/10.3390/polym10020208
Chicago/Turabian StyleSaporito, Francesca, Giuseppina Sandri, Maria Cristina Bonferoni, Silvia Rossi, Lorenzo Malavasi, Claudia Del Fante, Barbara Vigani, Lauren Black, and Franca Ferrari. 2018. "Electrospun Gelatin–Chondroitin Sulfate Scaffolds Loaded with Platelet Lysate Promote Immature Cardiomyocyte Proliferation" Polymers 10, no. 2: 208. https://doi.org/10.3390/polym10020208




