Microencapsulation of Phosphorylated Human-Like Collagen-Calcium Chelates for Controlled Delivery and Improved Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
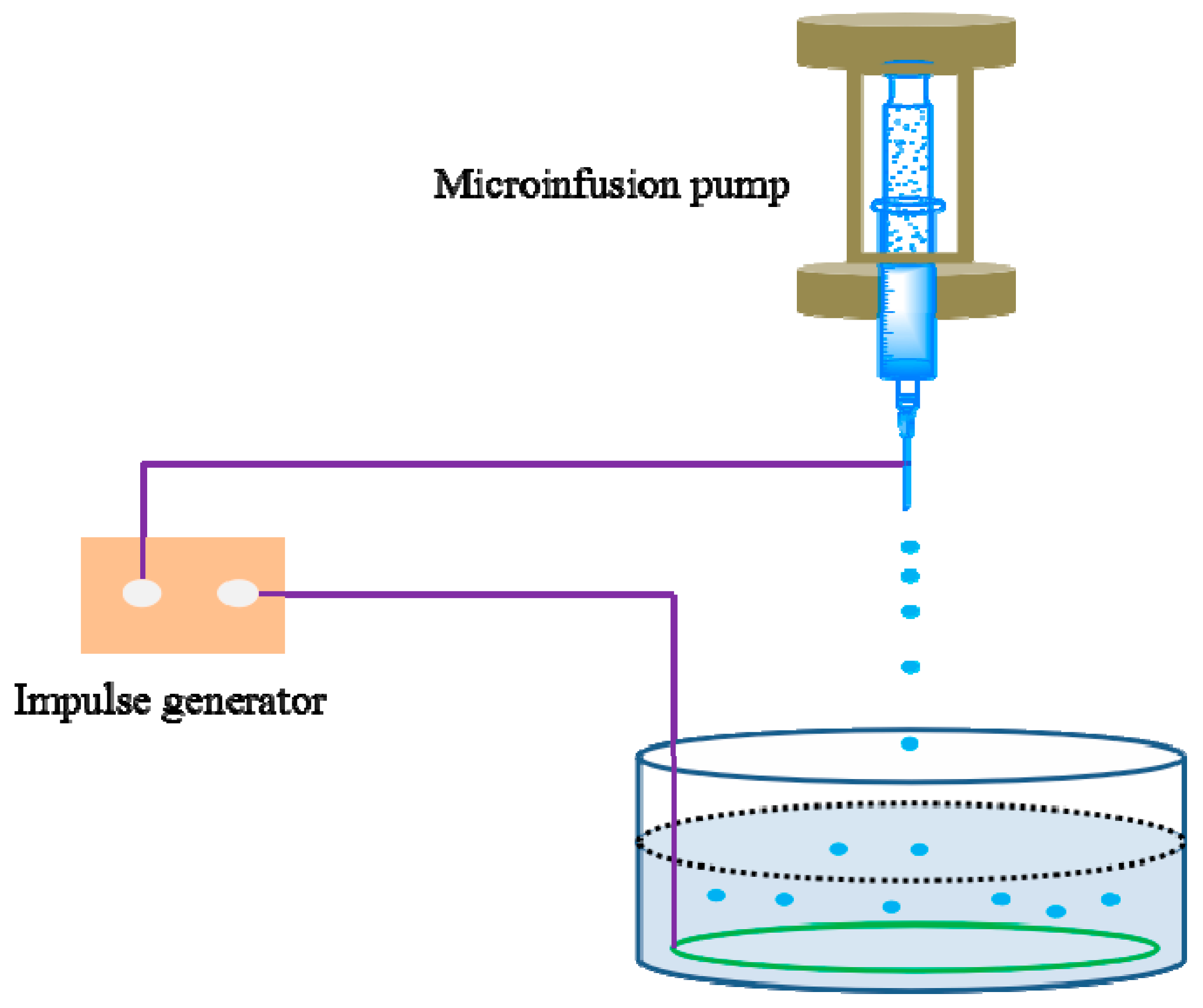
2.2. Preparation of Microcapsules
2.3. Determination of Encapsulation Efficiency (EE) and Loading Capacity (LC)
2.4. Characterization of Microcapsules
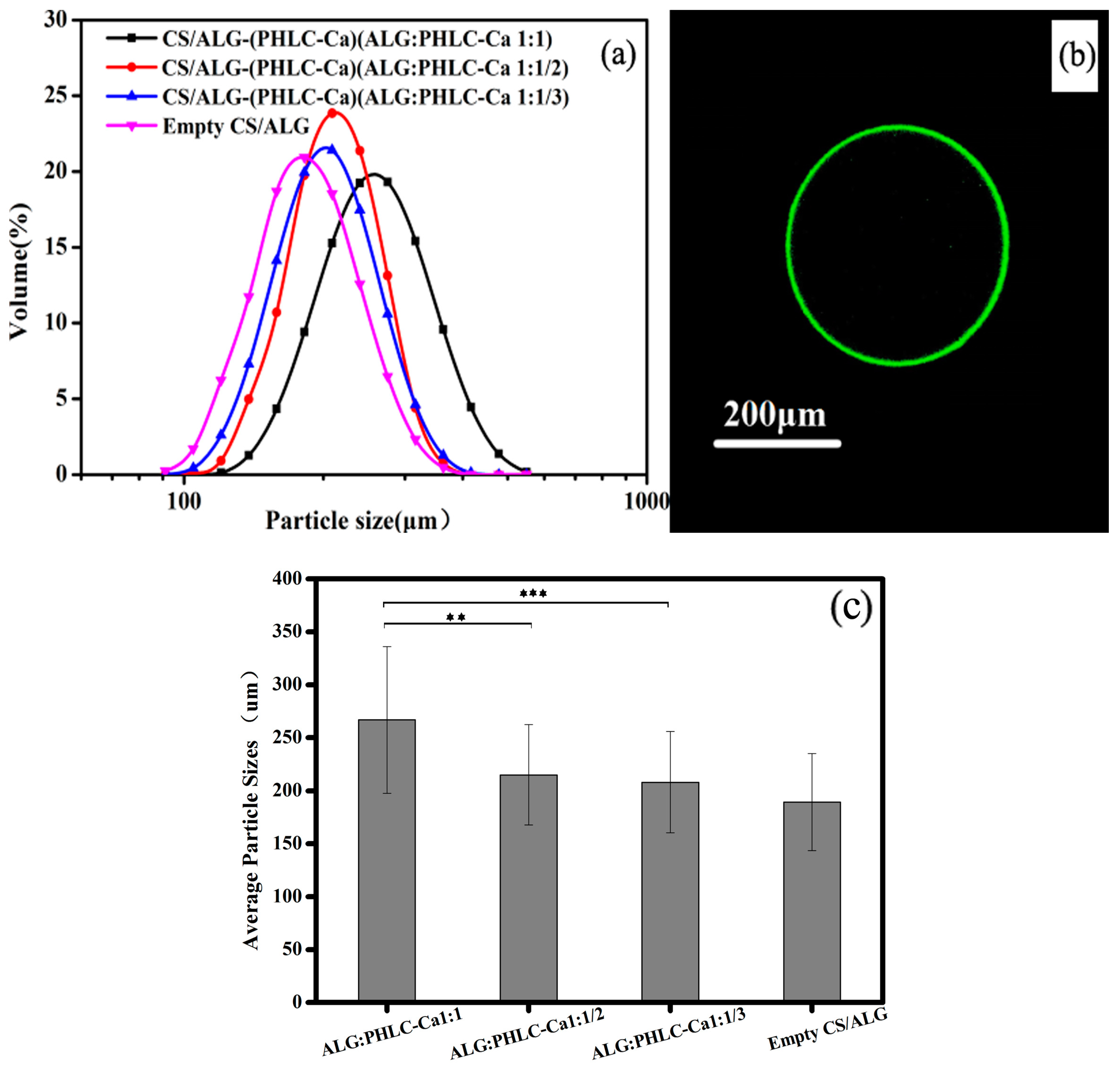
2.4.1. Particle Size Analysis
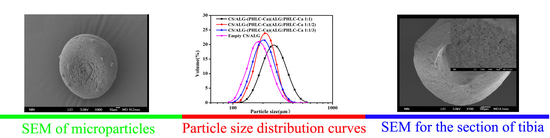
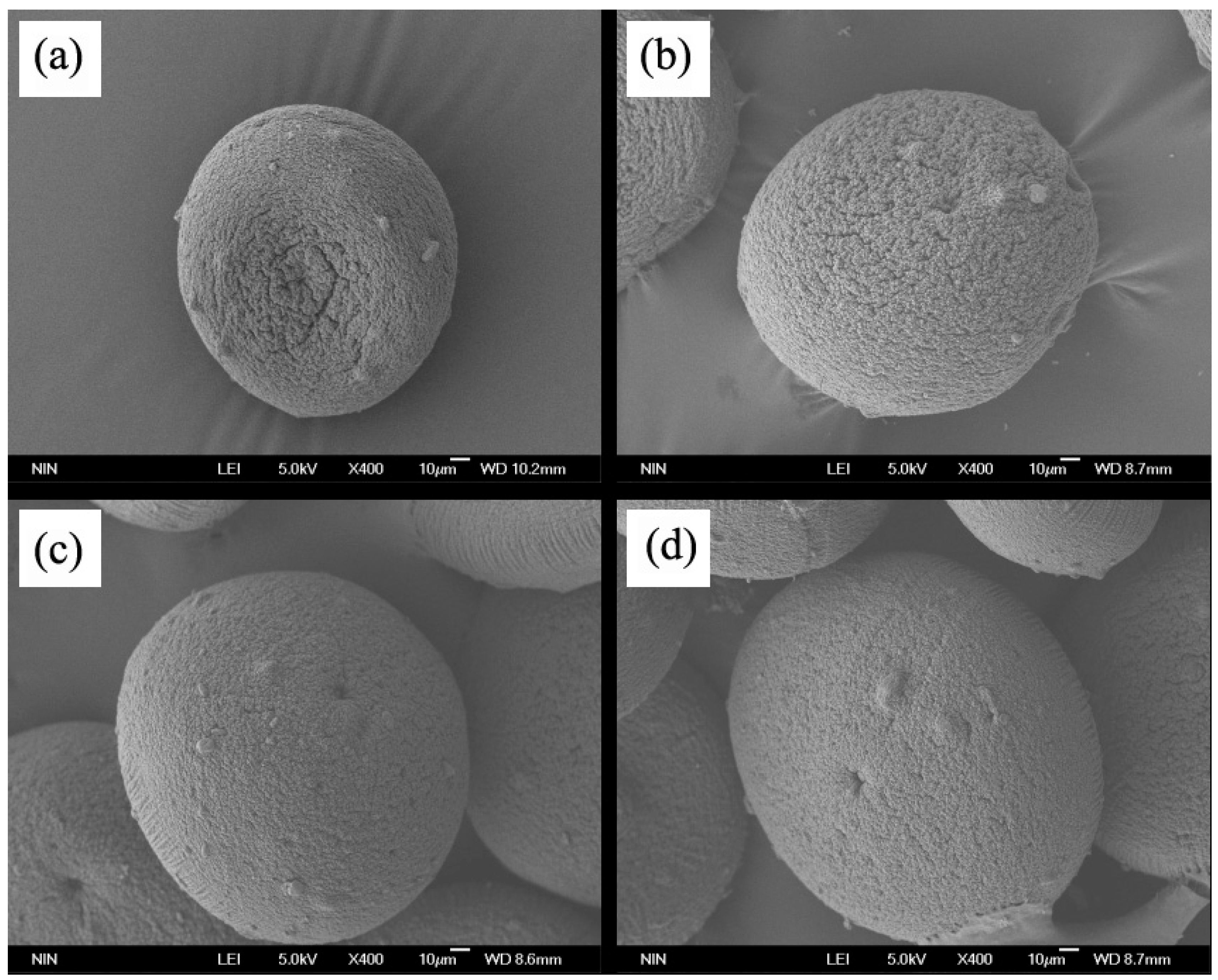
2.4.2. Microcapsules Morphology
2.4.3. Fourier Transform-Infrared Spectroscopy
2.4.4. Thermal Analysis
2.4.5. Confocal Laser Scanning Microscopy (CLSM)
2.5. Release Profiles of PHLC-Ca from Microcapsules In Vitro in the Gastrointestinal Environments
2.6. In Vitro Cell Cytotoxicity
2.7. Determination of PHLC-Ca Bioavailability after Oral Administration of CS/ALG-(PHLC-Ca) Microcapsules
2.8. Calculations and Data Analysis
3. Results and Discussion
3.1. Determination of Encapsulation Efficiency (EE) and Loading Capacity (LC)
3.2. Characterization of Microcapsules
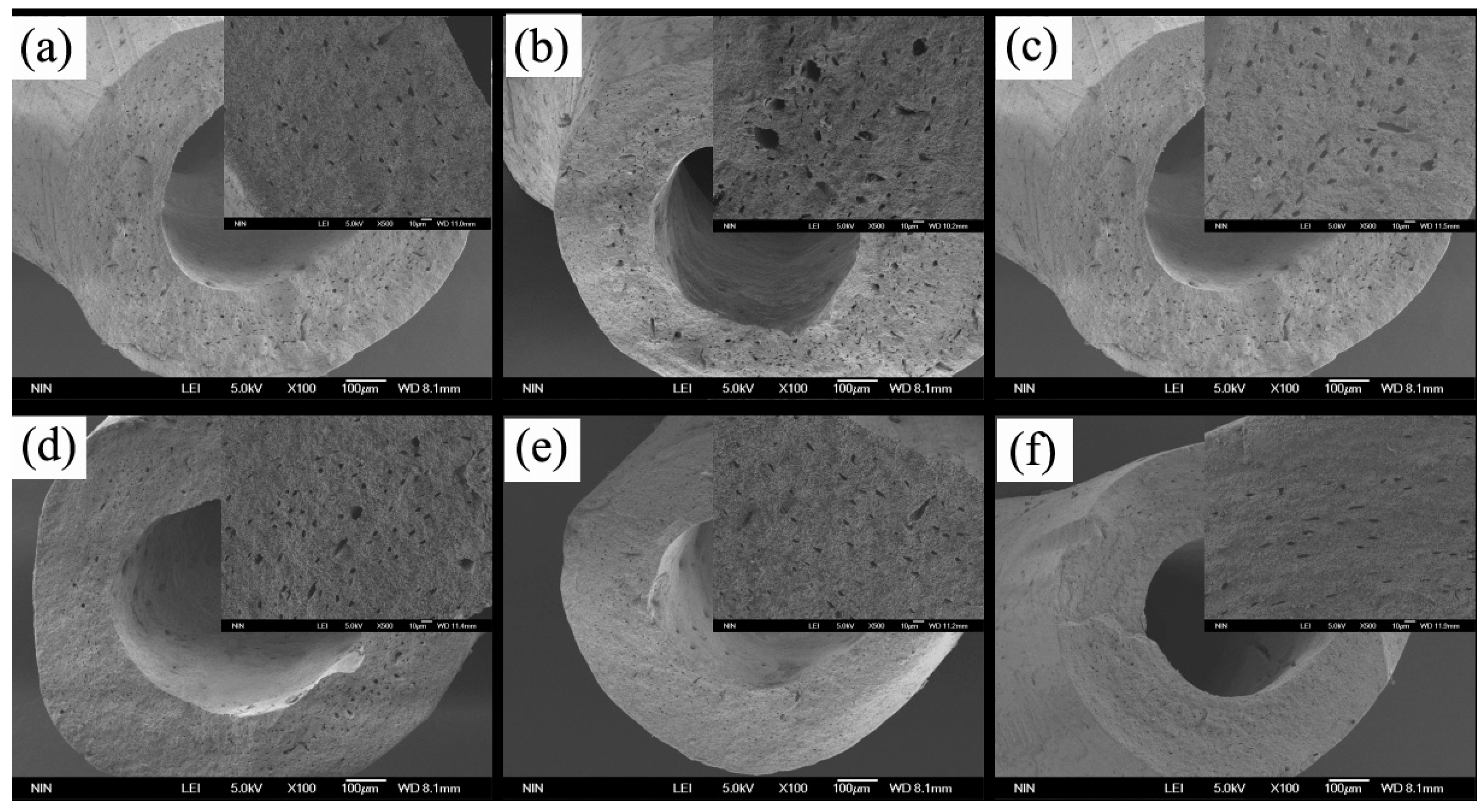
3.2.1. Morphology of Microcapsules
3.2.2. Size Distribution and CLSM of Microcapsules
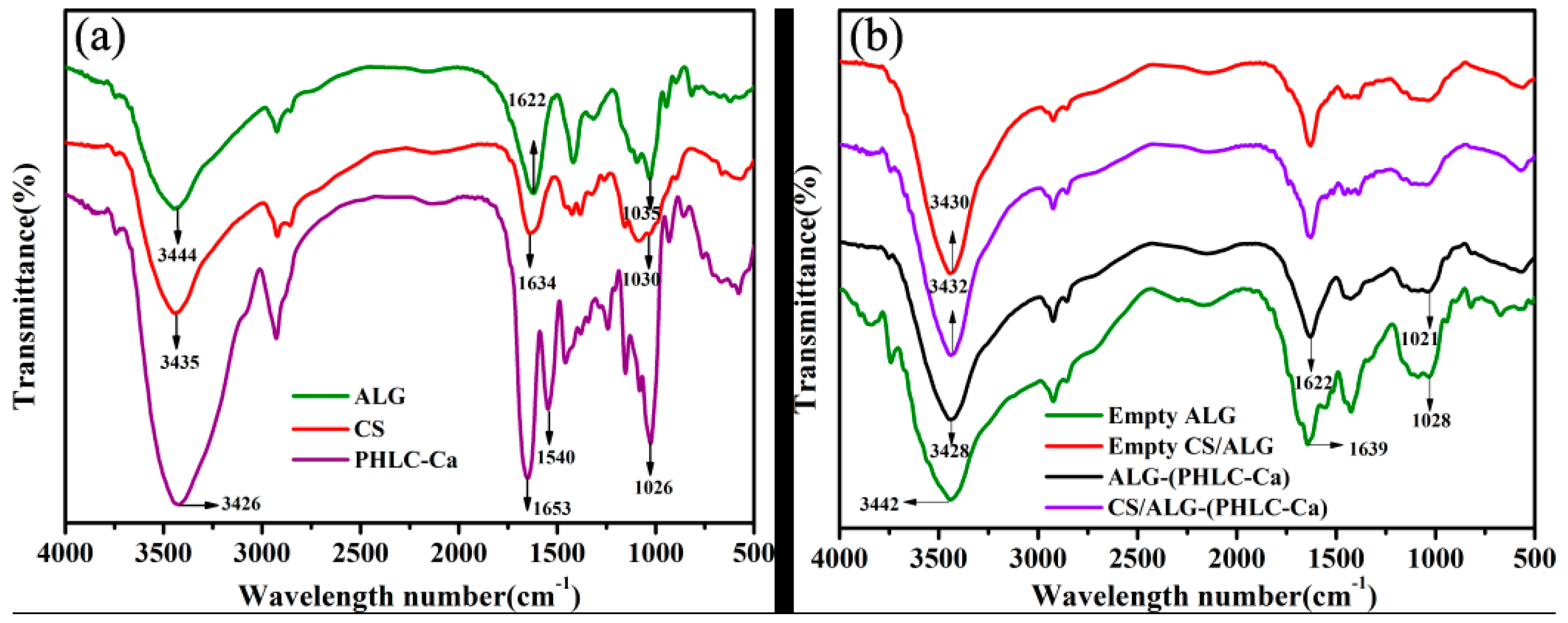
3.2.3. FT-IR Spectroscopic Analysis
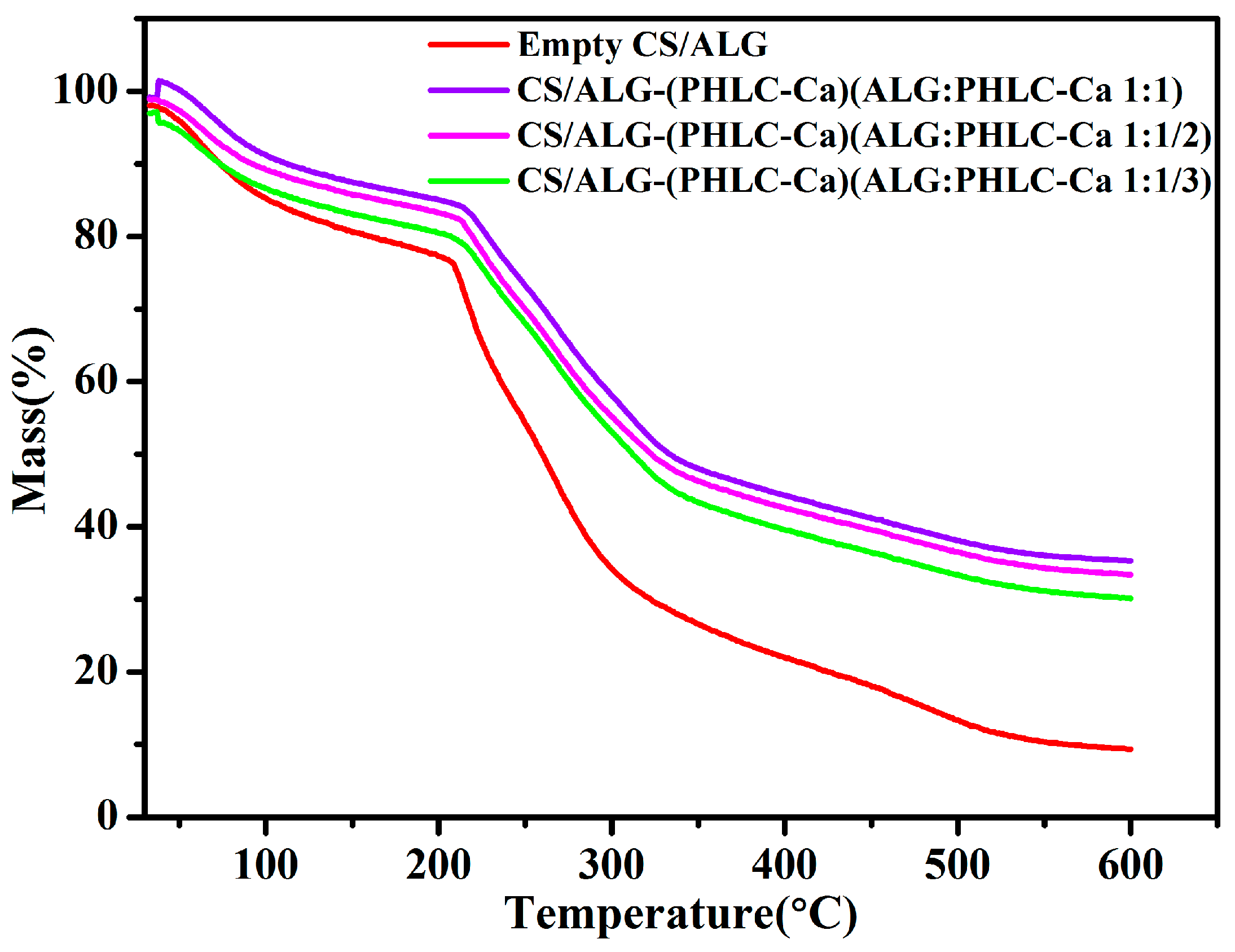
3.2.4. Thermal Analysis
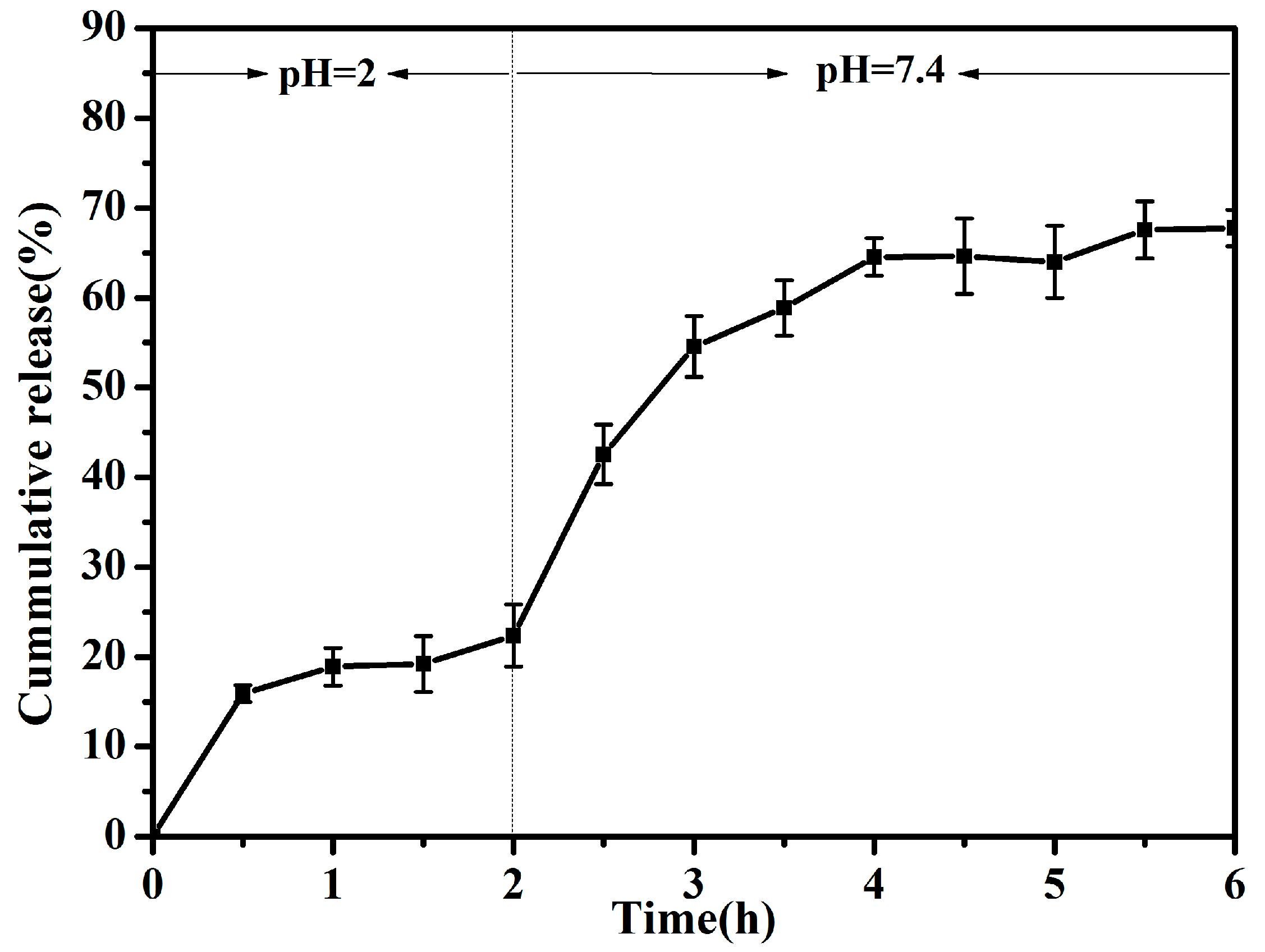
3.3. Release Profile In Vitro
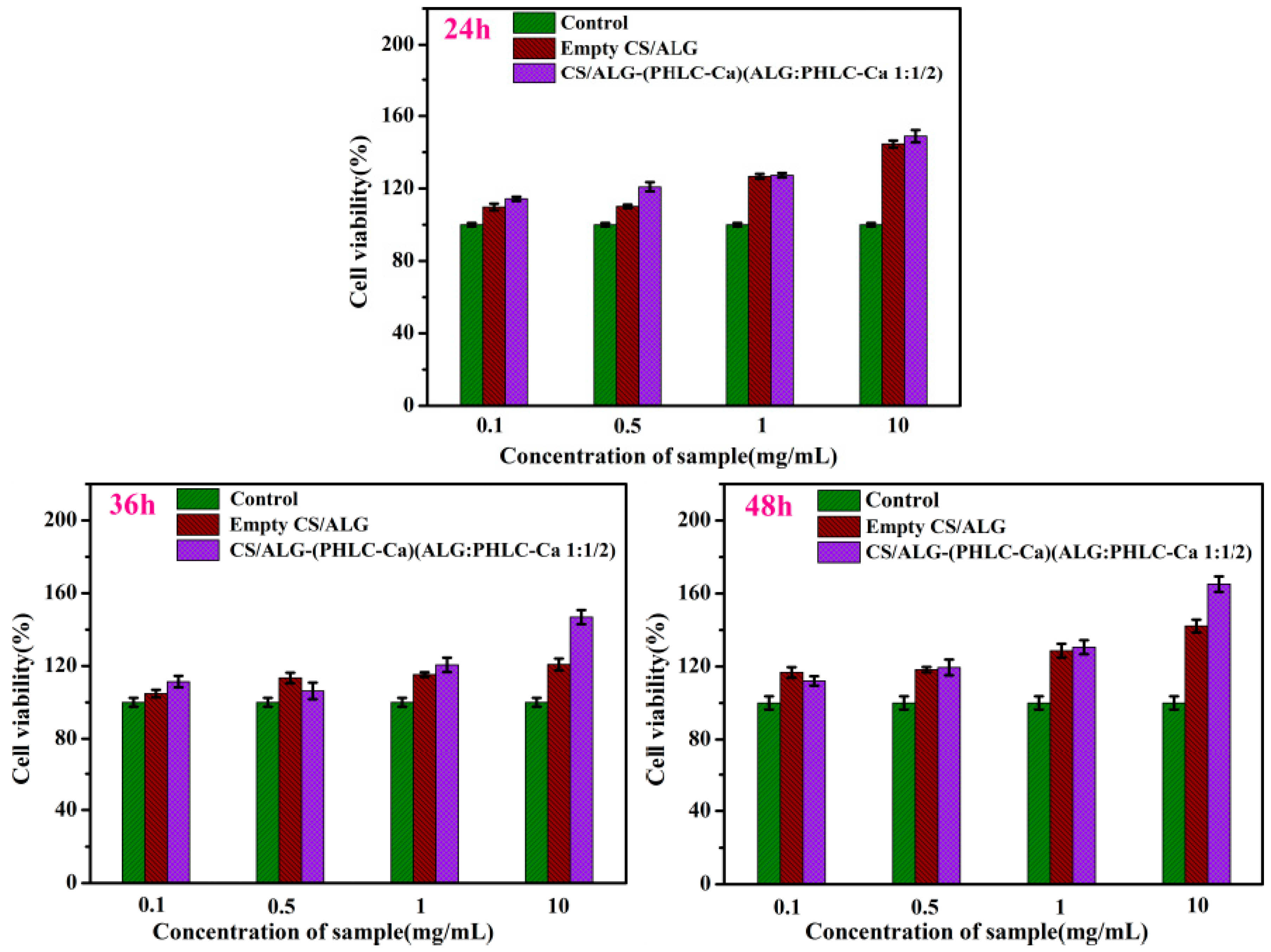
3.4. MTT Assay
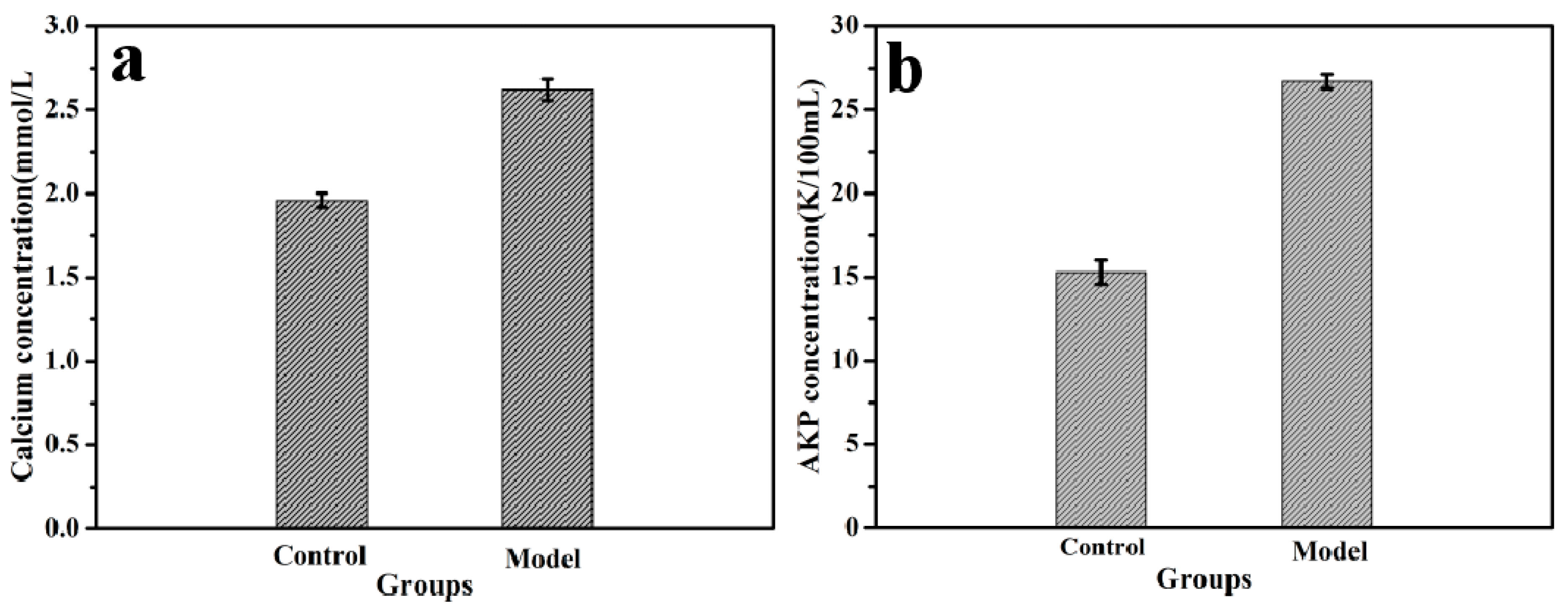
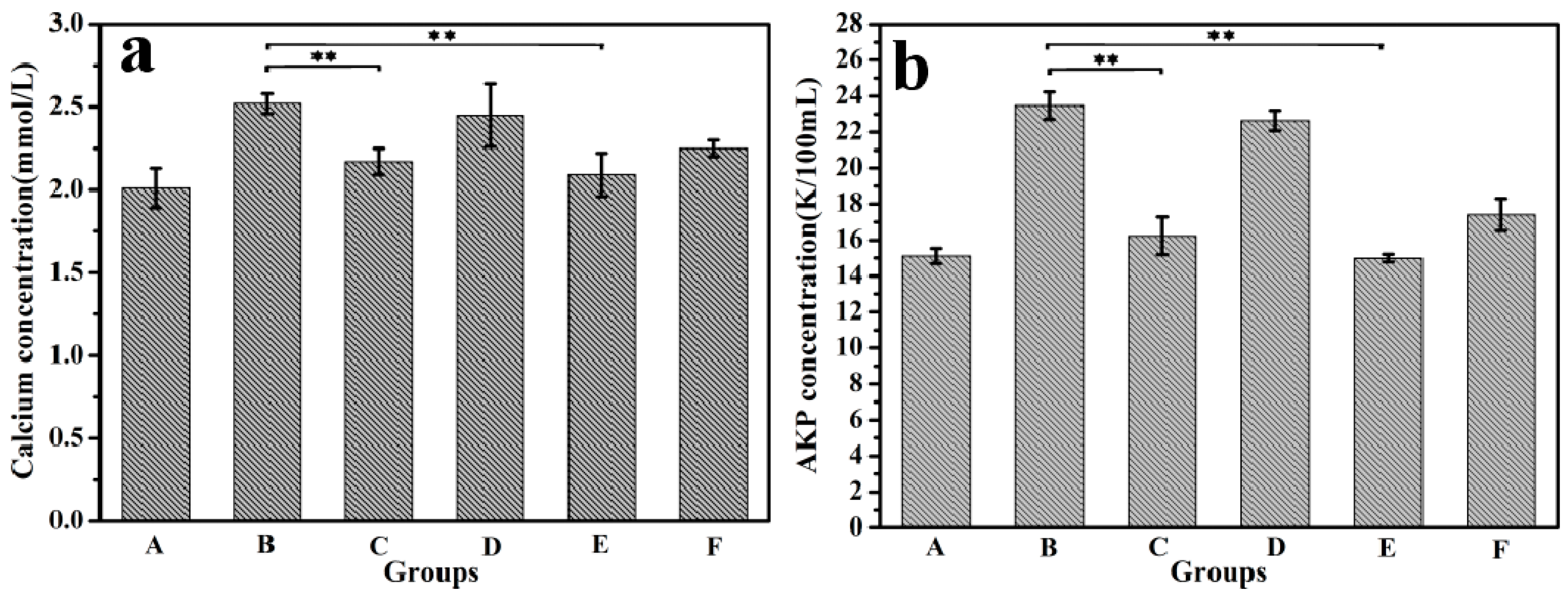
3.5. Animal Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Power, M.L.; Heaney, R.P.; Kalkwarf, H.J. The role of calcium in health and disease. Am. J. Obstet. Gynecol. 1999, 181, 1560–1569. [Google Scholar] [CrossRef]
- Cho, E.; Smith-Warner, S.A.; Spiegelman, D. Dairy foods, calcium, and colorectal cancer: A pooled analysis of 10 cohort studies. J. Natl. Cancer Inst. 2004, 96, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Vavrusova, M.; Skibsted, L.H. Calcium nutrition. Bioavailability and fortification. LWT Food Sci. Technol. 2014, 59, 1198–1204. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Rita, M.B.; Gomes, J.C.; Lobo, V.M.; Esteso, M.A. Diffusion of calcium gluconate in aqueous solutions of lactose at 298.15 K. Food Chem. 2011, 126, 1186–1189. [Google Scholar] [CrossRef]
- Chen, D.; Mu, X.; Huang, H.; Nie, R.; Liu, Z.; Zeng, M. Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Funct. Foods 2014, 6, 575–584. [Google Scholar] [CrossRef]
- Schinke, T.; Schilling, A.F.; Baranowsky, A. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat. Med. 2009, 15, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Touati, G.; Valayannopoulos, V.; Mention, K. Methylmalonic and propionic acidurias: Management without or with a few supplements of specific amino acid mixture. J. Inherit. Metab. Dis. 2006, 29, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Karawita, R.; Heo, S.J.; Lee, B.J.; Kim, S.K.; Jeon, Y.J. Recovery of a novel Ca-binding peptide from Alaska Pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Process Biochem. 2006, 41, 2097–2100. [Google Scholar] [CrossRef]
- Bass, J.K.; Chan, G.M. Calcium nutrition and metabolism during infancy. Nutrition 2006, 22, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Teucher, B. Calcium bioavailability in relation to bone health. Int. J. Vitam. Nutr. Res. 2002, 72, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, M.; Ye, A.; Singh, H. Characterisation of binding of iron to sodium caseinate and whey protein isolate. Food Chem. 2009, 114, 1007–1013. [Google Scholar] [CrossRef]
- Xiu, F.H.; Daidi, F.; Zhang, X. Kinetics of high cell density fed-batch culture of recombinant Escherichia coli producing human-like collagen. Chin. J. Chem. Eng. 2006, 14, 242–247. [Google Scholar] [CrossRef]
- Guo, J.; Luo, Y.; Fan, D. Medium optimization based on the metabolic-flux spectrum of recombinant Escherichia coli for high expression of human-like collagen II. Biotechnol. Appl. Biochem. 2010, 57, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Daidi, F.; Shang, L.; Xiaoxuan, M.; Wenjiao, X.; Pengfei, G. Optimization of fermentation process for human-like collagen production of recombinant Escherichia coli using response surface methodology. Chin. J. Chem. Eng. 2010, 18, 137–142. [Google Scholar] [CrossRef]
- Zhai, Y.; Cui, F. Recombinant human-like collagen directed growth of hydroxyapatite nanocrystals. J. Cryst. Growth 2006, 291, 202–206. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, D.; Duan, Z. Initial investigation of novel human-like collagen/chitosan scaffold for vascular tissue engineering. J. Biomed. Mater. Res. A 2009, 89, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, Y.; Deng, J. Preparation, characterization, and bioavailability of a phosphorylated human-like collagen calcium complex. Polym. Adv. Technol. 2015, 26, 1217–1225. [Google Scholar] [CrossRef]
- Sood, A.; Panchagnula, R. Peroral route: An opportunity for protein and peptide drug delivery. Chem. Rev. 2001, 101, 3275–3304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, W.; Lv, P.; Wang, L.; Ma, G. Preparation and evaluation of alginate–chitosan microspheres for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2011, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Wang, B.; Zhao, H. Microencapsulation peptide and protein drugs delivery system. Colloids Surf. B Biointerfaces 2005, 41, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Baek, N.; Park, K. Microencapsulation methods for delivery of protein drugs. Biotechnol. Bioprocess Eng. 2001, 6, 213–230. [Google Scholar] [CrossRef]
- Tran, V.T.; Benoît, J.P.; Venier-Julienne, M.C. Why and how to prepare biodegradable, monodispersed, polymeric microparticles in the field of pharmacy? Int. J. Pharm. 2011, 407, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G. Microencapsulation of protein drugs for drug delivery: Strategy, preparation, and applications. J. Control. Release 2014, 193, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Bueno, P.V.; Almeida, E.A.; Rodrigues, F.H.; Rubira, A.F.; Muniz, E.C. Characterization of N-trimethyl chitosan/alginate complexes and curcumin release. Int. J. Biol. Macromol. 2013, 57, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Jin, Y.; Heng, P. Cross-linking mechanisms of calcium and zinc in production of alginate microspheres. Int. J. Pharm. 2002, 242, 255–258. [Google Scholar] [CrossRef]
- Silva, C.M.; Ribeiro, A.J.; Figueiredo, I.V.; Gonçalves, A.R.; Veiga, F. Alginate microspheres prepared by internal gelation: Development and effect on insulin stability. Int. J. Pharm. 2006, 311, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Kundu, J.; Kundu, S.C. Biopolymeric nanoparticles. Sci. Technol. Adv. Mater. 2010, 11, 014101. [Google Scholar] [CrossRef] [PubMed]
- Vrignaud, S.; Benoit, J.P.; Saulnier, P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cha, D.; Park, H.J. Survival of freeze-dried Lactobacillus bulgaricus KFRI 673 in chitosan-coated calcium alginate microparticles. J. Agric. Food Chem. 2004, 52, 7300–7305. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H.C. Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT-and conventionally treated milk during storage. LWT Food Sci. Technol. 2006, 39, 177–183. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D.; Eufeld, R.N. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.; Le, T.N.T.; Nguyen, T.A.; Dang, M.C. Poly (ethylene glycol) grafted chitosan as new copolymer material for oral delivery of insulin. J. Nanosci. Nanotechnol. 2015, 6, 035004. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Peniche, C.; Argüelles, M.W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Mi, Y.; Su, R.; Fan, D.D.; Zhu, X.L.; Zhang, W.N. Preparation of N,O-carboxymethyl chitosan coated alginate microcapsules and their application to Bifidobacterium longum BIOMA 5920. Mater. Sci. Eng. C 2013, 33, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, P.; Chakrabort, S.; Bhattacharya, S.; Mishra, R.; Kundu, P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ding, X.; Sheng, S.; Yao, Z. Retinoic acid inhibits osteogenic differentiation of rat bone marrow strom al cells. Biochem. Biophys. Res. Commun. 2008, 375, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Angadi, S.C.; Manjeshwar, L.S.; Aminabhav, T.M. Novel composite blend microbeads of sodium alginate coated with chitosan for controlled release of amoxicillin. Int. J. Biol. Macromol. 2012, 51, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W.; Chan, L.W.; Kho, S.B.; Heng, P.W.S. Design of controlled-release solid dosage forms of alginate and chitosan using microwave. J. Control. Release 2002, 84, 99–114. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Monteiro, J.P. Preparation and cytotoxicity of N,N,N-trimethyl chitosan/alginate beads containing gold nanoparticles. Int. J. Biol. Macromol. 2015, 72, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Ahmad, M.S. Development of Ca-alginate-chitosan microcapsules for encapsulation and controlled release of imidacloprid to control dengue outbreaks. J. Ind. Eng. Chem. 2017, 56, 382–393. [Google Scholar] [CrossRef]
- Huo, W.; Xie, G.; Zhang, W. Preparation of a novel chitosan-microcapsules/starch blend film and the study of its drug-release mechanism. Int. J. Biol. Macromol. 2016, 87, 114–122. [Google Scholar] [CrossRef] [PubMed]

| ALG:PHLC-Ca (w:w) | EE (%) | LC (%) |
|---|---|---|
| 1:1/3 | 53.70 ± 0.97% | 13.42 ± 0.81% |
| 1:1/2 | 44.05 ± 1.69% | 16.31 ± 0.96% |
| 1:1 | 25.41 ± 1.02% | 16.54 ± 0.58% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Liu, Z.; Deng, J.; Lei, H.; Zhu, C.; Fan, D.; Lv, X. Microencapsulation of Phosphorylated Human-Like Collagen-Calcium Chelates for Controlled Delivery and Improved Bioavailability. Polymers 2018, 10, 185. https://doi.org/10.3390/polym10020185
Mi Y, Liu Z, Deng J, Lei H, Zhu C, Fan D, Lv X. Microencapsulation of Phosphorylated Human-Like Collagen-Calcium Chelates for Controlled Delivery and Improved Bioavailability. Polymers. 2018; 10(2):185. https://doi.org/10.3390/polym10020185
Chicago/Turabian StyleMi, Yu, Zhengfang Liu, Jianjun Deng, Huan Lei, Chenhui Zhu, Daidi Fan, and Xingqiang Lv. 2018. "Microencapsulation of Phosphorylated Human-Like Collagen-Calcium Chelates for Controlled Delivery and Improved Bioavailability" Polymers 10, no. 2: 185. https://doi.org/10.3390/polym10020185