Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering
Abstract
:1. Introduction
2. Materials and Method
2.1. Chitosan
2.2. Cellulose Nanofibers
2.3. Preparation of the CNF Filled-Chitosan Suspensions and Composite Hydrogels
2.4. Shear Rheological Tests on CNF/CHI Suspensions
2.4.1. Couette Geometry
Model and Determination of the Rheological Parameters
2.4.2. Capillary Rheometry Evaluation of Injectability of the CNF/CHI Suspensions
Determination of the Apparent Viscosity in the Injection Setup
2.5. Rheological Behavior of the CNF/CHI Composite Hydrogels
2.6. X-ray Synchrotron Scattering Analysis of CNF/CHI Formulations
2.7. Suitability of the CNF/CHI Sol/Gel Formulations for Application in IVD Tissue Engineering
2.7.1. Cell Culture of Human Fibroblasts on CNF/CHI Hydrogels
Live/Dead Staining of Cells
2.7.2. Cellulose Nanofibers Cytotoxicity Evaluation
2.8. Injection Experiments in Intervertebral Discs of Animal Models
2.8.1. MRI and Histological Observations
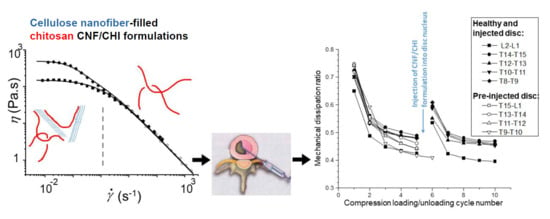
2.8.2. Biomechanical Studies on Discs Injected with CNF/CHI Formulations
3. Results and Discussion
3.1. Rheological Properties of CHI/CNF Injectable Formulations
3.1.1. Couette Rheometry
3.1.2. Building of Flow Diagrams in an Extended Shear Rate Range. Couette Geometry and Capillary Rheometry from the Injection Experiments
3.2. Viscoelastic Properties of the CNF/CHI Hydrogels
3.3. Assessment of the Suitability of the CNF/CHI Formulation for IVD Tissue Engineering
3.3.1. Cell Culture of Fibroblasts on the CNF/CHI Hydrogels
3.3.2. Application of the CNF/CHI Formulations to Restore the Disc Height Loss and Viscoelastic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharifi, S.; Bulstra, S.K.; Grijpma, D.W.; Kuijer, R. Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Sadosky, A.; Stacey, B.R.; Tai, K.-S.; Leslie, D. The burden of chronic low back pain: Clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012, 37, E668–E677. [Google Scholar] [CrossRef] [PubMed]
- Van Ooij, A.; Oner, F.C.; Verbout, A.J. Complications of artificial disc replacement: A report of 27 patients with the SB Charité disc. J. Spinal Disord. Tech. 2003, 16, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.G.; Smith, S.; Fairbank, J.C.T. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.W.; Gantenbein-Ritter, B. Intervertebral disc regeneration or repair with biomaterials and stem cell therapy—Feasible or fiction? Swiss Med. Wkly. 2012. [Google Scholar] [CrossRef] [PubMed]
- Whatley, B.R.; Wen, X. Intervertebral disc (IVD): Structure, degeneration, repair and regeneration. Mater. Sci. Eng. C 2012, 32, 61–77. [Google Scholar] [CrossRef]
- Rapoff, A.J.; Zdeblick, T.A. Biomechanical models of the cervical spine. In Frontiers in Head and Neck Trauma: Clinical and Biomechanical; Yoganandan, N., Pintar, F.A., Larson, S.J., Sances, A.J., Eds.; IOS Press: Clifton, VA, USA, 1998. [Google Scholar]
- Sato, K.; Kikuchi, S.; Yonezawa, T. In Vivo Intradiscal Pressure Measurement in Healthy Individuals and in Patients With Ongoing Back Problems. Spine 1999, 24, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Hukins, D.W.L.; Meakin, J.R. Relationship Between Structure and Mechanical Function of the Tissues of the Intervertebral Joint. Am. Zool. 2000, 40, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.A.; McNally, D.S.; Dolan, P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J. Bone Jt. Surg. Br. Vol. 1996, 78, 965–972. [Google Scholar] [CrossRef]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.I.; Nicoll, S.B. Characterization of photocrosslinked alginate hydrogels for nucleus pulposus cell encapsulation. J. Biomed. Mater. Res. Part A 2009, 91A, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Costalat, M.; Alcouffe, P.; David, L.; Delair, T. Macro-hydrogels versus nanoparticles by the controlled assembly of polysaccharides. Carbohydr. Polym. 2015, 134, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Pöppelmann, B.; Pappelbaum, K.; Moerschbacher, B.M.; Schneider, S.W. Human macrophage activation triggered by chitotriosidase-mediated chitin and chitosan degradation. Biomaterials 2010, 31, 8556–8563. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Mathews, S.; Gupta, P.K.; Bhonde, R.; Totey, S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011, 44, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.G.; Tahiri, K.; Montembault, A.S.; Domard, A.J.; Corvol, M.T.M. Multi-membrane chitosan hydrogels as chondrocytic cell bioreactors. Biomaterials 2011, 32, 5354–5364. [Google Scholar] [CrossRef] [PubMed]
- Montembault, A.; Tahiri, K.; Korwin-Zmijowska, C.; Chevalier, X.; Corvol, M.-T.; Domard, A. A material decoy of biological media based on chitosan physical hydrogels: Application to cartilage tissue engineering. Biochimie 2006, 88, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; Eder, M.; Rueggeberg, M.; Pandey, J.K.; Harrington, M.J.; Nishiyama, Y.; Putaux, J.-L.; Rochas, C.; Burgert, I. Reorientation of cellulose nanowhiskers in agarose hydrogels under tensile loading. Biomacromolecules 2012, 13, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; David, L.; Peniche-Covas, C.; Rochas, C.; Putaux, J.-L.; Trombotto, S.; Alcouffe, P.; Domard, A. Fine microstructure of processed chitosan nanofibril networks preserving directional packing and high molecular weight. Carbohydr. Polym. 2015, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Highly crystalline chitosan produced by multi-steps acid hydrolysis in the solid-state. Carbohydr. Polym. 2011, 83, 1730–1739. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; Laborie, M.-P. Morphological and Thermal Investigations of Cellulosic Bionanocomposites. In Biopolymer Nanocomposites; Dufresne, A., Thomas, S., Pothen, L.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 411–436. [Google Scholar]
- Samyn, P.; Osorio-Madrazo, A. Native Crystalline Polysaccharide Nanofibers: Processing and Properties. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–36. [Google Scholar]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaillé, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic Modulus and Stress-Transfer Properties of Tunicate Cellulose Whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.; Ching, Y.; Chuah, C.; Julai, S.; Liou, N.-S. Preparation and Characterization of Polyvinyl Alcohol-Chitosan Composite Films Reinforced with Cellulose Nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, M.; Ranginkar Jahromi, I.; Moallemi-Oreh, A.; Ebadi-Dehaghani, H.; Akbarzadeh, M. Evaluation of mixing efficiency in elaborating of chitosan/cellulose nanocomposite via statistical analyses. Int. J. Boil. Macromol. 2016, 93, 703–711. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, J.P.; Donnici, C.L.; Pereira, F.V. Biobased Nanocomposites from Layer-by-Layer Assembly of Cellulose Nanowhiskers with Chitosan. Biomacromolecules 2010, 11, 473–480. [Google Scholar] [CrossRef] [PubMed]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132, 42004. [Google Scholar] [CrossRef]
- Li, H.-Z.; Chen, S.-C.; Wang, Y.-Z. Preparation and characterization of nanocomposites of polyvinyl alcohol/cellulose nanowhiskers/chitosan. Compos. Sci. Technol. 2015, 115, 60–65. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Celebi, H.; Kurt, A. Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 2015, 133, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, H.; Chen, J.; Li, S.; Huang, J.; Zhou, C. Chitosan-chitin nanocrystal composite scaffolds for tissue engineering. Carbohydr. Polym. 2016, 152, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.; Naish, V.; O’Connor, B.; Blaise, C.; Gagne, F.; Hall, L.; Trudeau, V.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Pértile, R.A.; Moreira, S.; Gil, R.M.; Correia, A.; Guãrdao, L. Bacterial Cellulose: Long-Term Biocompatibility Studies. J. Biomater. Sci. Polym. Ed. 2012, 23, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, M.; Honkalampi, U.; von Wright, A.; Sneck, A.; Hentze, H-P.; Sievänen, J.; Hiltunen, J.; Hellén, E.K.O. Nanofibrillar Cellulose. In Vitro Study of Cytotoxic and Genotoxic Properties; TAPPI: London, UK, 2010. [Google Scholar]
- Eyholzer, C.; Borges de Couraça, A.; Duc, F.; Bourban, P.E.; Tingaut, P.; Zimmermann, T.; Månson, J.A.E.; Oksman, K. Biocomposite Hydrogels with Carboxymethylated, Nanofibrillated Cellulose Powder for Replacement of the Nucleus Pulposus. Biomacromolecules 2011, 12, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.C.; Eyholzer, C.; Duc, F.; Bourban, P.-E.; Tingaut, P.; Zimmermann, T.; Pioletti, D.P.; Månson, J.-A.E. Nanofibrillated cellulose composite hydrogel for the replacement of the nucleus pulposus. Acta Biomater. 2011, 7, 3412–3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Nguyen, T.H.M.; Abueva, C.; Ho, H.V.; Lee, S.-Y.; Lee, B.-T. In vitro and in vivo acute response towards injectable thermosensitive chitosan/TEMPO-oxidized cellulose nanofiber hydrogel. Carbohydr. Polym. 2018, 180, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Fiamingo, A.; Montembault, A.; Boitard, S.-E.; Naemetalla, H.; Agbulut, O.; Delair, T.; Campana-Filho, S.P.; Menasché, P.; David, L. Chitosan Hydrogels for the Regeneration of Infarcted Myocardium: Preparation, Physicochemical Characterization, and Biological Evaluation. Biomacromolecules 2016, 17, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.M. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Calero, N.; Muñoz, J.; Ramírez, P.; Guerrero, A. Flow behaviour, linear viscoelasticity and surface properties of chitosan aqueous solutions. Food Hydrocoll. 2010, 24, 659–666. [Google Scholar] [CrossRef]
- Halimi, C.; Montembault, A.; Guerry, A.; Delair, T.; Viguier, E.; Fulchiron, R.; David, L. Chitosan solutions as injectable systems for dermal filler applications: Rheological characterization and biological evidence. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 2596–2599. [Google Scholar]
- Eu, B.C. Generalization of the Hagen-Poiseuille velocity profile to non-Newtonian fluids and measurement of their viscosity. Am. J. Phys. 1990, 58, 83–84. [Google Scholar] [CrossRef]
- Christopher, R.H.; Middleman, S. Power-Law Flow through a Packed Tube. Ind. Eng. Chem. Fundam. 1965, 4, 422–426. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry; Gebrueder HAAKE GmbH: Karlsruhe, Germany, 1998; p. 291. [Google Scholar]
- Martins, S.A.A.; Barbosa, P.A.M.; Gaziola, D.L.T.L.; Andrade, S.M.H. Influence of particle size and fluid fraction on rheological and extrusion properties of crosslinked hyaluronic acid hydrogel dispersions. J. Appl. Polym. Sci. 2013, 128, 2180–2185. [Google Scholar]
- ISO10993-5. Biological Evaluation of Medical Devices. Test for Cytotoxicity: In Vitro Methods; ISO: Geneva, Switzerland, 1992. [Google Scholar]
- AFNOR6NF EN 30993-5. Evaluation Biologique des Dispositifs Médicaux. Partie 5: Essais Concernant la Cytotoxicité: Méthodes In Vitro; ISO 10993-10: France, 1994. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Parish, C.R.; Mullbacher, A. Automated colorimetric assay for T cell cytotoxicity. J. Immunol. Methods 1983, 58, 225–237. [Google Scholar] [CrossRef]
- Feuillet, T.; Seurin, M.-J.; Leveneur, O.; Viguier, E.; Beuf, O. Coil optimization for low-field MRI: A dedicated process for small animal preclinical studies. Lab. Anim. 2015, 49, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Halimi, C.; David, L.; Viguier, E.; Delair, T.; Montembault, A. Chitosan Aqueous Solution for Injection for the Prevention or the Treatment of Intervertebral Disc Degeneration. France Patent FR3039402B1, 2017. [Google Scholar]
- Montembault, A.; Viton, C.; Domard, A. Rheometric study of the gelation of chitosan in a hydroalcoholic medium. Biomaterials 2005, 26, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Montembault, A.; Viton, C.; Domard, A. Rheometric Study of the Gelation of Chitosan in Aqueous Solution without Cross-Linking Agent. Biomacromolecules 2005, 6, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Chornet, E.; Rodriguez, D. Steady-Shear Rheology of Concentrated Chitosan Solutions. J. Texture Stud. 2004, 35, 53–74. [Google Scholar] [CrossRef]
- Desorme, M.; Montembault, A.; Lucas, J.M.; Rochas, C.; Bouet, T.; David, L. Spinning of hydroalcoholic chitosan solutions. Carbohydr. Polym. 2013, 98, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Kienzle-Sterzer, C.A.; Rodriguez-Sanchez, D.; Rha, C.K. Flow behavior of a cationic biopolymer: Chitosan. Polym. Bull. 1985, 13, 1–6. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed]
- Gharehkhani, S.; Sadeghinezhad, E.; Kazi, S.N.; Yarmand, H.; Badarudin, A.; Safaei, M.R.; Zubir, M.N.M. Basic effects of pulp refining on fiber properties—A review. Carbohydr. Polym. 2015, 115, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Falcoz-Vigne, L.; Ogawa, Y.; Molina-Boisseau, S.; Nishiyama, Y.; Meyer, V.; Petit-Conil, M.; Mazeau, K.; Heux, L. Quantification of a tightly adsorbed monolayer of xylan on cellulose surface. Cellulose 2017, 24, 3725–3739. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kurki-Suonio, S.; Schacher, F.H.; Hietala, S.; Rojas, O.J.; Ikkala, O. Water-Resistant, Transparent Hybrid Nanopaper by Physical Cross-Linking with Chitosan. Biomacromolecules 2015, 16, 1062–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, R.; Saito, T.; Hondo, H.; Isogai, A. Influence of Flexibility and Dimensions of Nanocelluloses on the Flow Properties of Their Aqueous Dispersions. Biomacromolecules 2015, 16, 2127–2131. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.W.; Nerlich, A.G.; Lorenz, H.; Bruckner, T.; Richter, W.; Pfeiffer, M.; Gühring, T. Injection of a polymerized hyaluronic acid/collagen hydrogel matrix in an in vivo porcine disc degeneration model. Eur. Spine J. 2012, 21, 1700–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.M.; Hu, W.; Wang, X.D.; Gu, X.S. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. J. Mater. Sci. Mater. Med. 2007, 18, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Burns, W.C.; Thomas, M.C. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev. Mol. Med. 2010, 12, e17. [Google Scholar] [CrossRef] [PubMed]
- Hudson Katherine, D.; Mozia Robert, I.; Bonassar Lawrence, J. Dose-Dependent Response of Tissue-Engineered Intervertebral Discs to Dynamic Unconfined Compressive Loading. Tissue Eng. Part A 2015, 21, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Sample | nwr |
|---|---|
| CHI2 | 0.21 ± 0.1 |
| CHI2CNF0.2 | 0.21 ± 0.1 |
| CHI2CNF0.3 | 0.23 ± 0.1 |
| CHI2CNF0.4 | 0.21 ± 0.1 |
| Sample | η0, Chi (Pa.s) | τ Chi (s) | pChi | s | η0, CNF (Pa.s) | τ CNF (s) | p CNF |
|---|---|---|---|---|---|---|---|
| Hydroalcoholic conditions: | |||||||
| CHI1.7 | 62 | 1.0 | 0.71 | ||||
| CHI1.7CNF0.3 | 62 | 1.0 | 0.71 | 0.95 | 70 | 3.0 | 0.99 |
| CHI1.7CNF0.4 | 62 | 1.0 | 0.71 | 0.95 | 220 | 9.5 | 0.77 |
| CHI2 | 430 | 4.1 | 0.78 | ||||
| CHI2CNF0.2 | 430 | 4.1 | 0.78 | 0.95 | 385 | 8.5 | 0.93 |
| CHI2CNF0.3 | 430 | 4.1 | 0.78 | 0.95 | 591 | 9.1 | 0.99 |
| Water conditions: | |||||||
| CHI2 | 151 | 0.8 | 0.79 | ||||
| CHI2CNF0.2 | 151 | 0.8 | 0.79 | 0.97 | 257 | 8.3 | 0.93 |
| CHI2CNF0.3 | 151 | 0.8 | 0.79 | 0.99 | 282 | 8.3 | 1.54 |
| CHI2CNF0.4 | 151 | 0.8 | 0.79 | 1.03 | 343 | 8.3 | 1.30 |
| Sample | Relax. Frac.* | τ1 (s) | τ2 (s) | τ3 (s) | σ01 (MPa) | σ02 (MPa) | σ03 (MPa) | σ0 (MPa) | IVD Type |
|---|---|---|---|---|---|---|---|---|---|
| T8-T9 | 0.86 | 394.25 | 53.43 | 5.17 | 0.265 | 0.160 | 0.432 | 0.141 | S |
| T10-T11 | 0.91 | 629.51 | 101.35 | 7.65 | 0.217 | 0.211 | 0.367 | 0.074 | S |
| T12-T13 | 0.86 | 327.26 | 63.99 | 6.69 | 0.249 | 0.143 | 0.307 | 0.114 | S |
| T14-T15 | 0.90 | 605.27 | 94.24 | 8.09 | 0.166 | 0.140 | 0.352 | 0.071 | S |
| L1-L2 | 0.86 | 422.44 | 54.92 | 7.80 | 0.187 | 0.124 | 0.216 | 0.086 | S |
| Mean +/− sd | 0.88 +/− 0.02 | 475.74 +/− 134.12 | 73.59 +/− 22.61 | 7.08 +/− 1.19 | 0.217 +/− 0.04 | 0.156 +/− 0.03 | 0.335 +/− 0.08 | 0.097 +/− 0.03 | S |
| T8-T9 | 0.86 | 423.34 | 83.35 | 6.99 | 0.284 | 0.147 | 0.404 | 0.134 | I |
| T12-T13 | 0.91 | 383.18 | 62.85 | 6.71 | 0.271 | 0.162 | 0.312 | 0.076 | I |
| T14-T15 | 0.88 | 328.15 | 51.44 | 6.56 | 0.209 | 0.150 | 0.291 | 0.089 | I |
| L1-L2 | 0.88 | 429.04 | 66.29 | 8.12 | 0.198 | 0.119 | 0.216 | 0.075 | I |
| Mean +/− sd | 0.88 +/− 0.02 | 390.93 +/− 46.56 | 65.99 +/− 13.20 | 7.09 +/− 0.70 | 0.241 +/− 0.04 | 0.144 +/− 0.02 | 0.306 +/− 0.08 | 0.095 +/− 0.03 | I |
| T9-T10 | 0.92 | 370.91 | 72.19 | 6.48 | 0.256 | 0.234 | 0.326 | 0.069 | PI |
| T11-T12 | 0.92 | 353.52 | 64.99 | 6.27 | 0.221 | 0.188 | 0.314 | 0.061 | PI |
| T13-T14 | 0.92 | 352.23 | 55.87 | 6.36 | 0.182 | 0.159 | 0.315 | 0.056 | PI |
| T15-L1 | 0.92 | 381.10 | 56.11 | 6.45 | 0.170 | 0.161 | 0.294 | 0.057 | PI |
| Mean +/− sd | 0.920 +/− 0.02 | 364.44 +/− 14.00 | 62.29 +/− 7.85 | 6.39 +/− 0.09 | 0.207 +/− 0.04 | 0.185 +/− 0.03 | 0.312 +/− 0.01 | 0.061 +/− 0.01 | PI |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; Nunes de Oliveira, P.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers 2018, 10, 1202. https://doi.org/10.3390/polym10111202
Doench I, Torres-Ramos MEW, Montembault A, Nunes de Oliveira P, Halimi C, Viguier E, Heux L, Siadous R, Thiré RMSM, Osorio-Madrazo A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers. 2018; 10(11):1202. https://doi.org/10.3390/polym10111202
Chicago/Turabian StyleDoench, Ingo, Maria E. W. Torres-Ramos, Alexandra Montembault, Paula Nunes de Oliveira, Celia Halimi, Eric Viguier, Laurent Heux, Robin Siadous, Rossana M. S. M. Thiré, and Anayancy Osorio-Madrazo. 2018. "Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering" Polymers 10, no. 11: 1202. https://doi.org/10.3390/polym10111202