Electrospun Cellulose Nanocrystals/Chitosan/Polyvinyl Alcohol Nanofibrous Films and their Exploration to Metal Ions Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNC
2.3. Electrospinning
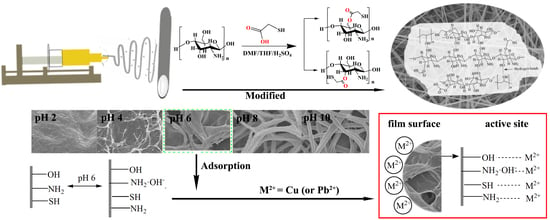
2.4. Modification of xCNC/CS/PVA with Thioglycolic Acid
2.5. The Stability of xCNC/CS/PVA-SH Nanofibrous Films
2.6. Adsorption of the Metalions
2.7. Characterization of xCNC/CS/PVA Composite Nanofibrous Films
3. Results and Discussion
3.1. Characterization of CNC
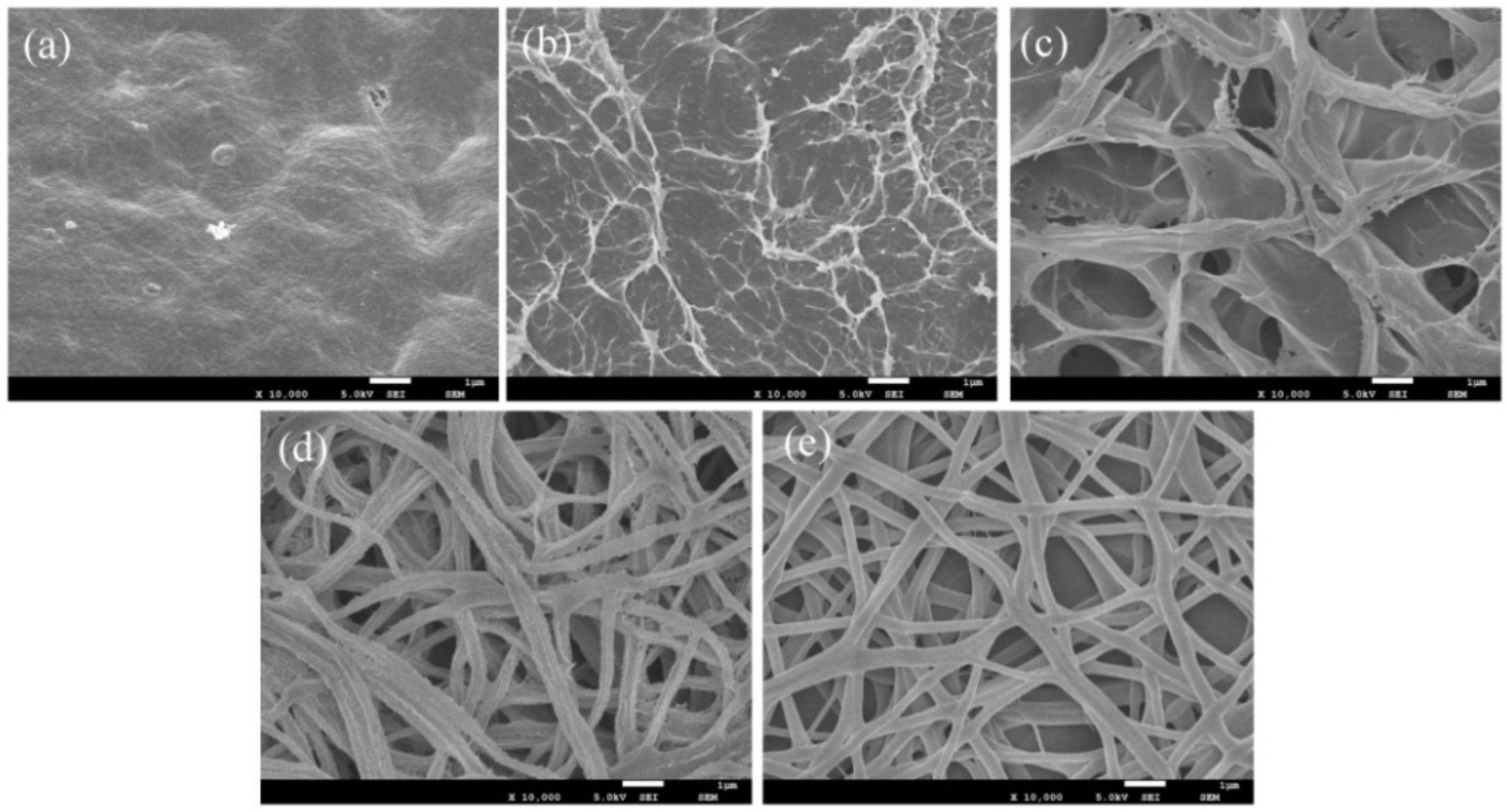
3.2. Surface Morphology of the xCNC/CS/PVA Composite Nanofibrous Films
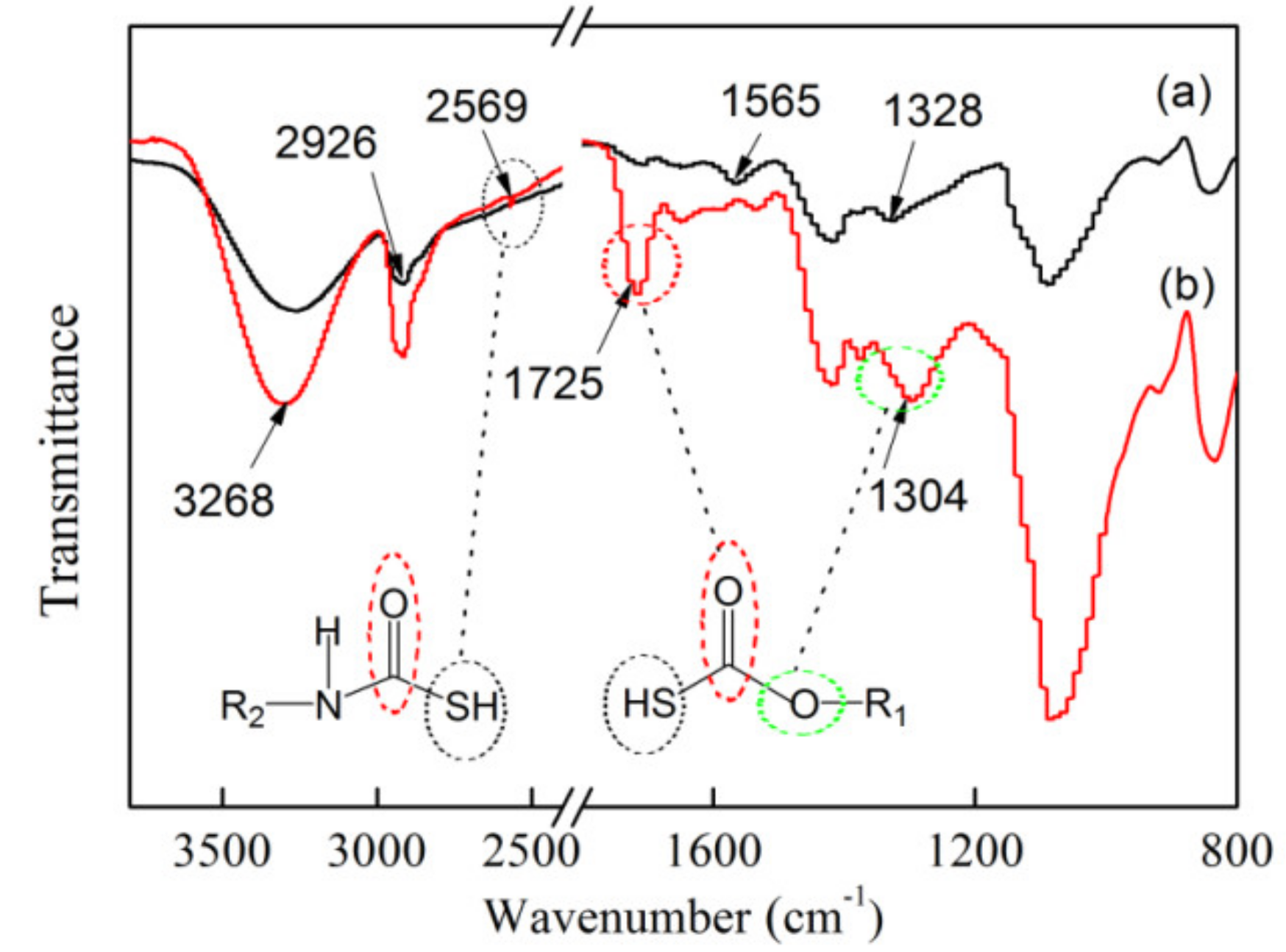
3.3. FTIR of Electrospun xCNC/CS/PVA-SH Nanofibrous Films
3.4. Surface Morphology of the CNC/CS/PVA-SH Composite Nanofibrous Films
3.5. Adsorption and Desorption of the Metalions
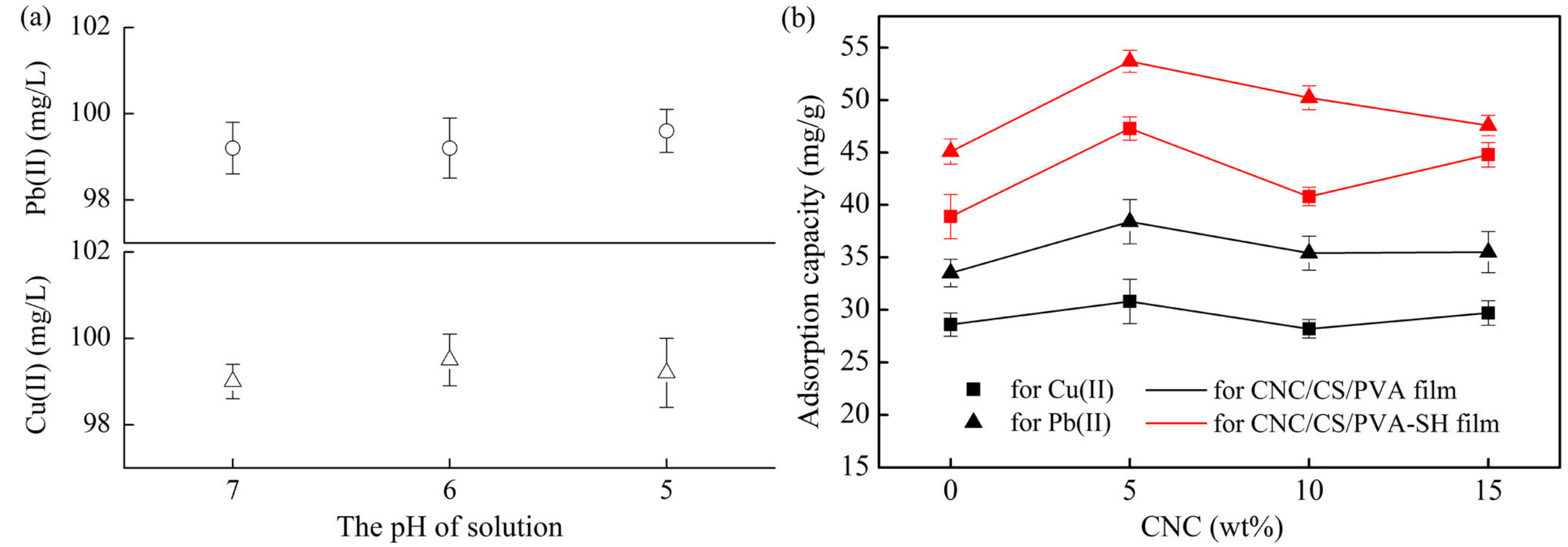
3.5.1. Effect of Different CNC Loading Levels
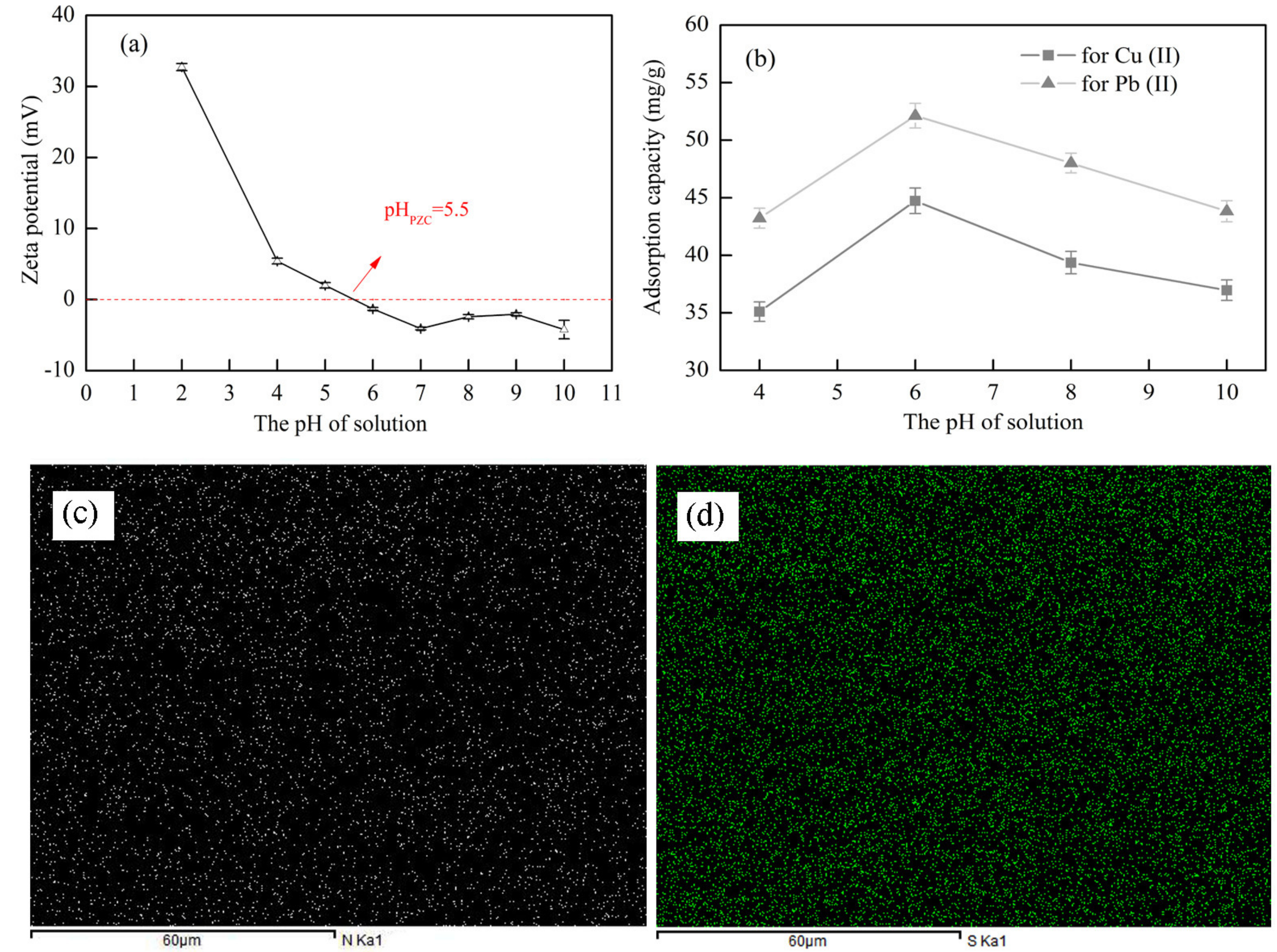
3.5.2. Effect of pH
3.5.3. Kinetics of Adsorption
3.5.4. Adsorption Isotherm
3.5.5. Reusability of the CNC/CS/PVA-SH Composite Nanofibrous Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chowdhury, I.H.; Chowdhury, A.H.; Bose, P.; Mandal, S.; Naskar, M.K. Effect of anion type on the synthesis of mesoporous nanostructured MgO, and its excellent adsorption capacity for the removal of toxic heavy metal ions from water. RSC Adv. 2016, 6, 6038–6047. [Google Scholar] [CrossRef]
- Nabi, S.A.; Shahadat, M.; Bushra, R.; Shalla, A.H.; Azam, A. Synthesis and characterization of nano-composite ion-exchanger; its adsorption behavior. Colloids Surf. B 2011, 87, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Saeed, A.; Zafar, S.I. Hybrid biosorbent: an innovative matrix to enhance the biosorption of Cd (II) from aqueous solution. J. Hazard. Mater. 2007, 148, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.L.; Fan, M.; Xu, M.; Brown, R.C.; Sung, S.; Saha, B.; Huang, C.P. Adsorption of arsenic (V) by activated carbon prepared from oat hulls. Chemosphere 2005, 61, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Katsou, E.; Malamis, S.; Tzanoudaki, M.; Haralambous, K.J.; Loizidou, M. Regeneration of natural zeolite polluted by lead and zinc in wastewater treatment systems. J. Hazard. Mater. 2010, 189, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Miretzky, P.; Cirelli, A.F. Hg (II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Vandenbossche, M.; Jimenez, M.; Casetta, M.; Bellayer, S.; Beaurain, A.; Bourbigot, S.; Traisnel, M. Chitosan-grafted nonwoven geotextile for heavy metals sorption in sediments. React. Funct. Polym. 2013, 73, 53–59. [Google Scholar] [CrossRef]
- Lai, Y.L.; Thirumavalavan, M.; Lee, J.-F. Effective adsorption of heavy metal ions (Cu2+, Pb2+, Zn2+) from aqueous solution by immobilization of adsorbents on Ca-alginate beads. Toxicol. Environ. Chem. 2011, 92, 697–705. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for coagulation/flocculation processes–an eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Jung, C.; Heo, J.; Han, J.; Her, N.; Lee, S.-J.; Oh, J.; Ryu, J.; Yoon, Y. Hexavalent chromium removal by various adsorbents: Powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Sep. Purif. Technol. 2013, 106, 63–71. [Google Scholar] [CrossRef]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Guibalc, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Haider, S.; Park, S.-Y. Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution. J. Membr. Sci. 2009, 328, 90–96. [Google Scholar] [CrossRef]
- Horzum, N.; Boyaci, E.; Eroglu, A.E.; Shahwan, T.; Demir, M.M. Sorption Efficienc of Chitosan Nanofibers toward Metal Ions at Low Concentrations. Biomacromolecules 2010, 11, 3301–3308. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.S.W.; Fatinathan, S. Adsorption of Cu (II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Ma, W.; Guo, Z.; Zhao, J.; Yu, Q.; Wang, F.; Han, J.; Pan, H.; Yao, J.; Zhang, Q.; Samal, S.K.; et al. Polyimide/cellulose acetate core/shell electrospun fibrous membranes for oil-water separation. Sep. Purif. Technol. 2017, 177, 71–85. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Joo, T.C.; Salleh, A.; Ang, B.C.; Afifi, A.M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium (VI) by flocculation/adsorption. Carbohydr. Polym. 2017, 157, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, X.; Lin, X.; Lu, X.; Zhou, Y.; Tang, Y. Polycaprolactone/chitosan blends: Simulation and experimental design. Mater. Des. 2016, 90, 396–402. [Google Scholar] [CrossRef]
- Klossner, R.R.; Queen, H.A.; Coughlin, A.J.; Krause, W.E. Correlation of chitosan’s rheological properties and its ability to electrospin. Biomacromolecules 2008, 9, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Razzaz, A.; Ghorban, S.; Hosayni, L.; Irani, M.; Aliabadi, M. Chitosan nanofibers functionalized by TiO2 nanoparticles for the removal of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2016, 58, 333–343. [Google Scholar] [CrossRef]
- Han, J.; Yue, Y.; Wu, Q.; Huang, C.; Pan, H.; Zhan, X.; Mei, C.; Xu, X. Effects of nanocellulose on the structure and properties of poly (vinyl alcohol)-borax hybrid foams. Cellulose 2017, 24, 4433–4448. [Google Scholar] [CrossRef]
- Han, J.; Lei, T.; Wu, Q. High-water-content mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Dynamic rheological properties and hydrogel formation mechanism. Carbohydr. Polym. 2014, 102, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, H.; Cao, M.; Hao, L.; Zhai, L.; Jiang, S.; Li, X. Synthesis and antimicrobial activity of mesoporous hydroxylapatite/zinc oxide nanofibers. Mater. Des. 2015, 87, 17–24. [Google Scholar] [CrossRef]
- Xu, R.; Tang, R.; Liu, S.; Li, F.; Zhang, B. An environmentally-friendly enzyme-based nanofibrous membrane for 3, 3′, 5, 5′-tetrabromobisphenol removal. RSC Adv. 2015, 5, 64091–64097. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, Q.; Han, J. Nanocellulose-mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef] [PubMed]
- Ridolfi, D.M.; Lemes, A.P.; de Oliveira, S.; Justo, G.Z.; Palladino, M.V.; Durán, N. Electrospun poly (ethylene oxide)/chitosan nanofibers with cellulose nanocrystals as support for cell culture of 3T3 fibroblasts. Cellulose 2017, 24, 3353–3365. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, M.; Liu, R.; Li, Y.; Wang, D.; Tan, J.; Wu, R.; Huang, Y. Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. Carbohydr. Polym. 2011, 83, 743–748. [Google Scholar] [CrossRef]
- Wu, S.; Li, F.; Wang, H.; Fu, L.; Zhang, B.; Li, G. Effects of poly (vinyl alcohol)(PVA) content on preparation of novel thiol-functionalized mesoporous PVA/SiO2 composite nanofiber membranes and their application for adsorption of heavy metal ions from aqueous solution. Polymer 2010, 51, 6203–6211. [Google Scholar] [CrossRef]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Zhang, Z.L.; Liu, H.Q.; Yin, Z.Z.; Li, L.; Liu, X.M. Characterization of cellulose-based electrospun nanofiber membrane and its adsorptive behaviours using Cu (II), Cd (II), Pb (II) as models. Sci. China Chem. 2013, 56, 567–575. [Google Scholar] [CrossRef]
- Huan, S.; Bai, L.; Liu, G.; Cheng, W.; Han, G. Electrospun nanofibrous composites of polystyrene and cellulose nanocrystals: manufacture and characterization. RSC Adv. 2015, 5, 50756–50766. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-assembling behavior of cellulose nanoparticles during freeze-drying: Effect of suspension concentration, particle size, crystal structure, and surface charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, G.; Zhang, J.; Liu, Y.; Nie, J.; Ma, G. Facile fabrication of core-shell polyelectrolyte complexes nanofibers based on electric field induced phase separation. Polymer 2017, 110, 80–86. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Yang, D.-Z.; Xu, F.; Zhang, Z.-P.; Yin, R.-X.; Nie, J. Electrospun core− shell structure nanofibers from homogeneous solution of poly (ethylene oxide)/chitosan. Macromolecules 2009, 42, 5278–5284. [Google Scholar] [CrossRef]
- Huan, S.; Bai, L.; Cheng, W.; Han, G. Manufacture of electrospun all-aqueous poly (vinyl alcohol)/cellulose nanocrystal composite nanofibrous mats with enhanced properties through controlling fibers arrangement and microstructure. Polymer 2016, 92, 25–35. [Google Scholar] [CrossRef]
- Bui, N.-N.; McCutcheon, J.R. Hydrophilic nanofibers as new supports for thin film composite membranes for engineered osmosis. Environ. Sci. Technol. 2013, 47, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Huan, S.; Liu, G.; Cheng, W.; Han, G.; Bai, L. Electrospun Poly (lactic acid)-Based Fibrous Nanocomposite Reinforced by Cellulose Nanocrystals: Impact of Fiber Uniaxial Alignment on Microstructure and Mechanical Properties. Biomacromolecules 2018, 19, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132, 42004. [Google Scholar] [CrossRef]
- Sharma, R.; Ahuja, M. Thiolated pectin: Synthesis, characterization and evaluation as a mucoadhesive polymer. Carbohydr. Polym. 2011, 85, 658–663. [Google Scholar] [CrossRef]
- Sun, K.; Li, Z.H. Preparations, properties and applications of chitosan based nanofibers fabricated by electrospinning. eXPRESS Polym. Lett. 2011, 5, 342–361. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wu, J.-X.; Lü, Q.-F.; Lin, T.-T. Facile preparation of lignosulfonate–graphene oxide–polyaniline ternary nanocomposite as an effective adsorbent for Pb (II) ions. ACS Sustain. Chem. Eng. 2014, 2, 1203–1211. [Google Scholar] [CrossRef]
- Wang, B.; Xia, J.; Mei, L.; Wang, L.; Zhang, Q. Highly efficient and rapid lead (II) scavenging by the natural Artemia cyst shell with unique three-dimensional porous structure and strong sorption affinity. ACS Sustain. Chem. Eng. 2017, 6, 1343–1351. [Google Scholar] [CrossRef]
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Synthesis of sodium acrylate and acrylamide copolymer/GO hydrogels and their effective adsorption for Pb2+ and Cd2+. ACS Sustain. Chem. Eng. 2016, 4, 3948–3959. [Google Scholar] [CrossRef]
- Jiang, N.; Xu, Y.; Dai, Y.; Luo, W.; Dai, L. Polyaniline nanofibers assembled on alginate microsphere for Cu2+ and Pb2+ uptake. J. Hazard. Mater. 2012, 215, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, N.; Valiyaveettil, S. Functionalized poly (vinyl alcohol) based nanofibers for the removal of arsenic from water. RSC Adv. 2013, 3, 2776–2783. [Google Scholar] [CrossRef]
- Jia, Y.-T.; Gong, J.; Gu, X.-H.; Kim, H.-Y.; Dong, J.; Shen, X.-Y. Fabrication and characterization of poly (vinyl alcohol)/chitosan blend nanofibers produced by electrospinning method. Carbohydr. Polym. 2007, 67, 403–409. [Google Scholar] [CrossRef]






| Solution | Conductivity (μs/cm) | Viscosity (mPa·s) | Surface Tension (mN/m) |
|---|---|---|---|
| 0-CNC/CS/PVA | 1283 | 1376 | 33.7 |
| 5-CNC/CS/PVA | 1308 | 1300 | 36.2 |
| 10-CNC/CS/PVA | 1315 | 1304 | 37.1 |
| 20-CNC/CS/PVA | 1332 | 1368 | 37.7 |
| Code | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Metal Ion | Qmax | b | R2 | K | n | R2 |
| Cu(II) | 484.06 | 0.013 | 0.986 | 45.124 | 2.745 | 0.911 |
| Pb(II) | 323.49 | 0.015 | 0.990 | 37.984 | 3.058 | 0.932 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Cheng, W.; Yue, Y.; Xuan, L.; Ni, X.; Han, G. Electrospun Cellulose Nanocrystals/Chitosan/Polyvinyl Alcohol Nanofibrous Films and their Exploration to Metal Ions Adsorption. Polymers 2018, 10, 1046. https://doi.org/10.3390/polym10101046
Wang D, Cheng W, Yue Y, Xuan L, Ni X, Han G. Electrospun Cellulose Nanocrystals/Chitosan/Polyvinyl Alcohol Nanofibrous Films and their Exploration to Metal Ions Adsorption. Polymers. 2018; 10(10):1046. https://doi.org/10.3390/polym10101046
Chicago/Turabian StyleWang, Dong, Wanli Cheng, Yiying Yue, Lihui Xuan, Xiaohui Ni, and Guangping Han. 2018. "Electrospun Cellulose Nanocrystals/Chitosan/Polyvinyl Alcohol Nanofibrous Films and their Exploration to Metal Ions Adsorption" Polymers 10, no. 10: 1046. https://doi.org/10.3390/polym10101046