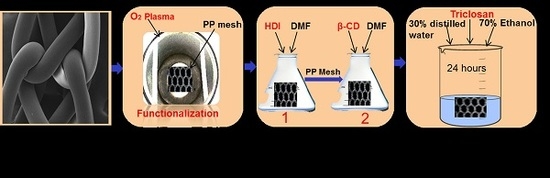
Preparation and Characterization of Antibacterial Polypropylene Meshes with Covalently Incorporated β-Cyclodextrins and Captured Antimicrobial Agent for Hernia Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cold Plasma Surface Functionalization
2.2.2. Two Grafting Steps and PP-HDI-CD Incorporation
2.2.3. Loading of Triclosan
2.3. Characterization Techniques
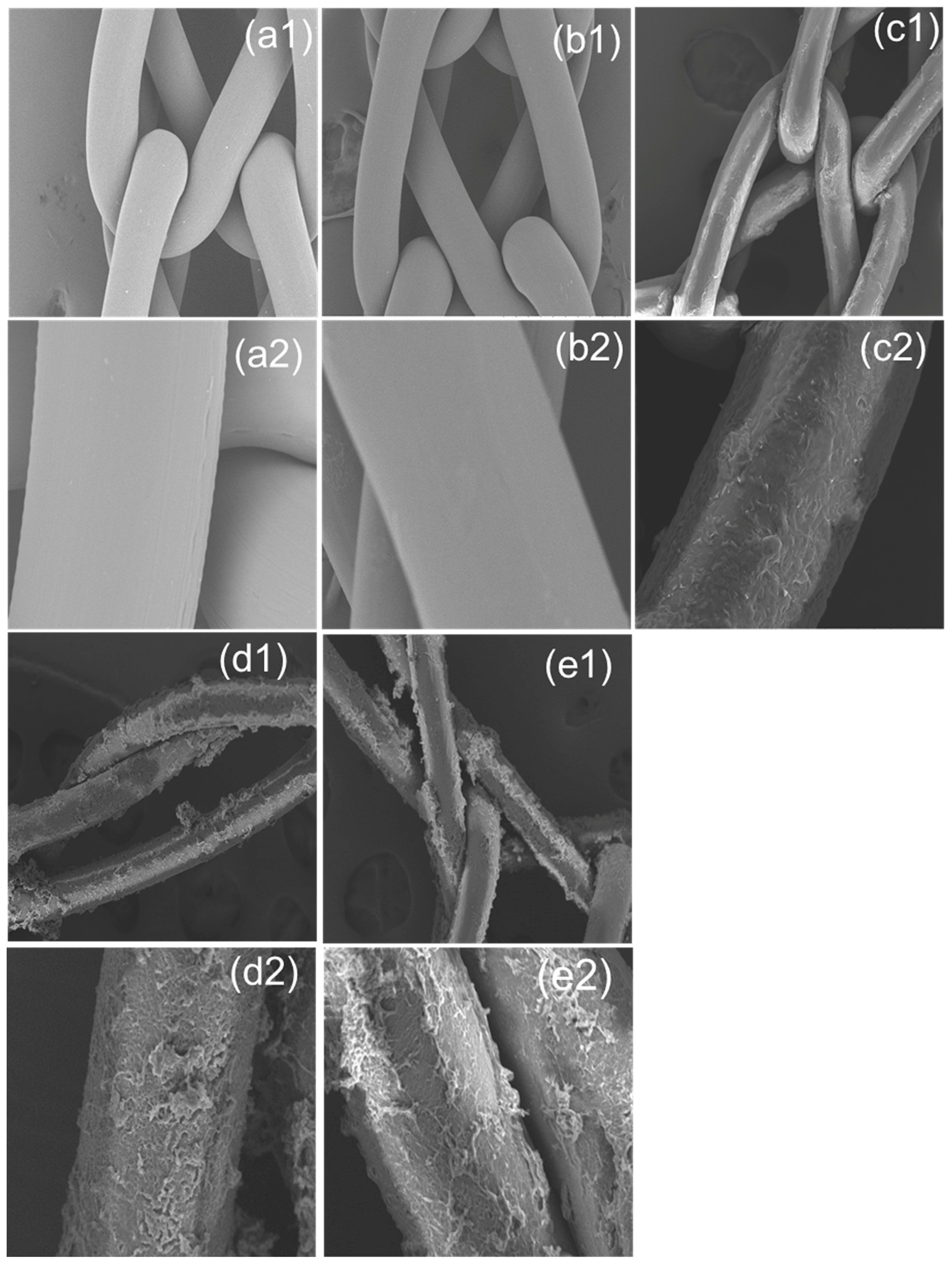
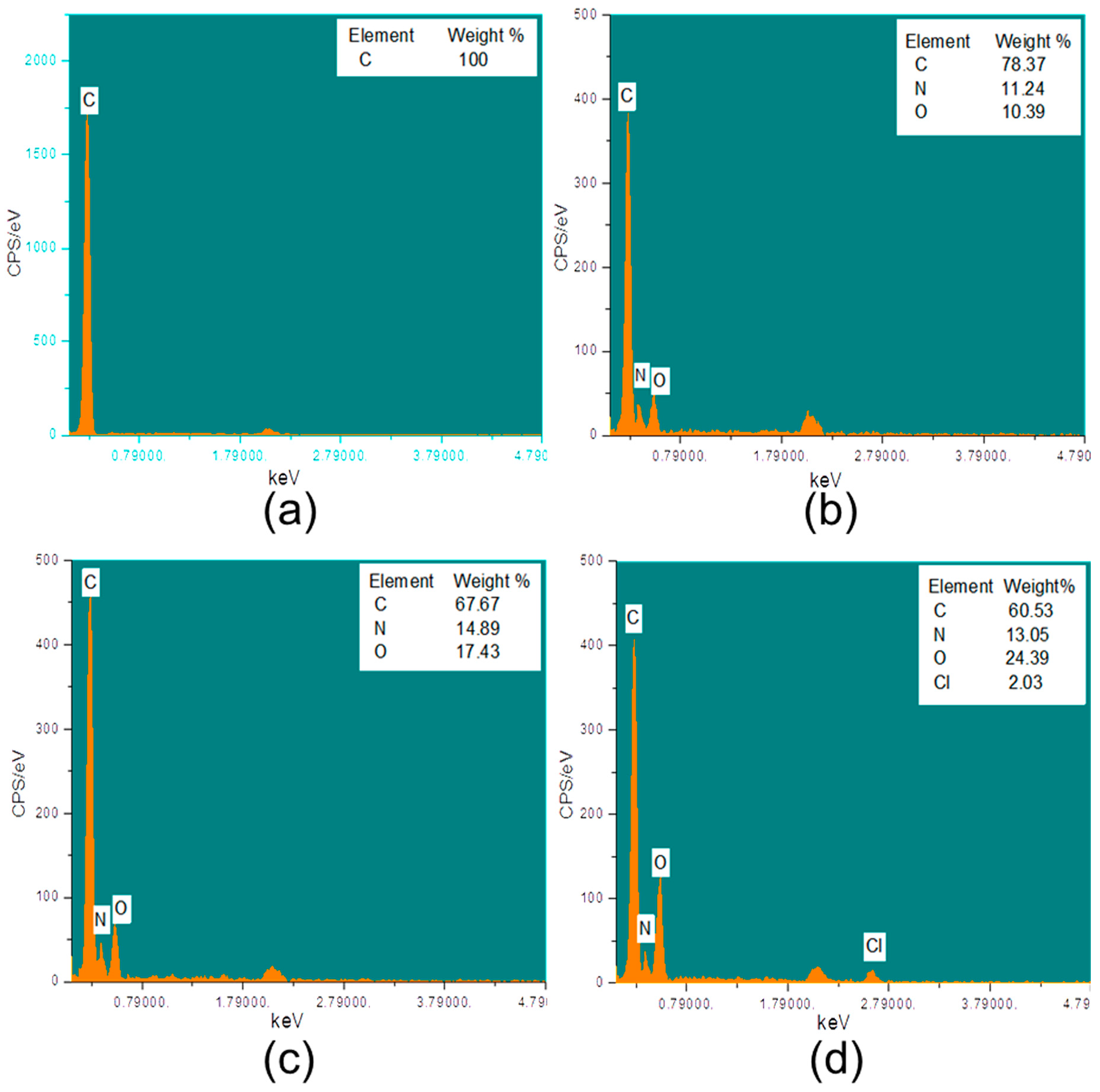
2.3.1. SEM and EDX
2.3.2. FTIR
2.3.3. Contact Angle
2.3.4. XRD
2.3.5. Thermal Analysis
2.3.6. Antibacterial Activity Assessment
Agar Diffusion Test Method (Qualitative Analysis)
Sustained Efficacy Test (Serial Plate Transfer Test)
2.3.7. Statistical Analysis
3. Results and Discussion
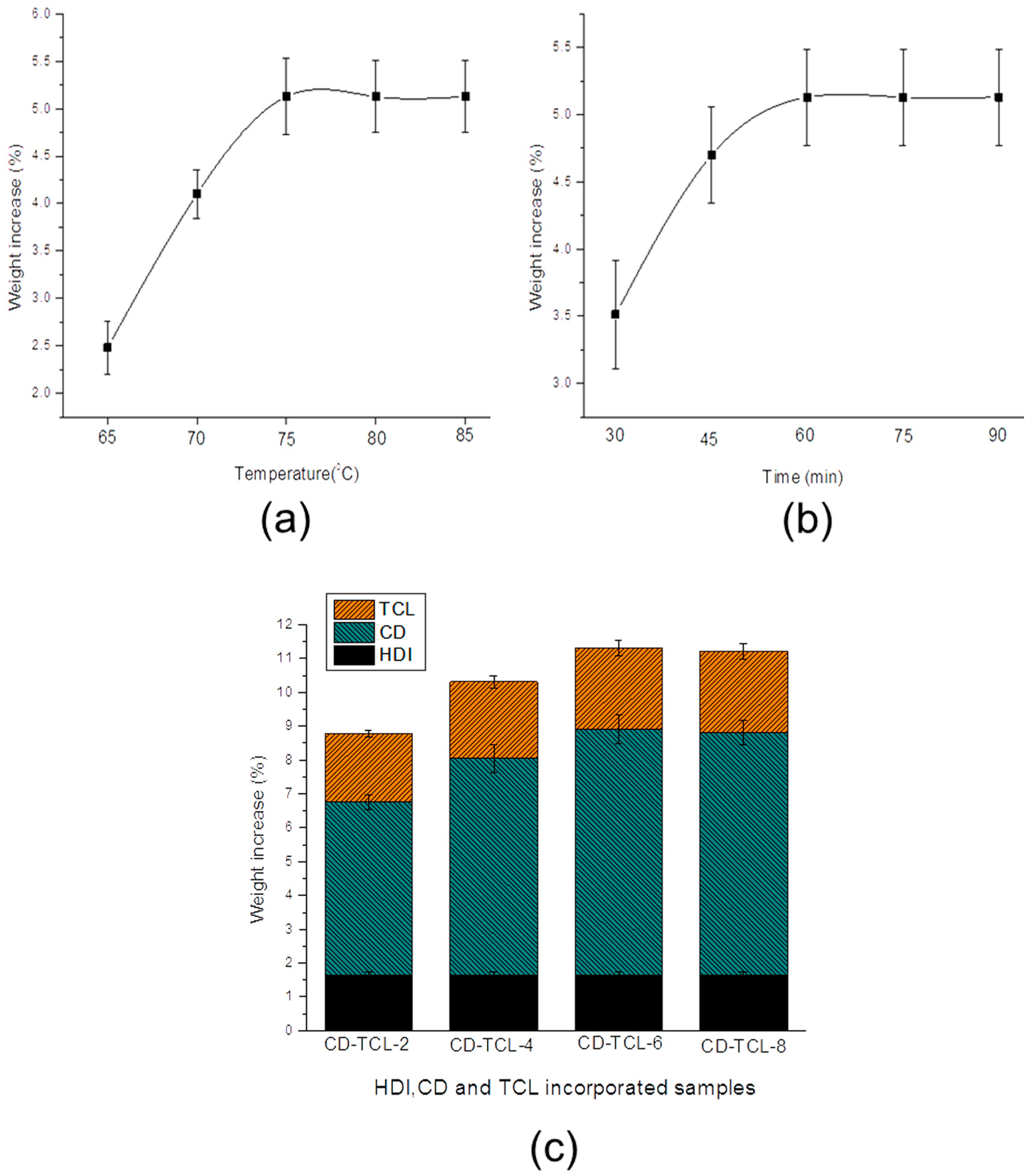
3.1. Cyclodextrin Grafting and Loading of Triclosan
3.2. Surface Morphology of PP Meshes
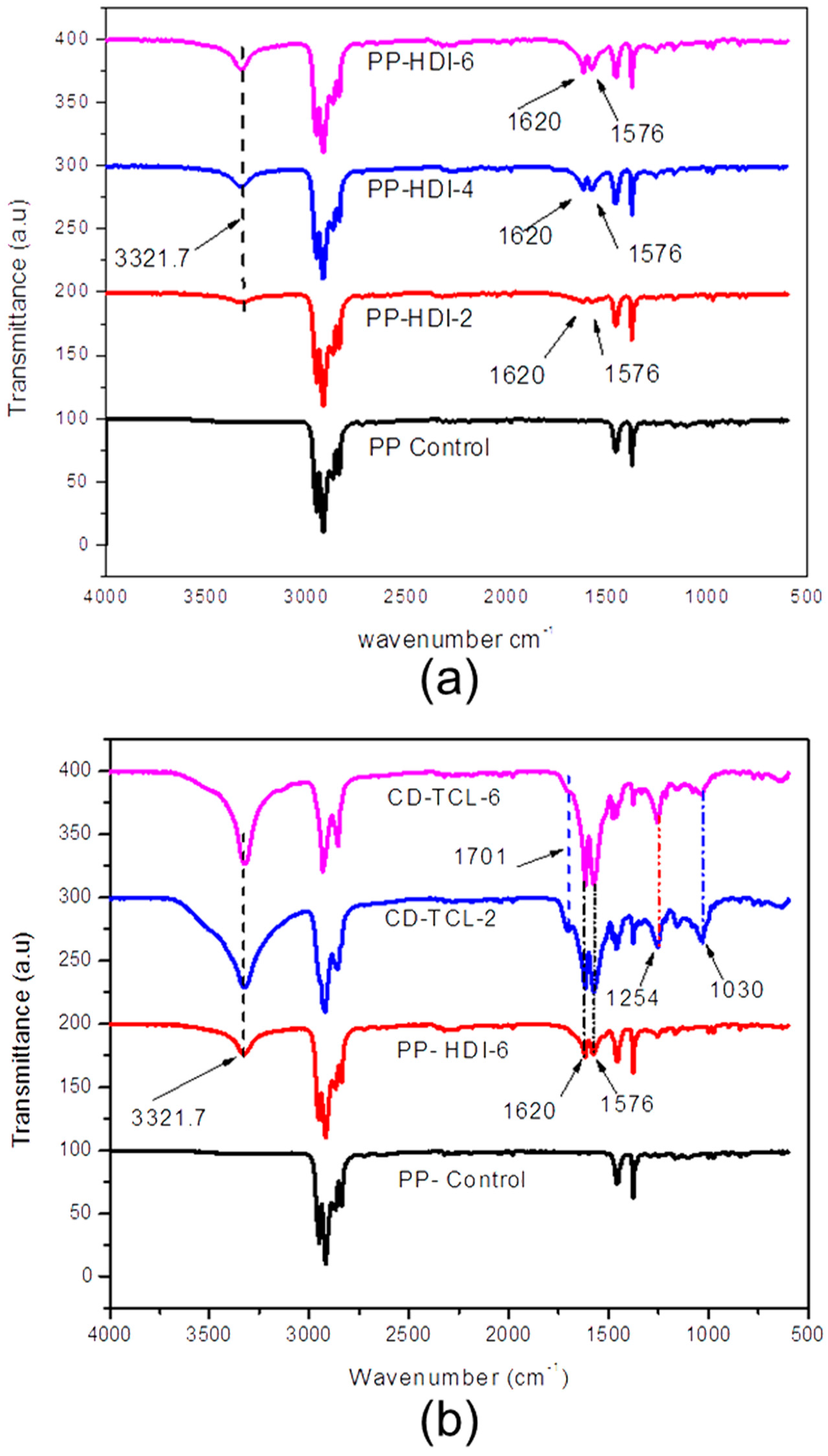
3.3. Characterization of HDI, CD, and Triclosan on Modified PP Mesh
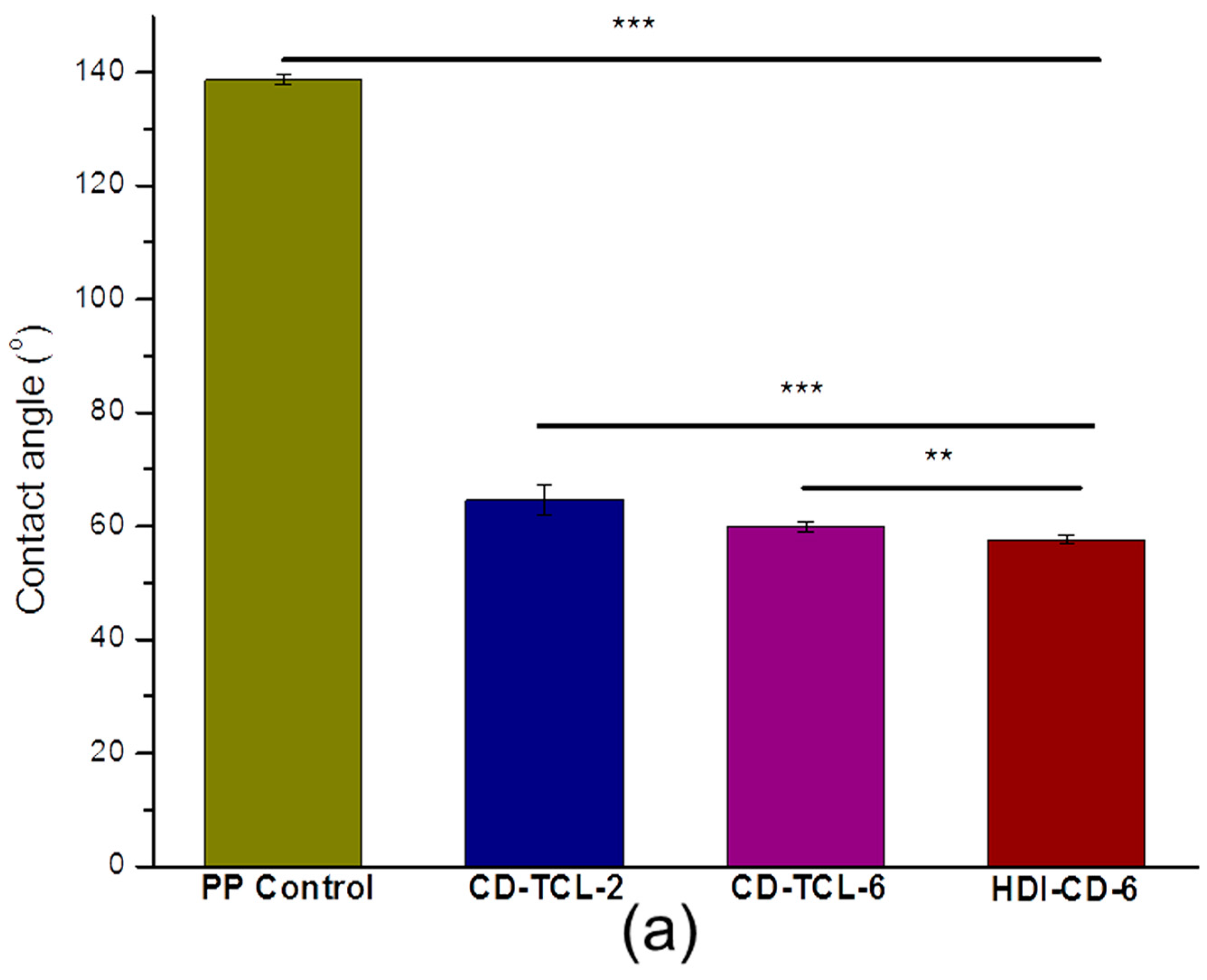
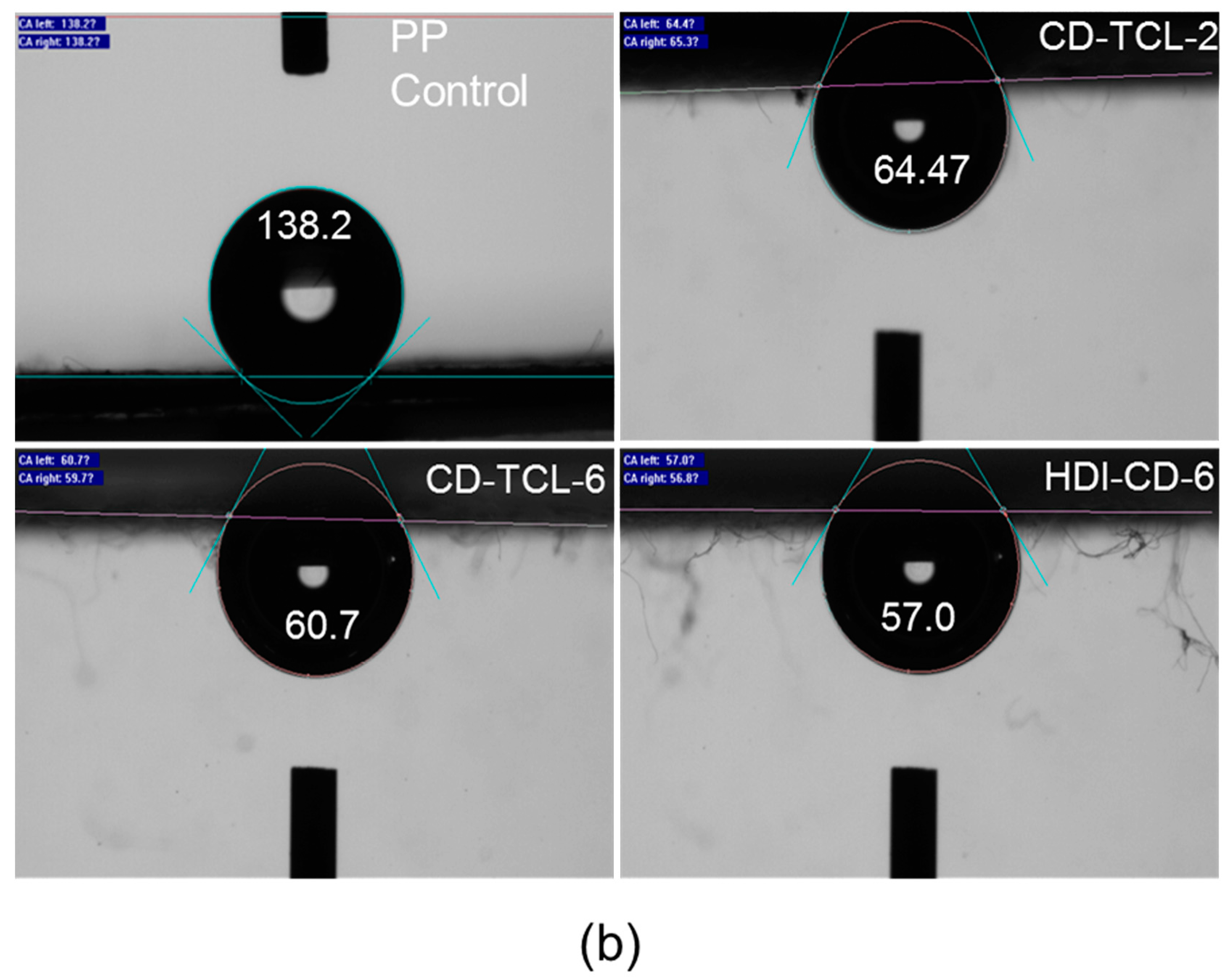
3.4. Hydrophilicity of PP Meshes (Water Contact Angle)
3.5. Structural and Thermal Properties
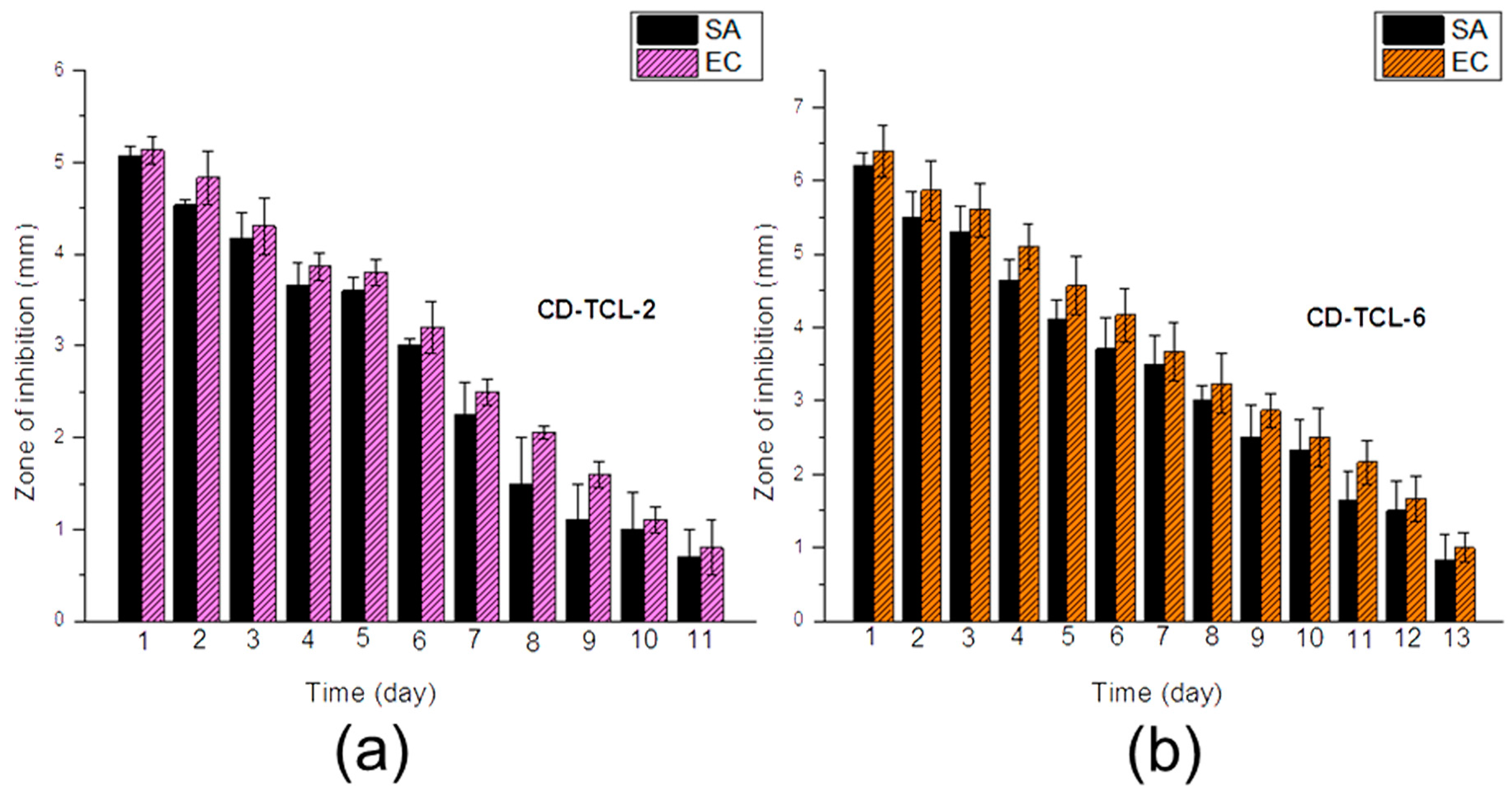
3.6. Antibacterial Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chughtai, B.; Thomas, D.; Mao, J.; Eilber, K.; Anger, J.; Clemens, J.Q.; Sedrakyan, A. Hernia repair with polypropylene mesh is not associated with an increased risk of autoimmune disease in adult men. Hernia 2017, 21, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, F.; Wang, L.; Zou, T.; Brochu, G.; Guidoin, R. Physical characteristics of medical textile prostheses designed for hernia repair: A comprehensive analysis of select commercial devices. Materials (Basel) 2015, 8, 8148–8168. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.N.; Finch, J.G. Which mesh for hernia repair? Ann. R. Coll. Surg. Engl. 2010, 92, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Bilsel, Y.; Abci, I. The search for ideal hernia repair; mesh materials and types. Int. J. Surg. 2012, 10, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Hazebroek, E.J.; Ng, A.; Yong, D.H.; Berry, H.; Leibman, S.; Smith, G.S. Evaluation of lightweight titanium-coated polypropylene mesh (timesh) for laparoscopic repair of large hiatal hernias. Surg. Endosc. 2008, 22, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Klinge, U.; Klosterhalfen, B.; Birkenhauer, V.; Junge, K.; Conze, J.; Schumpelick, V. Impact of polymer pore size on the interface scar formation in a rat model. J. Surg. Res. 2002, 103, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Gil, D.; Rex, J.; Cobb, W.; Reukov, V.; Vertegel, A. Anti-inflammatory coatings of hernia repair meshes: A pilot study. J. Biomed. Mater. Res. B 2018, 106, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sanbhal, N.; Miao, L.; Xu, R.; Khatri, A.; Wang, L. Physical structure and mechanical properties of knitted hernia mesh materials: A review. J. Ind. Text. 2017. [Google Scholar] [CrossRef]
- Kulaga, E.; Ploux, L.; Balan, L.; Schrodj, G.; Roucoules, V. Mechanically responsive antibacterial plasma polymer coatings for textile biomaterials. Plasma Process. Polym. 2014, 11, 63–79. [Google Scholar] [CrossRef]
- Knetsch, M.L.W.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Nisticò, R.; Magnacca, G.; Martorana, S. Surface science in hernioplasty: The role of plasma treatments. Appl. Surf. Sci. 2017, 419, 860–868. [Google Scholar] [CrossRef]
- Perez-Kohler, B.; Fernandez-Gutierrez, M.; Pascual, G.; Garcia-Moreno, F.; San Roman, J.; Bellon, J.M. In vitro assessment of an antibacterial quaternary ammonium-based polymer loaded with chlorhexidine for the coating of polypropylene prosthetic meshes. Hernia 2016, 20, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Labay, C.; Canal, J.M.; Modic, M.; Cvelbar, U.; Quiles, M.; Armengol, M.; Arbos, M.A.; Gil, F.J.; Canal, C. Antibiotic-loaded polypropylene surgical meshes with suitable biological behaviour by plasma functionalization and polymerization. Biomaterials 2015, 71, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Erdas, E.; Medas, F.; Pisano, G.; Nicolosi, A.; Calo, P.G. Antibiotic prophylaxis for open mesh repair of groin hernia: Systematic review and meta-analysis. Hernia 2016, 20, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, T.L.; Smith, P.W.; Gazoni, L.M.; Sawyer, R.G. The appropriate use of antibiotics in surgery: A review of surgical infections. Curr. Probl. Surg. 2007, 44, 635–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, J.; Wang, H.; Xia, Q.; Xu, S.; Han, C.C. Controlled antibiotics release system through simple blended electrospun fibers for sustained antibacterial effects. ACS Appl. Mater. Interfaces 2015, 7, 26400–26404. [Google Scholar] [CrossRef] [PubMed]
- Thatiparti, T.R.; Shoffstall, A.J.; von Recum, H.A. Cyclodextrin-based device coatings for affinity-based release of antibiotics. Biomaterials 2010, 31, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alvarez, L.; Matas, J.; Gomez-Galvan, F.; Ruiz-Rubio, L.; Leon, L.M.; Vilas-Vilela, J.L. Branched and ionic beta-cyclodextrins multilayer assembling onto polyacrylonitrile membranes for removal and controlled release of triclosan. Carbohydr. Polym. 2017, 156, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Galvan, F.; Perez-Alvarez, L.; Matas, J.; Alvarez-Bautista, A.; Poejo, J.; Duarte, C.M.; Ruiz-Rubio, L.; Vila-Vilela, J.L.; Leon, L.M. Preparation and characterization of soluble branched ionic beta-cyclodextrins and their inclusion complexes with triclosan. Carbohydr. Polym. 2016, 142, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Fidaleo, M.; Zuorro, A.; Lavecchia, R. Enhanced antibacterial and anti-quorum sensing activities of triclosan by complexation with modified beta-cyclodextrins. World J. Microbiol. Biotechnol. 2013, 29, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.; Kacem, I.; Blanchemain, N.; Cazaux, F.; Neut, C.; Hildebrand, H.F.; Martel, B. Cyclodextrin and maltodextrin finishing of a polypropylene abdominal wall implant for the prolonged delivery of ciprofloxacin. Acta Biomater. 2011, 7, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Avetta, P.; Nisticò, R.; Faga, M.G.; D’Angelo, D.; Boot, E.A.; Lamberti, R.; Martorana, S.; Calza, P.; Fabbri, D.; Magnacca, G. Hernia-repair prosthetic devices functionalised with chitosan and ciprofloxacin coating: Controlled release and antibacterial activity. J. Mater. Chem. B 2014, 2, 5287–5294. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, T.; Li, J.; Ji, Z.; Zhou, H.; Zhou, X.; Gu, N. Preparation of poly(l-lactic acid)-modified polypropylene mesh and its antiadhesion in experimental abdominal wall defect repair. J. Biomed. Mater. Res. B 2014, 102, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, L.; Carrino, L.; Napolitano, G. Oxygen cold plasma treatment on polypropylene: Influence of process parameters on surface wettability. Surf. Eng. 2013, 23, 247–252. [Google Scholar] [CrossRef]
- Shishoo, R. Plasma Technologies for Textiles; Woodhead Publishing Limited: Cornwall, UK, 2007. [Google Scholar]
- Fauland, G.; Constantin, F.; Gaffar, H.; Bechtold, T. Production scale plasma modification of polypropylene baselayer for improved water management properties. J. Appl. Polym. Sci. 2015, 132, 41294. [Google Scholar] [CrossRef]
- Jelil, R.A. A review of low-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Nava-Ortiz, C.A.; Alvarez-Lorenzo, C.; Bucio, E.; Concheiro, A.; Burillo, G. Cyclodextrin-functionalized polyethylene and polypropylene as biocompatible materials for diclofenac delivery. Int. J. Pharm. 2009, 382, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sarau, G.; Bochmann, A.; Lewandowska, R.; Christianse, S. From micro- to macro-raman spectroscopy: Solar silicon for a case study. In Advanced Aspects of Spectroscopy; InTech: Lodz, Poland, 2012. [Google Scholar]
- Gawish, S.M.; Matthews, S.R.; Wafa, D.M.; Breidt, F.; Bourham, M.A. Atmospheric plasma-aided biocidal finishes for nonwoven polypropylene fabrics. I. Synthesis and characterization. J. Appl. Polym. Sci. 2007, 103, 1900–1910. [Google Scholar] [CrossRef]
- Choi, J.M.; Park, K.; Lee, B.; Jeong, D.; Dindulkar, S.D.; Choi, Y.; Cho, E.; Park, S.; Yu, J.H.; Jung, S. Solubility and bioavailability enhancement of ciprofloxacin by induced oval-shaped mono-6-deoxy-6-aminoethylamino-beta-cyclodextrin. Carbohydr. Polym. 2017, 163, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Lizundia, E.; Hernáez, E.; León, L.M.; Vilas-Vilela, J.L. Active release coating of multilayer assembled branched and ionic β-cyclodextrins onto poly(ethylene terephthalate). Carbohydr. Polym. 2017, 174, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Henriques, M.; Soares, G. Cyclodextrin/cellulose hydrogel with gallic acid to prevent wound infection. Cellulose 2014, 21, 4519–4530. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-H.; Pan, Y.-J.; Liu, C.-F.; Huang, C.-L.; Hsieh, C.-T.; Chen, C.-K.; Lin, Z.-I.; Lou, C.-W. Preparation and compatibility evaluation of polypropylene/high density polyethylene polyblends. Materials 2015, 8, 8850–8859. [Google Scholar] [CrossRef] [PubMed]
- Muzio, G.; Miola, M.; Perero, S.; Oraldi, M.; Maggiora, M.; Ferraris, S.; Vernè, E.; Festa, V.; Festa, F.; Canuto, R.A.; et al. Polypropylene prostheses coated with silver nanoclusters/silica coating obtained by sputtering: Biocompatibility and antibacterial properties. Surf. Coat. Technol. 2017, 319, 326–334. [Google Scholar] [CrossRef]
- Majumdar, A.; Butola, B.S.; Thakur, S. Development and performance optimization of knitted antibacterial materials using polyester-silver nanocomposite fibres. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 26–31. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanbhal, N.; Mao, Y.; Sun, G.; Li, Y.; Peerzada, M.; Wang, L. Preparation and Characterization of Antibacterial Polypropylene Meshes with Covalently Incorporated β-Cyclodextrins and Captured Antimicrobial Agent for Hernia Repair. Polymers 2018, 10, 58. https://doi.org/10.3390/polym10010058
Sanbhal N, Mao Y, Sun G, Li Y, Peerzada M, Wang L. Preparation and Characterization of Antibacterial Polypropylene Meshes with Covalently Incorporated β-Cyclodextrins and Captured Antimicrobial Agent for Hernia Repair. Polymers. 2018; 10(1):58. https://doi.org/10.3390/polym10010058
Chicago/Turabian StyleSanbhal, Noor, Ying Mao, Gang Sun, Yan Li, Mazhar Peerzada, and Lu Wang. 2018. "Preparation and Characterization of Antibacterial Polypropylene Meshes with Covalently Incorporated β-Cyclodextrins and Captured Antimicrobial Agent for Hernia Repair" Polymers 10, no. 1: 58. https://doi.org/10.3390/polym10010058