Thermal and Chemical Expansion in Proton Ceramic Electrolytes and Compatible Electrodes
Abstract
:1. Introduction
2. Theory of Expansion of Solids
2.1. Basic Principles of Thermal Expansion
2.1.1. Thermal Expansion of Bulk Materials
2.1.2. Crystal Lattice Thermal Expansion
2.1.3. Significance and Relation between Bulk and Lattice Expansion
2.2. Chemical Expansion in Proton Conducting Oxides
2.2.1. Chemical Expansion upon Hydration
2.2.2. Chemical Expansion upon Reduction
3. Methods of Determining Expansion Coefficients
3.1. Bulk Thermal Expansion Coefficient Measurement
3.2. Measuring Thermal Expansion of Crystal Lattice
3.3. Chemical Expansion Coefficient Measurement
3.4. Chemical Expansion Coefficients from Computational Modelling
3.5. Thermal Expansion Coefficients from Computational Modelling
3.6. Assessment of the Influence of Heating Rate on the Thermal Expansion Coefficient—Experimental Details
4. Challenges in Assessing Measurement Data—Effects of Thermochemical Expansion, Non-Constant Thermal Expansion, Heating Rates and Porosity
4.1. Decoupling Thermal and Chemical Expansion Coefficients—Impossible?
4.1.1. Thermochemical Expansion upon Hydration—Case of Y-Doped BaZrO3 (BZY)
4.1.2. Thermochemical Expansion upon Reduction—Case of La1−xSrxCo0.2Fe0.8O3−δ (LSCF)
4.1.3. Non-Constant Thermal Expansion Coefficient
4.2. Thermal Expansion of a Composite
4.3. The Effect of Heating Rate on the Bulk Expansion Coefficients
5. Thermal and Chemical Expansion of Proton Conducting Ceramics (PCCs)
5.1. Proton Conducting Solid Electrolytes
5.1.1. ABO3 Perovskites and Perovskite-Related Materials
5.1.1.1. Barium Cerate and Barium Zirconate Based Perovskites
5.1.1.2. Other Perovskites and Perovskite-Related Compounds
5.1.2. ABO4 Oxides
5.1.3. Pyrochlore- and Fluorite-Related Structures
5.2. Mixed Ionic Electronic Conductors
5.2.1. Electrode Materials Used on PCCs
5.2.2. Expansion of Air Electrodes
5.2.2.1. General Trends
5.2.2.2. Selected Air Side Electrode Materials
5.2.3. Expansion of Fuel Side Electrodes
6. Thermal Compatibility and Mismatch: Symmetries, Issues, Methods of Mitigation
6.1. Issues That Can Lead to Cell Failure
6.2. Mitigating Strategies
7. Summary
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols List
| Abbr. | Meaning |
| a, b, c | lattice parameters |
| Acc | acceptor dopant |
| BCN | Ba3Ca1+xNb2−xO9−δ |
| BCY | BaCe1−xYxO3−δ |
| BZCY | BaZr1−x−yCexYyO3−δ |
| BZCY72 | BaZr0.7Ce0.2Y0.1O3−δ |
| BSCF | Ba1−xSrxCo0.8Fe0.2O3−δ |
| BZO | barium zirconate |
| BZY | BaZr1−xYxO3−δ |
| cercer | ceramic ceramic composite |
| cermet | ceramic metal composite |
| CTE | coefficient of thermal expansion |
| DFT | density functional theory |
| DIL | Dilatometry |
| EoS | equation of state |
| G | Gibbs free energy |
| HA | harmonic approximation |
| HT | high temperature |
| HTPC | high temperature proton conductor |
| HT-XRD | high temperature X-ray diffraction |
| hydr | hydration/hydrated |
| K | bulk modulus |
| L | Length |
| LSCF | La1−xSrxCoyFe1−yO3−δ |
| Ln | lanthanide |
| LT | low temperature |
| LWO | lanthanum tungstate |
| MD | molecular dynamics |
| MIEC | mixed ionic electronic conductor |
| ND | neutron diffraction |
| PCC | proton conducting ceramic |
| PCE | proton ceramic electrolyte |
| PCEC | proton ceramic electrolyser cell |
| PCFC | proton ceramic fuel cell |
| QHA | quasi-harmonic approximation |
| RE | rare earth |
| RT | room temperature |
| SOEC | solid oxide electrolyser cell |
| SOFC | solid oxide fuel cell |
| ΔhydrH | standard hydration enthalpy |
| ΔhydrSo | standard hydration entropy |
| T | temperature |
| t | transport number |
| TCO | triple conducting oxide |
| TEC | thermal expansion coefficient |
| TG | thermogravimetric analysis |
| TPB | triple-phase boundary |
| V | volume |
| XRD | X-ray diffraction |
| YSZ | yttria stabilized zirconia |
| α | coefficient of thermal expansion |
| β | coefficient of chemical expansion |
| δ | oxygen non-stoichiometry |
| ε | uniaxial strain |
| μ | shear modulus |
| v | volume fraction |
References
- Desaguliers, J.T. A Course of Experimental Philosophy; W. Innys: London, UK, 1745. [Google Scholar]
- Touloukian, Y.S.; Kirby, R.K.; Taylor, R.E.; Desai, P.D. Thermal Expansion; Springer US: Boston, MA, USA, 1975; ISBN 978-1-4757-1624-5. [Google Scholar]
- Marrony, M.; Berger, P.; Mauvy, F.; Grenier, J.C.; Sata, N.; Magrasó, A.; Haugsrud, R.; Slater, P.R.; Taillades, G.; Roziere, J.; et al. Proton-Conducting Ceramics. From Fundamentals to Applied Research; Marrony, M., Ed.; Pan Stanford Publishing: Singapore, 2016; ISBN 978-981-4613-84-2. [Google Scholar]
- Colomban, P. Proton Conductors: Solids, Membranes and Gels-Materials and Devices; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Iwahara, H.; Yajima, T.; Hibino, T.; Ozaki, K.; Suzuki, H. Protonic conduction in calcium, strontium and barium zirconates. Solid State Ion. 1993, 61, 65–69. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Ono, K.; Ogaki, K. Proton Conduction in Sintered Oxides Based on BaCeO3. J. Electrochem. Soc. 1988, 135, 529–533. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; OHayre, R.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Morejudo, S.H.; Zanón, R.; Escolástico, S.; Yuste-Tirados, I.; Malerød-Fjeld, H.; Vestre, P.K.; Coors, W.G.; Martínez, A.; Norby, T.; Serra, J.M.; et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 2016, 353, 563–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coors, W.G. Protonic ceramic fuel cells for high-efficiency operation with methane. J. Power Sources 2003, 118, 150–156. [Google Scholar] [CrossRef]
- Malerød-Fjeld, H.; Clark, D.; Yuste-Tirados, I.; Zanón, R.; Catalán-Martinez, D.; Beeaff, D.; Morejudo, S.H.; Vestre, P.K.; Norby, T.; Haugsrud, R.; et al. Thermo-electrochemical production of compressed hydrogen from methane with near-zero energy loss. Nat. Energy 2017, 2, 923–931. [Google Scholar] [CrossRef]
- Iwahara, H. High temperature proton conducting oxides and their applications to solid electrolyte fuel cells and steam electrolyzer for hydrogen production. Solid State Ion. 1988, 28–30, 573–578. [Google Scholar] [CrossRef]
- Molenda, J.; Kupecki, J.; Baron, R.; Blesznowski, M.; Brus, G.; Brylewski, T.; Bucko, M.; Chmielowiec, J.; Cwieka, K.; Gazda, M.; et al. Status report on high temperature fuel cells in Poland—Recent advances and achievements. Int. J. Hydrog. Energy 2017, 42, 4366–4403. [Google Scholar] [CrossRef]
- Iwahara, H. Proton conducting ceramics and their applications. Solid State Ion. 1996, 86–88, 9–15. [Google Scholar] [CrossRef]
- Norby, T. Solid-state protonic conductors: Principles, properties, progress and prospects. Solid State Ion. 1999, 125, 1–11. [Google Scholar] [CrossRef]
- Zagórski, K.; Wachowski, S.; Szymczewska, D.; Mielewczyk-Gryń, A.; Jasiński, P.; Gazda, M. Performance of a single layer fuel cell based on a mixed proton-electron conducting composite. J. Power Sources 2017, 353, 230–236. [Google Scholar] [CrossRef]
- Wachowski, S.; Li, Z.; Polfus, J.M.; Norby, T. Performance and stability in H2S of SrFe0.75Mo0.25O3−δ as electrode in proton ceramic fuel cells. J. Eur. Ceram. Soc. 2018, 38, 163–171. [Google Scholar] [CrossRef]
- Sakai, T.; Matsushita, S.; Matsumoto, H.; Okada, S.; Hashimoto, S.; Ishihara, T. Intermediate temperature steam electrolysis using strontium zirconate-based protonic conductors. Int. J. Hydrog. Energy 2009, 34, 56–63. [Google Scholar] [CrossRef]
- Katahira, K.; Matsumoto, H.; Iwahara, H.; Koide, K.; Iwamoto, T. Solid electrolyte hydrogen sensor with an electrochemically-supplied hydrogen standard. Sens. Actuators B Chem. 2001, 73, 130–134. [Google Scholar] [CrossRef]
- Yajima, T.; Koide, K.; Takai, H.; Fukatsu, N.; Iwahara, H. Application of hydrogen sensor using proton conductive ceramics as a solid electrolyte to aluminum casting industries. Solid State Ion. 1995, 79, 333–337. [Google Scholar] [CrossRef]
- Serret, P.; Colominas, S.; Reyes, G.; Abellà, J. Characterization of ceramic materials for electrochemical hydrogen sensors. Fusion Eng. Des. 2011, 86, 2446–2449. [Google Scholar] [CrossRef]
- Volkov, A.; Gorbova, E.; Vylkov, A.; Medvedev, D.; Demin, A.; Tsiakaras, P. Design and applications of potentiometric sensors based on proton-conducting ceramic materials. A brief review. Sens. Actuators B Chem. 2017, 244, 1004–1015. [Google Scholar] [CrossRef]
- Phair, J.W.; Badwal, S.P.S. Review of proton conductors for hydrogen separation. Ionics 2006, 12, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Lundin, S.T.B.; Patki, N.S.; Fuerst, T.F.; Ricote, S.; Wolden, C.A.; Way, J.D. Dense Inorganic Membranes for Hydrogen Separation. In Membranes for Gas Separations; World Scientific: Singapore, 2017; pp. 271–363. [Google Scholar]
- Tao, Z.; Yan, L.; Qiao, J.; Wang, B.; Zhang, L.; Zhang, J. A review of advanced proton-conducting materials for hydrogen separation. Prog. Mater. Sci. 2015, 74, 1–50. [Google Scholar] [CrossRef]
- Fontaine, M.L.; Norby, T.; Larring, Y.; Grande, T.; Bredesen, R. Oxygen and Hydrogen Separation Membranes Based on Dense Ceramic Conductors. Membr. Sci. Technol. 2008, 13, 401–458. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3–4, 359–363. [Google Scholar] [CrossRef]
- Vasileiou, E.; Kyriakou, V.; Garagounis, I.; Vourros, A.; Stoukides, M. Ammonia synthesis at atmospheric pressure in a BaCe0.2Zr0.7Y0.1O2.9 solid electrolyte cell. Solid State Ion. 2015, 275, 110–116. [Google Scholar] [CrossRef]
- Marnellos, G.; Stoukides, M. Ammonia Synthesis at Atmospheric Pressure. Science 1998, 282, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Gocha, A. CeramicTechToday from The American Ceramic Society; The American Ceramic Society: Westerville, OH, USA, 2017. [Google Scholar]
- Dubois, A.; Ricote, S.; Braun, R.J. Benchmarking the expected stack manufacturing cost of next generation, intermediate-temperature protonic ceramic fuel cells with solid oxide fuel cell technology. J. Power Sources 2017, 369, 65–77. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, A.; Stiller, C.; Härkegård, G.; Bolland, O. Modeling of thermal stresses and probability of survival of tubular SOFC. J. Power Sources 2006, 158, 287–294. [Google Scholar] [CrossRef]
- Tietz, F. Thermal expansion of SOFC materials. Ionics 1999, 5, 129–139. [Google Scholar] [CrossRef]
- Selimovic, A.; Kemm, M.; Torisson, T.; Assadi, M. Steady state and transient thermal stress analysis in planar solid oxide fuel cells. J. Power Sources 2005, 145, 463–469. [Google Scholar] [CrossRef]
- Lin, C.K.; Chen, T.T.; Chyou, Y.P.; Chiang, L.K. Thermal stress analysis of a planar SOFC stack. J. Power Sources 2007, 164, 238–251. [Google Scholar] [CrossRef]
- Laurencin, J.; Delette, G.; Lefebvre-Joud, F.; Dupeux, M. A numerical tool to estimate SOFC mechanical degradation: Case of the planar cell configuration. J. Eur. Ceram. Soc. 2008, 28, 1857–1869. [Google Scholar] [CrossRef]
- Carter, B.; Norton, G. Ceramic Materials; Springer: New York, NY, USA, 2007; ISBN 978-0-387-46270-7. [Google Scholar]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed.; Wiley: Hoboken, NJ, USA, 1976; ISBN 978-0-471-47860-7. [Google Scholar]
- Kittel, C.; McEuen, P. Introduction to Solid State Physics; Willey: Hoboken, NJ, USA, 1998; Volume 8, ISBN 047141526X. [Google Scholar]
- Levy, R.A. Principles of Solid State Physics; Academic Press: Cambridge, MA, USA, 1968; ISBN 9780124457508. [Google Scholar]
- Brown, F.C. The Physics of Solids; W.A. Benjamin: New York, NY, USA, 1967. [Google Scholar]
- Krishnan, R.S.; Srinivasan, R.; Devanarayan, S. Thermal Expansion of Crystals; Pergamon Press: Oxford, UK, 1979; ISBN 0-08-021405-3. [Google Scholar]
- Belousov, R.I.; Filatov, S.K. Algorithm for calculating the thermal expansion tensor and constructing the thermal expansion diagram for crystals. Glas. Phys. Chem. 2007, 33, 271–275. [Google Scholar] [CrossRef]
- Paufler, P.; Weber, T. On the determination of linear expansion coefficients of triclinic crystals using X-ray diffraction. Eur. J. Mineral. 1999, 11, 721–730. [Google Scholar] [CrossRef]
- Branson, D.L. Thermal Expansion Coefficients of Zirconate Ceramics. J. Am. Ceram. Soc. 1965, 48, 441–442. [Google Scholar] [CrossRef]
- Adler, S.B. Chemical Expansivity of Electrochemical Ceramics. J. Am. Ceram. Soc. 2004, 84, 2117–2119. [Google Scholar] [CrossRef]
- Garai, J. Correlation between thermal expansion and heat capacity. Calphad 2006, 30, 354–356. [Google Scholar] [CrossRef] [Green Version]
- Mohazzabi, P.; Behroozi, F. Thermal expansion of solids: A simple classical model. Eur. J. Phys. 1997, 18, 237–240. [Google Scholar] [CrossRef]
- Suzuki, I. Thermal expansion of periclase and olivine and their anharmonic properties. In Elastic Properties and Equations of State; American Geophysical Union: Washington, DC, USA, 1988; Volume 23, pp. 361–375. ISBN 0875902405. [Google Scholar]
- Samara, G.A.; Morosin, B. Anharmonic Effects in KTaO3: Ferroelectric Mode, Thermal Expansion, and Compressibility. Phys. Rev. B 1973, 8, 1256–1264. [Google Scholar] [CrossRef]
- Li, C.W.; Tang, X.; Muñoz, J.A.; Keith, J.B.; Tracy, S.J.; Abernathy, D.L.; Fultz, B. Structural Relationship between Negative Thermal Expansion and Quartic Anharmonicity of Cubic ScF3. Phys. Rev. Lett. 2011, 107, 195504. [Google Scholar] [CrossRef] [PubMed]
- Janio de Castro Lima, J.; Paraguassu, A.B. Linear thermal expansion of granitic rocks: Influence of apparent porosity, grain size and quartz content. Bull. Eng. Geol. Environ. 2004, 63, 215–220. [Google Scholar] [CrossRef]
- Parker, F.J.; Rice, R.W. Correlation between Grain Size and Thermal Expansion for Aluminum Titanate Materials. J. Am. Ceram. Soc. 1989, 72, 2364–2366. [Google Scholar] [CrossRef]
- Antal, D.; Húlan, T.; Štubňa, I.; Záleská, M.; Trník, A. The influence of texture on elastic and thermophysical properties of kaolin- and illite-based ceramic bodies. Ceram. Int. 2017, 43, 2730–2736. [Google Scholar] [CrossRef]
- Paulik, S.W.; Faber, K.T.; Fuller, E.R. Development of Textured Microstructures in Ceramics with Large Thermal Expansion Anisotropy. J. Am. Ceram. Soc. 1994, 77, 454–458. [Google Scholar] [CrossRef]
- Mogensen, M.; Sammes, N.M.; Tompsett, G.A. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Perry, N.H.; Bishop, S.R. Understanding chemical expansion in perovskite-structured oxides. Phys. Chem. Chem. Phys. 2015, 17, 10028–10039. [Google Scholar] [CrossRef] [PubMed]
- Marrocchelli, D.; Bishop, S.R.; Tuller, H.L.; Yildiz, B. Understanding Chemical Expansion in Non-Stoichiometric Oxides: Ceria and Zirconia Case Studies. Adv. Funct. Mater. 2012, 22, 1958–1965. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Bishop, S.R.; Tuller, H.L.; Watson, G.W.; Yildiz, B. Charge localization increases chemical expansion in cerium-based oxides. Phys. Chem. Chem. Phys. 2012, 14, 12070–12074. [Google Scholar] [CrossRef] [PubMed]
- Haugsrud, R. On the high-temperature oxidation of nickel. Corros. Sci. 2003, 45, 211–235. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Vullum, F.; Nitsche, F.; Selbach, S.M.; Grande, T. Solid solubility and phase transitions in the system LaNb1−xTaxO4. J. Solid State Chem. 2008, 181, 2580–2585. [Google Scholar] [CrossRef]
- Wachowski, S.; Mielewczyk-Gryn, A.; Gazda, M. Effect of isovalent substitution on microstructure and phase transition of LaNb1−xMxO4 (M = Sb, V or Ta; x = 0.05–0.3). J. Solid State Chem. 2014, 219, 201–209. [Google Scholar] [CrossRef]
- Haugsrud, R.; Norby, T. High-temperature proton conductivity in acceptor-doped LaNbO4. Solid State Ion. 2006, 177, 1129–1135. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Yang, C.K.; Haile, S.M. Unraveling the defect chemistry and proton uptake of yttrium-doped barium zirconate. Scr. Mater. 2011, 65, 102–107. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- Omata, T.; Noguchi, Y.; Otsuka-Yao-Matsuo, S. Infrared Study of High-Temperature Proton-Conducting Aliovalently Doped SrZrO3 and BaZrO3. J. Electrochem. Soc. 2005, 152, E200–E205. [Google Scholar] [CrossRef]
- Imashuku, S.; Uda, T.; Nose, Y.; Awakura, Y. To journal of phase equilibria and diffusion phase relationship of the BaO-ZrO2-YO1.5 system at 1500 and 1600 °C. J. Phase Equilibria Diffus. 2010, 31, 348–356. [Google Scholar] [CrossRef]
- Giannici, F.; Shirpour, M.; Longo, A.; Martorana, A.; Merkle, R.; Maier, J. Long-range and short-range structure of proton-conducting Y:BaZrO3. Chem. Mater. 2011, 23, 2994–3002. [Google Scholar] [CrossRef] [Green Version]
- Shirpour, M.; Rahmati, B.; Sigle, W.; Van Aken, P.A.; Merkle, R.; Maier, J. Dopant segregation and space charge effects in proton-conducting BaZrO3 perovskites. J. Phys. Chem. C 2012, 116, 2453–2461. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Adams, S.; Münch, W.; Fuchs, A.; Klock, U.; Maier, J. Proton conducting alkaline earth zirconates and titanates for high drain electrochemical applications. Solid State Ion. 2001, 145, 295–306. [Google Scholar] [CrossRef]
- Oikawa, I.; Takamura, H. Correlation among Oxygen Vacancies, Protonic Defects, and the Acceptor Dopant in Sc-Doped BaZrO3 Studied by 45Sc Nuclear Magnetic Resonance. Chem. Mater. 2015, 27, 6660–6667. [Google Scholar] [CrossRef]
- Han, D.; Shinoda, K.; Uda, T. Dopant Site Occupancy and Chemical Expansion in Rare Earth-Doped Barium Zirconate. J. Am. Ceram. Soc. 2014, 97, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.J.; Virkar, A.V. Lattice Parameters and Densities of Rare-Earth Oxide Doped Ceria Electrolytes. J. Am. Ceram. Soc. 1995, 78, 433–439. [Google Scholar] [CrossRef]
- Andersson, A.K.E.; Selbach, S.M.; Knee, C.S.; Grande, T. Chemical Expansion Due to Hydration of Proton-Conducting Perovskite Oxide Ceramics. J. Am. Ceram. Soc. 2014, 97, 2654–2661. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Hatada, N.; Uda, T. Chemical Expansion of Yttrium-Doped Barium Zirconate and Correlation with Proton Concentration and Conductivity. J. Am. Ceram. Soc. 2016, 99, 3745–3753. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yamada, N. Thermal lattice expansion behavior of Yb-doped BaCeO3. Solid State Ion. 2003, 162–163, 23–29. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Kinyanjui, F.G.; Norberg, S.T.; Ahmed, I.; Eriksson, S.G.; Hull, S. In-situ conductivity and hydration studies of proton conductors using neutron powder diffraction. Solid State Ion. 2012, 225, 312–316. [Google Scholar] [CrossRef]
- Jedvik, E.; Lindman, A.; Benediktsson, M.Þ.; Wahnström, G. Size and shape of oxygen vacancies and protons in acceptor-doped barium zirconate. Solid State Ion. 2015, 275, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Bjørheim, T.S.; Kotomin, E.A.; Maier, J. Hydration entropy of BaZrO3 from first principles phonon calculations. J. Mater. Chem. A 2015, 3, 7639–7648. [Google Scholar] [CrossRef]
- Bjørheim, T.S.; Løken, A.; Haugsrud, R. On the relationship between chemical expansion and hydration thermodynamics of proton conducting perovskites. J. Mater. Chem. A 2016, 4, 5917–5924. [Google Scholar] [CrossRef] [Green Version]
- Løken, A.; Saeed, S.W.; Getz, M.N.; Liu, X.; Bjørheim, T.S. Alkali metals as efficient A-site acceptor dopants in proton conducting BaZrO3. J. Mater. Chem. A 2016, 4, 9229–9235. [Google Scholar] [CrossRef]
- Løken, A.; Haugsrud, R.; Bjørheim, T.S. Unravelling the fundamentals of thermal and chemical expansion of BaCeO3 from first principles phonon calculations. Phys. Chem. Chem. Phys. 2016, 18, 31296–31303. [Google Scholar] [CrossRef] [PubMed]
- Løken, A.; Bjørheim, T.S.; Haugsrud, R. The pivotal role of the dopant choice on the thermodynamics of hydration and associations in proton conducting BaCe0.9X0.1O3−δ (X = Sc, Ga, Y, In, Gd and Er). J. Mater. Chem. A 2015, 3, 23289–23298. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, A.; Choi, S.Y.; Jung, W.; Chung, S.Y. Vacancy-Induced Electronic Structure Variation of Acceptors and Correlation with Proton Conduction in Perovskite Oxides. Angew. Chem. Int. Ed. 2016, 55, 13499–13503. [Google Scholar] [CrossRef] [PubMed]
- Løken, A. Hydration Thermodynamics of Oxides. Effects of Defect Associations. Ph.D. Thesis, University of Oslo, Oslo, Norway, 2017. [Google Scholar]
- Bishop, S.R.; Duncan, K.L.; Wachsman, E.D. Defect equilibria and chemical expansion in non-stoichiometric undoped and gadolinium-doped cerium oxide. Electrochim. Acta 2009, 54, 1436–1443. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Chatzichristodoulou, C.; Bishop, S.R. Defining chemical expansion: The choice of units for the stoichiometric expansion coefficient. Phys. Chem. Chem. Phys. 2014, 16, 9229–9232. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Ramos, T.M.G.M. Chemically-induced stresses in ceramic oxygen ion-conducting membranes. Solid State Ion. 2000, 129, 259–269. [Google Scholar] [CrossRef]
- Jiang, S.P. A comparison of O2 reduction reactions on porous (La,Sr)MnO3 and (La,Sr)(Co,Fe)O3 electrodes. Solid State Ion. 2002, 146, 1–22. [Google Scholar] [CrossRef]
- Esquirol, A.; Brandon, N.P.; Kilner, J.A.; Mogensen, M. Electrochemical Characterization of La0.6Sr0.4Co0.2Fe0.8O3 Cathodes for Intermediate-Temperature SOFCs. J. Electrochem. Soc. 2004, 151, A1847–A1855. [Google Scholar] [CrossRef]
- Tietz, F.; Haanappel, V.A.C.; Mai, A.; Mertens, J.; Stöver, D. Performance of LSCF cathodes in cell tests. J. Power Sources 2006, 156, 20–22. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Rørvik, P.M.; Haavik, C. Microstructure and performance of La0.58Sr0.4Co0.2Fe0.8O3−δ cathodes deposited on BaCe0.2Zr0.7Y0.1O3−δ by infiltration and spray pyrolysis. J. Power Sources 2012, 209, 172–179. [Google Scholar] [CrossRef]
- Sun, S.; Cheng, Z. Electrochemical Behaviors for Ag, LSCF and BSCF as Oxygen Electrodes for Proton Conducting IT-SOFC. J. Electrochem. Soc. 2017, 164, F3104–F3113. [Google Scholar] [CrossRef] [Green Version]
- Bishop, S.R.; Duncan, K.L.; Wachsman, E.D. Surface and Bulk Defect Equilibria in Strontium-Doped Lanthanum Cobalt Iron Oxide. J. Electrochem. Soc. 2009, 156, B1242–B1248. [Google Scholar] [CrossRef]
- Bishop, S.R.; Duncan, K.L.; Wachsman, E.D. Thermo-Chemical Expansion in Strontium-Doped Lanthanum Cobalt Iron Oxide. J. Am. Ceram. Soc. 2010, 93, 4115–4121. [Google Scholar] [CrossRef]
- Kuhn, M.; Hashimoto, S.; Sato, K.; Yashiro, K.; Mizusaki, J. Thermo-chemical lattice expansion in La0.6Sr0.4Co1−yFeyO3−δ. Solid State Ion. 2013, 241, 12–16. [Google Scholar] [CrossRef]
- James, J.D.; Spittle, J.A.; Brown, S.G.R.; Evans, R.W. A review of measurement techniques for the thermal expansion coefficient of metals and alloys at elevated temperatures. Meas. Sci. Technol. 2001, 12, R1–R15. [Google Scholar] [CrossRef]
- Mielewczyk-Gryn, A.; Gdula-Kasica, K.; Kusz, B.; Gazda, M. High temperature monoclinic-to-tetragonal phase transition in magnesium doped lanthanum ortho-niobate. Ceram. Int. 2013, 39, 4239–4244. [Google Scholar] [CrossRef]
- Huse, M.; Skilbred, A.W.B.; Karlsson, M.; Eriksson, S.G.; Norby, T.; Haugsrud, R.; Knee, C.S. Neutron diffraction study of the monoclinic to tetragonal structural transition in LaNbO4 and its relation to proton mobility. J. Solid State Chem. 2012, 187, 27–34. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Oxygen nonstoichiometry and defect structure of the double perovskite GdBaCo2O6−δ. Solid State Ion. 2010, 180, 1620–1625. [Google Scholar] [CrossRef]
- Zuev, A.Y.; Tsvetkov, D.S. Conventional Methods for Measurements of Chemo-Mechanical Coupling. In Electro-Chemo-Mechanics of Solids; Bishop, S.R., Perry, N.H., Marrocchelli, D., Sheldon, B., Eds.; Springer: New York, NY, USA, 2017; pp. 5–33. ISBN 9783319514055. [Google Scholar]
- Nedeltcheva, T.; Simeonova, P.; Lovchinov, V. Improved iodometric method for simultaneous determination of non-stoichiometric oxygen and total copper content in YBCO superconductors. Anal. Chim. Acta 1995, 312, 227–229. [Google Scholar] [CrossRef]
- Rørmark, L.; Wiik, K.; Stølen, S.; Grande, T. Oxygen stoichiometry and structural properties of La1−xAxMnO3±δ (A = Ca or Sr and 0 ≤ x ≤ 1). J. Mater. Chem. 2002, 12, 1058–1067. [Google Scholar] [CrossRef]
- Baroni, S.; de Gironcoli, S.; Dal Corso, A.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef] [Green Version]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Vinet, P.; Smith, J.R.; Ferrante, J.; Rose, J.H. Temperature effects on the universal equation of state of solids. Phys. Rev. B 1987, 35, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Zhao, Y.; Weidner, D.J. Thermal expansion of SrZrO3 and BaZrO3 perovskites. Phys. Chem. Miner. 1991, 18, 294–301. [Google Scholar] [CrossRef]
- Hudish, G.; Manerbino, A.; Coors, W.G.; Ricote, S. Chemical expansion in BaZr0.9−xCexY0.1O3−δ (x = 0 and 0.2) upon hydration determined by high-temperature X-ray diffraction. J. Am. Ceram. Soc. 2018, 101, 1298–1309. [Google Scholar] [CrossRef]
- Hiraiwa, C.; Han, D.; Kuramitsu, A.; Kuwabara, A.; Takeuchi, H.; Majima, M.; Uda, T. Chemical Expansion and Change in Lattice Constant of Y-Doped BaZrO3 by Hydration/Dehydration Reaction and Final Heat-Treating Temperature. J. Am. Ceram. Soc. 2013, 96, 879–884. [Google Scholar] [CrossRef]
- Lein, H.L.; Wiik, K.; Grande, T. Thermal and chemical expansion of mixed conducting La0.5Sr0.5Fe1−xCoxO3−δ materials. Solid State Ion. 2006, 177, 1795–1798. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Adler, S.B. Thermal and Chemical Expansion of Sr-Doped Lanthanum Cobalt Oxide (La1−xSrxCoO3−δ). Chem. Mater. 2005, 17, 4537–4546. [Google Scholar] [CrossRef]
- Fossdal, A.; Menon, M.; Waernhus, I.; Wiik, K.; Einarsrud, M.A.; Grande, T. Crystal Structure and Thermal Expansion of La1−xSrxFeO3−δ Materials. J. Am. Ceram. Soc. 2005, 87, 1952–1958. [Google Scholar] [CrossRef]
- Hashimoto, S.; Fukuda, Y.; Kuhn, M.; Sato, K.; Yashiro, K.; Mizusaki, J. Thermal and chemical lattice expansibility of La0.6Sr0.4Co1−yFeyO3−δ (y = 0.2, 0.4, 0.6 and 0.8). Solid State Ion. 2011, 186, 37–43. [Google Scholar] [CrossRef]
- Kuhn, M.; Hashimoto, S.; Sato, K.; Yashiro, K.; Mizusaki, J. Oxygen nonstoichiometry, thermo-chemical stability and lattice expansion of La0.6Sr0.4FeO3−δ. Solid State Ion. 2011, 195, 7–15. [Google Scholar] [CrossRef]
- Mather, G.C.; Heras-Juaristi, G.; Ritter, C.; Fuentes, R.O.; Chinelatto, A.L.; Pérez-Coll, D.; Amador, U. Phase Transitions, Chemical Expansion, and Deuteron Sites in the BaZr0.7Ce0.2Y0.1O3−δ Proton Conductor. Chem. Mater. 2016, 28, 4292–4299. [Google Scholar] [CrossRef]
- Kerner, E.H. The Elastic and Thermo-elastic Properties of Composite Media. Proc. Phys. Soc. Sect. B 1956, 69, 808–813. [Google Scholar] [CrossRef]
- Pratihar, S.K.; Dassharma, A.; Maiti, H.S. Properties of Ni/YSZ porous cermets prepared by electroless coating technique for SOFC anode application. J. Mater. Sci. 2007, 42, 7220–7226. [Google Scholar] [CrossRef] [Green Version]
- Coble, R.L.; Kingery, W.D. Effect of Porosity on Physical Properties of Sintered Alumina. J. Am. Ceram. Soc. 1956, 39, 377–385. [Google Scholar] [CrossRef]
- Shyam, A.; Bruno, G.; Watkins, T.R.; Pandey, A.; Lara-curzio, E.; Parish, C.M.; Stafford, R.J. Journal of the European Ceramic Society The effect of porosity and microcracking on the thermomechanical properties of cordierite. J. Eur. Ceram. Soc. 2015, 35, 4557–4566. [Google Scholar] [CrossRef]
- Mori, M.; Yamamoto, T.; Itoh, H.; Inaba, H.; Tagawa, H. Thermal Expansion of Nickel-Zirconia Anodes in Solid Oxide Fuel Cells during Fabrication and Operation. J. Electrochem. Soc. 1998, 145, 1374–1381. [Google Scholar] [CrossRef]
- Elomari, S.; Skibo, M.D.; Sundarrajan, A.; Richards, H. Thermal expansion behavior of particulate metal-matrix composites. Compos. Sci. Technol. 1998, 58, 369–376. [Google Scholar] [CrossRef]
- Sevostianov, I. On the thermal expansion of composite materials and cross-property connection between thermal expansion and thermal conductivity. Mech. Mater. 2012, 45, 20–33. [Google Scholar] [CrossRef]
- Hayashi, H.; Saitou, T.; Maruyama, N.; Inaba, H.; Kawamura, K.; Mori, M. Thermal expansion coefficient of yttria stabilized zirconia for various yttria contents. Solid State Ion. 2005, 176, 613–619. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem. Soc. Rev. 2010, 39, 4355–4369. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, L.; Fisher, C.A.J.; Islam, M.S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: Structural and mechanistic features. Chem. Soc. Rev. 2010, 39, 4370–4387. [Google Scholar] [CrossRef] [PubMed]
- Norby, T. Proton Conductivity in Perovskite Oxides. In Perovskite Oxide for Solid Oxide Fuel Cells; Ishihara, T., Ed.; Springer: Boston, MA, USA, 2009; pp. 217–241. ISBN 978-0-387-77708-5. [Google Scholar]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, F.; Zhang, L.; Chen, F. Synthesis of BaCe0.7Zr0.1Y0.1Yb0.1O3−δ proton conducting ceramic by a modified Pechini method. Solid State Ion. 2012, 213, 29–35. [Google Scholar] [CrossRef]
- Lagaeva, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. Insights on thermal and transport features of BaCe0.8−xZrxY0.2O3−δ proton-conducting materials. J. Power Sources 2015, 278, 436–444. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. High Total Proton Conductivity in Large-Grained Yttrium-Doped Barium Zirconate. Chem. Mater. 2009, 21, 2755–2762. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Blanc, F.; Okuyama, Y.; Buannic, L.; Lucio-Vega, J.C.; Grey, C.P.; Haile, S.M. Proton trapping in yttrium-doped barium zirconate. Nat. Mater. 2013, 12, 647–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, K.H.; Haile, S.M. Chemical stability and proton conductivity of doped BaCeO3–BaZrO3 solid solutions. Solid State Ion. 1999, 125, 355–367. [Google Scholar] [CrossRef]
- Haugsrud, R. High Temperature Proton Conductors—Fundamentals and Functionalities. Diffus. Found. 2016, 8, 31–79. [Google Scholar] [CrossRef]
- Akbarzadeh, A.R.; Kornev, I.; Malibert, C.; Bellaiche, L.; Kiat, J.M. Combined theoretical and experimental study of the low-temperature properties of BaZrO3. Phys. Rev. B 2005, 72, 205104. [Google Scholar] [CrossRef]
- Yamanaka, S.; Fujikane, M.; Hamaguchi, T.; Muta, H.; Oyama, T.; Matsuda, T.; Kobayashi, S.; Kurosaki, K. Thermophysical properties of BaZrO3 and BaCeO3. J. Alloys Compd. 2003, 359, 109–113. [Google Scholar] [CrossRef]
- Mathews, M.D.; Mirza, E.B.; Momin, A.C. High-temperature X-ray diffractometric studies of CaZrO3, SrZrO3 and BaZrO3. J. Mater. Sci. Lett. 1991, 10, 305–306. [Google Scholar] [CrossRef]
- Taglieri, G.; Tersigni, M.; Villa, P.L.; Mondelli, C. Synthesis by the citrate route and characterisation of BaZrO3, a high tech ceramic oxide: Preliminary results. Int. J. Ind. Chem. 1999, 1, 103–110. [Google Scholar] [CrossRef]
- Braun, A.; Ovalle, A.; Pomjakushin, V.; Cervellino, A.; Erat, S.; Stolte, W.C.; Graule, T. Yttrium and hydrogen superstructure and correlation of lattice expansion and proton conductivity in the BaZr0.9Y0.1O2.95 proton conductor. Appl. Phys. Lett. 2009, 95, 224103. [Google Scholar] [CrossRef]
- Goupil, G.; Delahaye, T.; Gauthier, G.; Sala, B.; Joud, F.L. Stability study of possible air electrode materials for proton conducting electrochemical cells. Solid State Ion. 2012, 209–210, 36–42. [Google Scholar] [CrossRef]
- Lyagaeva, Y.G.; Medvedev, D.A.; Demin, A.K.; Tsiakaras, P.; Reznitskikh, O.G. Thermal expansion of materials in the barium cerate-zirconate system. Phys. Solid State 2015, 57, 285–289. [Google Scholar] [CrossRef]
- Han, D.; Majima, M.; Uda, T. Structure analysis of BaCe0.8Y0.2O3−δ in dry and wet atmospheres by high-temperature X-ray diffraction measurement. J. Solid State Chem. 2013, 205, 122–128. [Google Scholar] [CrossRef]
- Malavasi, L.; Ritter, C.; Chiodelli, G. Correlation between Thermal Properties, Electrical Conductivity, and Crystal Structure in the BaCe0.80Y0.20O2.9 Proton Conductor. Chem. Mater. 2008, 20, 2343–2351. [Google Scholar] [CrossRef]
- Zhu, Z.; Tao, Z.; Bi, L.; Liu, W. Investigation of SmBaCuCoO5+δ double-perovskite as cathode for proton-conducting solid oxide fuel cells. Mater. Res. Bull. 2010, 45, 1771–1774. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Zhen, J.; Zhu, S.; Li, B.; Sun, K.; Wang, P. Ionic conductivity, sintering and thermal expansion behaviors of mixed ion conductor BaZr0.1Ce0.7Y0.1Yb0.1O3−δ prepared by ethylene diamine tetraacetic acid assisted glycine nitrate process. J. Power Sources 2011, 196, 5000–5006. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Balakireva, V.B.; Kuz’min, A.V.; Plaksin, S.V. Electrical conductivity of CaZr1−xScxO3-delta (x = 0.01–0.20) in dry and humid air. Inorg. Mater. 2014, 50, 495–502. [Google Scholar] [CrossRef]
- Yajima, T.; Suzuki, H.; Yogo, T.; Iwahara, H. Protonic conduction in SrZrO3- based oxides. Solid State Ion. 1992, 51, 101–107. [Google Scholar] [CrossRef]
- Hibino, T.; Mizutani, K.; Yajima, T.; Iwahara, H. Evaluation of proton conductivity in SrCeO3, BaCeO3, CaZrO3 and SrZrO3 by temperature programmed desorption method. Solid State Ion. 1992, 57, 303–306. [Google Scholar] [CrossRef]
- Matsuda, T.; Yamanaka, S.; Kurosaki, K.; Kobayashi, S. High temperature phase transitions of SrZrO3. J. Alloys Compd. 2003, 351, 43–46. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Yamauchi, T.; Ogaki, K. High temperature type protonic conductor based on SrCeO3 and its application to the extraction of hydrogen gas. Solid State Ion. 1986, 18–19, 1003–1007. [Google Scholar] [CrossRef]
- Yamanaka, S.; Kurosaki, K.; Maekawa, T.; Matsuda, T.; Kobayashi, S.; Uno, M. Thermochemical and thermophysical properties of alkaline-earth perovskites. J. Nucl. Mater. 2005, 344, 61–66. [Google Scholar] [CrossRef]
- Li, L.; Nino, J.C. Proton-conducting barium stannates: Doping strategies and transport properties. Int. J. Hydrog. Energy 2013, 38, 1598–1606. [Google Scholar] [CrossRef]
- Maekawa, T.; Kurosaki, K.; Yamanaka, S. Thermal and mechanical properties of polycrystalline BaSnO3. J. Alloys Compd. 2006, 416, 214–217. [Google Scholar] [CrossRef]
- Snijkers, F.M.M.; Buekenhoudt, A.; Luyten, J.J.; Cooymans, J.; Mertens, M. Proton conductivity in perovskite type yttrium doped barium hafnate. Scr. Mater. 2004, 51, 1129–1134. [Google Scholar] [CrossRef]
- Maekawa, T.; Kurosaki, K.; Yamanaka, S. Thermal and mechanical properties of perovskite-type barium hafnate. J. Alloys Compd. 2006, 407, 44–48. [Google Scholar] [CrossRef]
- Furøy, K.A.; Haugsrud, R.; Hänsel, M.; Magrasó, A.; Norby, T. Role of protons in the electrical conductivity of acceptor-doped BaPrO3, BaTbO3, and BaThO3. Solid State Ion. 2007, 178, 461–467. [Google Scholar] [CrossRef]
- Purohit, R.D.; Tyagi, A.K.; Mathews, M.D.; Saha, S. Combustion synthesis and bulk thermal expansion studies of Ba and Sr thorates. J. Nucl. Mater. 2000, 280, 51–55. [Google Scholar] [CrossRef]
- Fu, W.T.; Visser, D.; Knight, K.S.; IJdo, D.J.W. Temperature-induced phase transitions in BaTbO3. J. Solid State Chem. 2004, 177, 1667–1671. [Google Scholar] [CrossRef]
- Nomura, K.; Takeuchi, T.; Tanase, S.; Kageyama, H.; Tanimoto, K.; Miyazaki, Y. Proton conduction in (La0.9Sr0.1)MIIIO3−d (MIII = Sc, In, and Lu) perovskites. Solid State Ion. 2002, 155, 647–652. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Stroeva, A.Y. Solid proton conducting electrolytes based on LaScO3. Russ. J. Electrochem. 2012, 48, 949–960. [Google Scholar] [CrossRef]
- Okuyama, Y.; Kozai, T.; Ikeda, S.; Matsuka, M.; Sakai, T.; Matsumoto, H. Incorporation and conduction of proton in Sr-doped LaMO3 (M = Al, Sc, In, Yb, Y). Electrochim. Acta 2014, 125, 443–449. [Google Scholar] [CrossRef]
- Danilov, N.; Vdovin, G.; Reznitskikh, O.; Medvedev, D.; Demin, A.; Tsiakaras, P. Physicо-chemical characterization and transport features of proton-conducting Sr-doped LaYO3 electrolyte ceramics. J. Eur. Ceram. Soc. 2016, 36, 2795–2800. [Google Scholar] [CrossRef]
- Dietrich, M.; Vassen, R.; Stover, D. LaYbO3, A Candidate for Thermal Barrier Coating Materials. In 27th Annual Cocoa Beach Conference on Advanced Ceramics and Composites: A: Ceramic Engineering and Science Proceedings, Volume 24, Issue 3; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 637–643. [Google Scholar]
- Ovanesyan, K.; Petrosyan, A.; Shirinyan, G.; Pedrini, C.; Zhang, L. Czochralski single crystal growth of Ce- and Pr-doped LaLuO3 double oxide. J. Cryst. Growth 1999, 198–199, 497–500. [Google Scholar] [CrossRef]
- Inaba, H.; Hayashi, H.; Suzuki, M. Structural phase transition of perovskite oxides LaMO3 and La0.9Sr0.1MO3 with different size of B-site ions. Solid State Ion. 2001, 144, 99–108. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Zhao, Y.; Weidner, D.J.; Parise, J.B.; Cox, D.E. Thermal expansion and structural distortion of perovskite—Data for NaMgF3 perovskite. Part I. Phys. Earth Planet. Inter. 1993, 76, 1–16. [Google Scholar] [CrossRef]
- Bohn, H.; Schober, T.; Mono, T.; Schilling, W. The high temperature proton conductor Ba3Ca1.18Nb1.82O9−δ. I. Electrical conductivity. Solid State Ion. 1999, 117, 219–228. [Google Scholar] [CrossRef]
- Krug, F.; Schober, T. The high-temperature proton conductor Ba3(Ca1.18Nb1.82)O9−gd: Thermogravimetry of the water uptake. Solid State Ion. 1996, 92, 297–302. [Google Scholar] [CrossRef]
- Schober, T.; Friedrich, J. The mixed perovskites BaCa(1+x)/3Nb(2−x)/3O3−x/2 (x = 0…0.18): Proton uptake. Solid State Ion. 2000, 136–137, 161–165. [Google Scholar] [CrossRef]
- Bhella, S.S.; Thangadurai, V. Investigations on the thermo-chemical stability and electrical conductivity of K-doped Ba3−xKxCaNb2O9−δ (x = 0.5, 0.75, 1, 1.25). Solid State Ion. 2011, 192, 229–234. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, F.; Zhang, L.; Brinkman, K.; Chen, F. Doping effects on complex perovskite Ba3Ca1.18Nb1.82O9−δ intermediate temperature proton conductor. J. Power Sources 2011, 196, 7917–7923. [Google Scholar] [CrossRef]
- Hassan, D.; Janes, S.; Clasen, R. Proton-conducting ceramics as electrode/electrolyte materials for SOFC’s-part I: Preparation, mechanical and thermal properties of sintered bodies. J. Eur. Ceram. Soc. 2003, 23, 221–228. [Google Scholar] [CrossRef]
- Mono, T.; Schober, T. Lattice parameter change in water vapor exposed Ba3Ca1.18Nb1.82O9−δ. Solid State Ion. 1996, 91, 155–159. [Google Scholar] [CrossRef]
- Schober, T.; Friedrich, J.; Triefenbach, D.; Tietz, F. Dilatometry of the high-temperature proton conductor Ba3Ca1.18Nb1.82O9−δ. Solid State Ion. 1997, 100, 173–181. [Google Scholar] [CrossRef]
- Jayaraman, V.; Magrez, A.; Caldes, M.; Joubert, O.; Ganne, M.; Piffard, Y.; Brohan, L. Characterization of perovskite systems derived from Ba2In2O5□: Part I: The oxygen-deficient Ba2In2(1−x)Ti2xO5+x□1−x (0 ≤ x ≤ 1) compounds. Solid State Ion. 2004, 170, 17–24. [Google Scholar] [CrossRef]
- Bjørheim, T.S.; Rahman, S.M.H.; Eriksson, S.G.; Knee, C.S.; Haugsrud, R. Hydration Thermodynamics of the Proton Conducting Oxygen-Deficient Perovskite Series BaTi1−xMxO3−x/2 with M = In or Sc. Inorg. Chem. 2015, 54, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.H.; Knee, C.S.; Ahmed, I.; Eriksson, S.G.; Haugsrud, R. 50 mol% indium substituted BaTiO3: Characterization of structure and conductivity. Int. J. Hydrog. Energy 2012, 37, 7975–7982. [Google Scholar] [CrossRef]
- Quarez, E.; Noirault, S.; Caldes, M.T.; Joubert, O. Water incorporation and proton conductivity in titanium substituted barium indate. J. Power Sources 2010, 195, 1136–1141. [Google Scholar] [CrossRef]
- Rahman, S.M.H.; Ahmed, I.; Haugsrud, R.; Eriksson, S.G.; Knee, C.S. Characterisation of structure and conductivity of BaTi0.5Sc0.5O3−δ. Solid State Ion. 2014, 255, 140–146. [Google Scholar] [CrossRef]
- Rahman, S.M.H.; Norberg, S.T.; Knee, C.S.; Biendicho, J.J.; Hull, S.; Eriksson, S.G. Proton conductivity of hexagonal and cubic BaTi1−xScxO3−δ (0.1 ≤ x ≤ 0.8). Dalton Trans. 2014, 43, 15055–15064. [Google Scholar] [CrossRef] [PubMed]
- Noirault, S.; Quarez, E.; Piffard, Y.; Joubert, O. Water incorporation into the Ba2(In1−xMx)2O5 (M = Sc3+ 0 ≤ x < 0.5 and M = Y3+ 0 ≤ x < 0.35) system and protonic conduction. Solid State Ion. 2009, 180, 1157–1163. [Google Scholar] [CrossRef]
- Haugsrud, R.; Norby, T. High-Temperature Proton Conductivity in Acceptor-Substituted Rare-Earth Ortho-Tantalates, LnTaO4. J. Am. Ceram. Soc. 2007, 90, 1116–1121. [Google Scholar] [CrossRef]
- Haugsrud, R.; Norby, T. Proton conduction in rare-earth ortho-niobates and ortho-tantalates. Nat. Mater. 2006, 5, 193–196. [Google Scholar] [CrossRef]
- Bi, Z.; Bridges, C.A.; Kim, J.H.; Huq, A.; Paranthaman, M.P. Phase stability and electrical conductivity of Ca-doped LaNb1−xTaxO4−δ high temperature proton conductors. J. Power Sources 2011, 196, 7395–7403. [Google Scholar] [CrossRef]
- Norby, T.; Christiansen, N. Proton conduction in Ca- and Sr-substituted LaPO4. Solid State Ion. 1995, 77, 240–243. [Google Scholar] [CrossRef]
- Bjørheim, T.S.; Norby, T.; Haugsrud, R. Hydration and proton conductivity in LaAsO4. J. Mater. Chem. 2012, 22, 1652–1661. [Google Scholar] [CrossRef]
- Toyoura, K.; Matsunaga, K. Hydrogen Bond Dynamics in Proton-Conducting Lanthanum Arsenate. J. Phys. Chem. C 2013, 117, 18006–18012. [Google Scholar] [CrossRef]
- Amezawa, K.; Tomii, Y.; Yamamoto, N. High temperature protonic conduction in Ca-doped YPO4. Solid State Ion. 2003, 162–163, 175–180. [Google Scholar] [CrossRef]
- Mokkelbost, T.; Lein, H.L.; Vullum, P.E.; Holmestad, R.; Grande, T.; Einarsrud, M.A. Thermal and mechanical properties of LaNbO4-based ceramics. Ceram. Int. 2009, 35, 2877–2883. [Google Scholar] [CrossRef]
- Fjeld, H.; Kepaptsoglou, D.M.; Haugsrud, R.; Norby, T. Charge carriers in grain boundaries of 0.5% Sr-doped LaNbO4. Solid State Ion. 2010, 181, 104–109. [Google Scholar] [CrossRef]
- Mielewczyk-Gryn, A.; Wachowski, S.; Zagórski, K.; Jasiński, P.; Gazda, M. Characterization of magnesium doped lanthanum orthoniobate synthesized by molten salt route. Ceram. Int. 2015, 41, 7847–7852. [Google Scholar] [CrossRef]
- Brandão, A.D.; Gracio, J.; Mather, G.C.; Kharton, V.V.; Fagg, D.P. B-site substitutions in LaNb1−xMxO4−δ materials in the search for potential proton conductors (M = Ga, Ge, Si, B, Ti, Zr, P, Al). J. Solid State Chem. 2011, 184, 863–870. [Google Scholar] [CrossRef]
- Syvertsen, G.E.; Magrasó, A.; Haugsrud, R.; Einarsrud, M.A.; Grande, T. The effect of cation non-stoichiometry in LaNbO4 materials. Int. J. Hydrog. Energy 2012, 37, 8017–8026. [Google Scholar] [CrossRef]
- Huse, M.; Norby, T.; Haugsrud, R. Effects of A and B site acceptor doping on hydration and proton mobility of LaNbO4. Int. J. Hydrog. Energy 2012, 37, 8004–8016. [Google Scholar] [CrossRef]
- Depero, L.E.; Sangaletti, L. Cation Sublattice and Coordination Polyhedra in ABO4 type of Structures. J. Solid State Chem. 1997, 129, 82–91. [Google Scholar] [CrossRef]
- Errandonea, D.; Manjon, F. Pressure effects on the structural and electronic properties of ABX4 scintillating crystals. Prog. Mater. Sci. 2008, 53, 711–773. [Google Scholar] [CrossRef]
- Li, H.; Zhou, S.; Zhang, S. The relationship between the thermal expansions and structures of ABO4 oxides. J. Solid State Chem. 2007, 180, 589–595. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Hara, K.; Sawada, A. The ferroelastic transition in some scheelite-type crystals. Phys. B+C 1988, 150, 258–264. [Google Scholar] [CrossRef]
- David, W.I.F. High Resolution Neutron Powder Diffraction Studies of the Ferroelastic Phase Transition in LaNbO4. MRS Proc. 1989, 166, 203. [Google Scholar] [CrossRef]
- Mokkelbost, T.; Kaus, I.; Haugsrud, R.; Norby, T.; Grande, T.; Einarsrud, M.A. High-Temperature Proton-Conducting Lanthanum Ortho-Niobate-Based Materials. Part II: Sintering Properties and Solubility of Alkaline Earth Oxides. J. Am. Ceram. Soc. 2008, 91, 879–886. [Google Scholar] [CrossRef]
- Ivanova, M.; Ricote, S.; Meulenberg, W.A.; Haugsrud, R.; Ziegner, M. Effects of A- and B-site (co-)acceptor doping on the structure and proton conductivity of LaNbO4. Solid State Ion. 2012, 213, 45–52. [Google Scholar] [CrossRef]
- Mielewczyk-Gryn, A.; Wachowski, S.; Strychalska, J.; Zagórski, K.; Klimczuk, T.; Navrotsky, A.; Gazda, M. Heat capacities and thermodynamic properties of antimony substituted lanthanum orthoniobates. Ceram. Int. 2016, 42, 7054–7059. [Google Scholar] [CrossRef]
- Wachowski, S.; Mielewczyk-Gryń, A.; Zagórski, K.; Li, C.; Jasiński, P.; Skinner, S.J.; Haugsrud, R.; Gazda, M. Influence of Sb-substitution on ionic transport in lanthanum orthoniobates. J. Mater. Chem. A 2016, 4, 11696–11707. [Google Scholar] [CrossRef] [Green Version]
- Syvertsen, G.E.; Estournès, C.; Fjeld, H.; Haugsrud, R.; Einarsrud, M.; Grande, T.; Menon, M. Spark Plasma Sintering and Hot Pressing of Hetero-Doped LaNbO4. J. Am. Ceram. Soc. 2012, 95, 1563–1571. [Google Scholar] [CrossRef]
- Mokkelbost, T.; Andersen, Ø.; Strøm, R.A.; Wiik, K.; Grande, T.; Einarsrud, M. High-Temperature Proton-Conducting LaNbO4-Based Materials: Powder Synthesis by Spray Pyrolysis. J. Am. Ceram. Soc. 2007, 90, 3395–3400. [Google Scholar] [CrossRef]
- Magrasó, A.; Xuriguera, H.; Varela, M.; Sunding, M.F.; Strandbakke, R.; Haugsrud, R.; Norby, T. Novel Fabrication of Ca-Doped LaNbO4 Thin-Film Proton-Conducting Fuel Cells by Pulsed Laser Deposition. J. Am. Ceram. Soc. 2010, 93, 1874–1878. [Google Scholar] [CrossRef]
- Amsif, M.; Marrero-López, D.; Ruiz-Morales, J.C.; Savvin, S.; Núñez, P. Low temperature sintering of LaNbO4 proton conductors from freeze-dried precursors. J. Eur. Ceram. Soc. 2012, 32, 1235–1244. [Google Scholar] [CrossRef]
- Mielewczyk-Gryń, A.; Gdula, K.; Molin, S.; Jasinski, P.; Kusz, B.; Gazda, M. Structure and electrical properties of ceramic proton conductors obtained with molten-salt and solid-state synthesis methods. J. Non. Cryst. Solids 2010, 356, 1976–1979. [Google Scholar] [CrossRef]
- Brandão, A.D.; Antunes, I.; Frade, J.R.; Torre, J.; Kharton, V.V.; Fagg, D.P. Enhanced Low-Temperature Proton Conduction in Sr0.02La0.98NbO4−δ by Scheelite Phase Retention. Chem. Mater. 2010, 22, 6673–6683. [Google Scholar] [CrossRef]
- Santibáñez-Mendieta, A.B.; Fabbri, E.; Licoccia, S.; Traversa, E. Tailoring phase stability and electrical conductivity of Sr0.02La0.98Nb1−xTaxO4 for intermediate temperature fuel cell proton conducting electrolytes. Solid State Ion. 2012, 216, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Wachowski, S.L.; Kamecki, B.; Winiarz, P.; Dzierzgowski, K.; Mielewczyk-Gryń, A.; Gazda, M.; Wachowski, S.L.; Jasiński, P.; Witkowska, A.; Gazda, M.; et al. Tailoring structural properties of lanthanum orthoniobates through an isovalent substitution on the Nb-site. Inorg. Chem. Front. 2018, 24, 1–16. [Google Scholar] [CrossRef]
- Jian, L.; Wayman, C.M. Compressive behavior and domain-related shape memory effect in LaNbO4 ceramics. Mater. Lett. 1996, 26, 1–7. [Google Scholar] [CrossRef]
- Parlinski, K.; Hashi, Y.; Tsunekawa, S.; Kawazoe, Y. Computer simulation of ferroelastic phase transition in LaNbO4. J. Mater. Res. 1997, 12, 2428–2437. [Google Scholar] [CrossRef]
- Sarin, P.; Hughes, R.W.; Lowry, D.R.; Apostolov, Z.D.; Kriven, W.M. High-Temperature Properties and Ferroelastic Phase Transitions in Rare-Earth Niobates (LnNbO4). J. Am. Ceram. Soc. 2014, 97, 3307–3319. [Google Scholar] [CrossRef]
- Hikichi, Y.; Ota, T.; Daimon, K.; Hattori, T. Thermal, Mechanical, and Chemical Properties of Sintered Xenotime-Type RPO4 (R = Y, Er, Yb, or Lu). J. Am. Ceram. Soc. 1998, 81, 2216–2218. [Google Scholar] [CrossRef]
- Bayer, G. Thermal expansion of ABO4-compounds with zircon-and scheelite structures. J. Less Common Met. 1972, 26, 255–262. [Google Scholar] [CrossRef]
- Hikichi, Y.; Ota, T.; Hattori, T. Thermal, mechanical and chemical properties of sintered monazite-(La, Ce, Nd or Sm). Mineral. J. 1997, 19, 123–130. [Google Scholar] [CrossRef]
- Omori, M.; Kobayashi, Y.; Hirai, T. Dilatometric behavior of martensitic transformation of NdNbO4 polycrystals. J. Mater. Sci. 2000, 35, 719–721. [Google Scholar] [CrossRef]
- Filatov, S.K. General concept of increasing crystal symmetry with an increase in temperature. Crystallogr. Rep. 2011, 56, 953–961. [Google Scholar] [CrossRef]
- Akiyama, K.; Nagano, I.; Shida, M.; Ota, S. Thermal Barrier Coating Material. U.S. Patent No. 7,622,411, 24 November 2009. [Google Scholar]
- Yang, H.; Peng, F.; Zhang, Q.; Guo, C.; Shi, C.; Liu, W.; Sun, G.; Zhao, Y.; Zhang, D.; Sun, D.; et al. A promising high-density scintillator of GdTaO4 single crystal. CrystEngComm 2014, 16, 2480–2485. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Zhou, S.; Cao, X. Bonding characteristics, thermal expansibility, and compressibility of RXO4 (R = Rare Earths, X = P, As) within monazite and zircon structures. Inorg. Chem. 2009, 48, 4542–4548. [Google Scholar] [CrossRef] [PubMed]
- Huse, M.; Norby, T.; Haugsrud, R. Proton Conductivity in Acceptor-Doped LaVO4. J. Electrochem. Soc. 2011, 158, B857–B865. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, S.; Li, H.; Li, N. Investigation of thermal expansion and compressibility of rare-earth orthovanadates using a dielectric chemical bond method. Inorg. Chem. 2008, 47, 7863–7867. [Google Scholar] [CrossRef] [PubMed]
- Bjørheim, T.S.; Besikiotis, V.; Haugsrud, R. Hydration thermodynamics of pyrochlore structured oxides from TG and first principles calculations. Dalton Trans. 2012, 41, 13343–13351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omata, T.; Ikeda, K.; Tokashiki, R.; Otsuka-Yao-Matsuo, S. Proton solubility for La2Zr2O7 with a pyrochlore structure doped with a series of alkaline-earth ions. Solid State Ion. 2004, 167, 389–397. [Google Scholar] [CrossRef]
- Eurenius, K.E.J.; Ahlberg, E.; Knee, C.S. Proton conductivity in Ln1.96Ca0.04Sn2O7−δ(Ln = La, Sm, Yb) pyrochlores as a function of the lanthanide size. Solid State Ion. 2010, 181, 1258–1263. [Google Scholar] [CrossRef]
- Eurenius, K.E.J.; Ahlberg, E.; Ahmed, I.; Eriksson, S.G.; Knee, C.S. Investigation of proton conductivity in Sm1.92Ca0.08Ti2O7−δ and Sm2Ti1.92Y0.08O7−δ pyrochlores. Solid State Ion. 2010, 181, 148–153. [Google Scholar] [CrossRef]
- Eurenius, K.E.J.; Ahlberg, E.; Knee, C.S. Proton conductivity in Sm2Sn2O7 pyrochlores. Solid State Ion. 2010, 181, 1577–1585. [Google Scholar] [CrossRef]
- Omata, T.; Otsuka-Yao-Matsuo, S. Electrical Properties of Proton-Conducting Ca-Doped La2Zr2O7 with a Pyrochlore-Type Structure. J. Electrochem. Soc. 2001, 148, E252–E261. [Google Scholar] [CrossRef]
- Shimura, T.; Komori, M.; Iwahara, H. Ionic conduction in pyrochlore-type oxides containing rare earth elements at high temperature. Solid State Ion. 1996, 86–88, 685–689. [Google Scholar] [CrossRef]
- Ma, W.; Gong, S.; Xu, H.; Cao, X. On improving the phase stability and thermal expansion coefficients of lanthanum cerium oxide solid solutions. Scr. Mater. 2006, 54, 1505–1508. [Google Scholar] [CrossRef]
- Besikiotis, V.; Ricote, S.; Jensen, M.H.; Norby, T.; Haugsrud, R. Conductivity and hydration trends in disordered fluorite and pyrochlore oxides: A study on lanthanum cerate–zirconate based compounds. Solid State Ion. 2012, 229, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Kalland, L.E.; Norberg, S.T.; Kyrklund, J.; Hull, S.; Eriksson, S.G.; Norby, T.; Mohn, C.E.; Knee, C.S. C-type related order in the defective fluorites La2Ce2O7 and Nd2Ce2O7 studied by neutron scattering and ab initio MD simulations. Phys. Chem. Chem. Phys. 2016, 18, 24070–24080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.X.; Tracy, C.L.L.; Lang, M.; Ewing, R.C.C. Stability of fluorite-type La2Ce2O7 under extreme conditions. J. Alloys Compd. 2016, 674, 168–173. [Google Scholar] [CrossRef]
- Wang, J.; Bai, S.; Zhang, H.; Zhang, C. The structure, thermal expansion coefficient and sintering behavior of Nd3+-doped La2Zr2O7 for thermal barrier coatings. J. Alloys Compd. 2009, 476, 89–91. [Google Scholar] [CrossRef]
- Lehmann, H.; Pitzer, D.; Pracht, G.; Vassen, R.; Stöver, D. Thermal conductivity and thermal expansion coefficients of the lanthanum rare-earth-element zirconate system. J. Am. Ceram. Soc. 2003, 86, 1338–1344. [Google Scholar] [CrossRef]
- Haugsrud, R. Defects and transport properties in Ln6WO12 (Ln = La, Nd, Gd, Er). Solid State Ion. 2007, 178, 555–560. [Google Scholar] [CrossRef]
- Zayas-Rey, M.J.; dos Santos-Gómez, L.; Marrero-López, D.; León-Reina, L.; Canales-Vázquez, J.; Aranda, M.A.G.; Losilla, E.R. Structural and Conducting Features of Niobium-Doped Lanthanum Tungstate, La27(W1−xNbx)5O55.55−δ. Chem. Mater. 2013, 25, 448–456. [Google Scholar] [CrossRef]
- Magrasó, A.; Haugsrud, R. Effects of the La/W ratio and doping on the structure, defect structure, stability and functional properties of proton-conducting lanthanum tungstate La28−xW4+xO54+δ. A review. J. Mater. Chem. A 2014, 2, 12630–12641. [Google Scholar] [CrossRef]
- Magrasó, A.; Hervoches, C.H.; Ahmed, I.; Hull, S.; Nordström, J.; Skilbred, A.W.B.; Haugsrud, R. In situ high temperature powder neutron diffraction study of undoped and Ca-doped La28−xW4+xO54+3x/2 (x = 0.85). J. Mater. Chem. A 2013, 1, 3774–3782. [Google Scholar] [CrossRef]
- Hancke, R.; Magrasó, A.; Norby, T.; Haugsrud, R. Hydration of lanthanum tungstate (La/W=5.6 and 5.3) studied by TG and simultaneous TG–DSC. Solid State Ion. 2013, 231, 25–29. [Google Scholar] [CrossRef]
- Quarez, E.; Kravchyk, K.V.; Joubert, O. Compatibility of proton conducting La6WO12 electrolyte with standard cathode materials. Solid State Ion. 2012, 216, 19–24. [Google Scholar] [CrossRef]
- Seeger, J.; Ivanova, M.E.; Meulenberg, W.A.; Sebold, D.; Stöver, D.; Scherb, T.; Schumacher, G.; Escolástico, S.; Solís, C.; Serra, J.M. Synthesis and characterization of nonsubstituted and substituted proton-conducting La6−xWO12−y. Inorg. Chem. 2013, 52, 10375–10386. [Google Scholar] [CrossRef] [PubMed]
- Zayas-Rey, M.J.; dos Santos-Gómez, L.; Cabeza, A.; Marrero-López, D.; Losilla, E.R. Proton conductors based on alkaline-earth substituted La28−xW4+xO54+3x/2. Dalton Trans. 2014, 43, 6490–6499. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Wu, T.; Liu, W.; Liu, X.; Meng, G. Cathode processes and materials for solid oxide fuel cells with proton conductors as electrolytes. J. Mater. Chem. 2010, 20, 6218–6225. [Google Scholar] [CrossRef]
- Merkle, R.; Poetzsch, D.; Maier, J. Oxygen Reduction Reaction at Cathodes on Proton Conducting Oxide Electrolytes: Contribution from Three Phase Boundary Compared to Bulk Path. ECS Trans. 2015, 66, 95–102. [Google Scholar] [CrossRef]
- Téllez Lozano, H.; Druce, J.; Cooper, S.J.; Kilner, J.A. Double perovskite cathodes for proton-conducting ceramic fuel cells: Are they triple mixed ionic electronic conductors? Sci. Technol. Adv. Mater. 2017, 18, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Strandbakke, R.; Cherepanov, V.A.; Zuev, A.Y.; Tsvetkov, D.S.; Argirusis, C.; Sourkouni, G.; Prünte, S.; Norby, T. Gd- and Pr-based double perovskite cobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ion. 2015, 278, 120–132. [Google Scholar] [CrossRef]
- Zohourian, R.; Merkle, R.; Maier, J. Proton uptake into the protonic cathode material BaCo0.4Fe0.4Zr0.2O3−δ and comparison to protonic electrolyte materials. Solid State Ion. 2017, 299, 64–69. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Rioja-Monllor, L.; Nakamura, T.; Ricote, S.; O’Hayre, R.; Amezawa, K.; Einarsrud, M.A.; Grande, T. Effect of Cation Ordering on the Performance and Chemical Stability of Layered Double Perovskite Cathodes. Materials 2018, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; He, B.; Ling, Y.; Xun, Z.; Peng, R.; Meng, G.; Liu, X. Cobalt-free oxide Ba0.5Sr0.5Fe0.8Cu0.2O3−δ for proton-conducting solid oxide fuel cell cathode. Int. J. Hydrog. Energy 2010, 35, 3769–3774. [Google Scholar] [CrossRef]
- Grimaud, A.; Mauvy, F.; Bassat, J.M.; Fourcade, S.; Rocheron, L.; Marrony, M.; Grenier, J.C. Hydration Properties and Rate Determining Steps of the Oxygen Reduction Reaction of Perovskite-Related Oxides as H+-SOFC Cathodes. J. Electrochem. Soc. 2012, 159, B683–B694. [Google Scholar] [CrossRef]
- Dailly, J.; Fourcade, S.; Largeteau, A.; Mauvy, F.; Grenier, J.C.; Marrony, M. Perovskite and A2MO4-type oxides as new cathode materials for protonic solid oxide fuel cells. Electrochim. Acta 2010, 55, 5847–5853. [Google Scholar] [CrossRef]
- Shang, M.; Tong, J.; O’Hayre, R. A promising cathode for intermediate temperature protonic ceramic fuel cells: BaCo0.4Fe0.4Zr0.2O3−δ. RSC Adv. 2013, 3, 15769–15775. [Google Scholar] [CrossRef]
- Tao, Z.; Bi, L.; Zhu, Z.; Liu, W. Novel cobalt-free cathode materials BaCexFe1−xO3−δ for proton-conducting solid oxide fuel cells. J. Power Sources 2009, 194, 801–804. [Google Scholar] [CrossRef]
- Poetzsch, D.; Merkle, R.; Maier, J. Proton conductivity in mixed-conducting BSFZ perovskite from thermogravimetric relaxation. Phys. Chem. Chem. Phys. 2014, 16, 16446–16453. [Google Scholar] [CrossRef] [PubMed]
- Poetzsch, D.; Merkle, R.; Maier, J. Proton uptake in the H+-SOFC cathode material Ba0.5Sr0.5Fe0.8Zn0.2O3−δ: Transition from hydration to hydrogenation with increasing oxygen partial pressure. Faraday Discuss. 2015, 182, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Mukundan, R.; Davies, P.K.; Worrell, W.L. Electrochemical Characterization of Mixed Conducting Ba(Ce0.8−yPryGd0.2)O2.9 Cathodes. J. Electrochem. Soc. 2001, 148, A82–A86. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Wang, S.; Choi, Y.; Zuo, C.; Liu, M. A mixed proton, oxygen ion, and electron conducting cathode for SOFCs based on oxide proton conductors. J. Power Sources 2010, 195, 471–474. [Google Scholar] [CrossRef]
- Tao, Z.; Bi, L.; Yan, L.; Sun, W.; Zhu, Z.; Peng, R.; Liu, W. A novel single phase cathode material for a proton-conducting SOFC. Electrochem. Commun. 2009, 11, 688–690. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, Y.; Peng, R.; Xia, C. Nano-sized Sm0.5Sr0.5CoO3−δ as the cathode for solid oxide fuel cells with proton-conducting electrolytes of BaCe0.8Sm0.2O2.9. Electrochim. Acta 2009, 54, 4888–4892. [Google Scholar] [CrossRef]
- Upasen, S.; Batocchi, P.; Mauvy, F.; Slodczyk, A.; Colomban, P. Chemical and structural stability of La0.6Sr0.4Co0.2Fe0.8O3−δ ceramic vs. medium/high water vapor pressure. Ceram. Int. 2015, 41, 14137–14147. [Google Scholar] [CrossRef]
- Pu, T.; Tan, W.; Shi, H.; Na, Y.; Lu, J.; Zhu, B. Steam/CO2 electrolysis in symmetric solid oxide electrolysis cell with barium cerate-carbonate composite electrolyte. Electrochim. Acta 2016, 190, 193–198. [Google Scholar] [CrossRef]
- Lin, B.; Dong, Y.; Yan, R.; Zhang, S.; Hu, M.; Zhou, Y.; Meng, G. In situ screen-printed BaZr0.1Ce0.7Y0.2O3−δ electrolyte-based protonic ceramic membrane fuel cells with layered SmBaCo2O5+x cathode. J. Power Sources 2009, 186, 446–449. [Google Scholar] [CrossRef]
- Kim, J.; Sengodan, S.; Kwon, G.; Ding, D.; Shin, J.; Liu, M.; Kim, G. Triple-Conducting Layered Perovskites as Cathode Materials for Proton-Conducting Solid Oxide Fuel Cells. ChemSusChem 2014, 7, 2811–2815. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhang, S.; Bi, L.; Ding, H.; Liu, X.; Gao, J.; Meng, G. Prontonic ceramic membrane fuel cells with layered GdBaCo2O5+x cathode prepared by gel-casting and suspension spray. J. Power Sources 2008, 177, 330–333. [Google Scholar] [CrossRef]
- Brieuc, F.; Dezanneau, G.; Hayoun, M.; Dammak, H. Proton diffusion mechanisms in the double perovskite cathode material GdBaCo2O5.5: A molecular dynamics study. Solid State Ion. 2017, 309, 187–191. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Lin, B.; Ding, H.; Wang, S.; Ling, Y.; Peng, R.; Meng, G.; Liu, X. High performance of proton-conducting solid oxide fuel cell with a layered PrBaCo2O5+δ cathode. J. Power Sources 2009, 194, 835–837. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X.; Liu, X.; Meng, G. A novel layered perovskite cathode for proton conducting solid oxide fuel cells. J. Power Sources 2010, 195, 775–778. [Google Scholar] [CrossRef]
- Nian, Q.; Zhao, L.; He, B.; Lin, B.; Peng, R.; Meng, G.; Liu, X. Layered SmBaCuCoO5+δ and SmBaCuFeO5+δ perovskite oxides as cathode materials for proton-conducting SOFCs. J. Alloys Compd. 2010, 492, 291–294. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Nian, Q.; Xun, Z.; Peng, R.; Meng, G.; Liu, X. In situ drop-coated BaZr0.1Ce0.7Y0.2O3−δ electrolyte-based proton-conductor solid oxide fuel cells with a novel layered PrBaCuFeO5+δ cathode. J. Power Sources 2009, 194, 291–294. [Google Scholar] [CrossRef]
- Taillades, G.; Dailly, J.; Taillades-Jacquin, M.; Mauvy, F.; Essouhmi, A.; Marrony, M.; Lalanne, C.; Fourcade, S.; Jones, D.J.; Grenier, J.C.; et al. Intermediate temperature anode-supported fuel cell based on BaCe0.9Y0.1O3 electrolyte with novel Pr2NiO4 cathode. Fuel Cells 2010, 10, 166–173. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Mikhalev, S.; Kovalevsky, A.V.; Fagg, D.P. Fabrication and electrochemical performance of a stable, anode supported thin BaCe0.4Zr0.4Y0.2O3−δ electrolyte Protonic Ceramic Fuel Cell. J. Power Sources 2015, 278, 582–589. [Google Scholar] [CrossRef]
- Upasen, S.; Batocchi, P.; Mauvy, F.; Slodczyk, A.; Colomban, P. Protonation and structural/chemical stability of Ln2NiO4+δ ceramics vs. H2O/CO2: High temperature/water pressure ageing tests. J. Alloys Compd. 2014, 622, 1074–1085. [Google Scholar] [CrossRef]
- Zhao, H.; Mauvy, F.; Lalanne, C.; Bassat, J.M.; Fourcade, S.; Grenier, J.C. New cathode materials for ITSOFC: Phase stability, oxygen exchange and cathode properties of La2−xNiO4+δ. Solid State Ion. 2008, 179, 2000–2005. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Wang, T.; Chen, G.; Wu, K.; Cheng, Y. Decreasing the polarization resistance of LaSrCoO4 cathode by Fe substitution for Ba(Zr0.1Ce0.7Y0.2)O3 based protonic ceramic fuel cells. J. Alloys Compd. 2016, 689, 581–586. [Google Scholar] [CrossRef]
- Acuña, W.; Tellez, J.F.; Macías, M.A.; Roussel, P.; Ricote, S.; Gauthier, G.H. Synthesis and characterization of BaGa2O4 and Ba3Co2O6(CO3)0.6 compounds in the search of alternative materials for Proton Ceramic Fuel Cell (PCFC). Solid State Sci. 2017, 71, 61–68. [Google Scholar] [CrossRef]
- Danilov, N.A.; Tarutin, A.P.; Lyagaeva, J.G.; Pikalova, E.Y.; Murashkina, A.A.; Medvedev, D.A.; Patrakeev, M.V.; Demin, A.K. Affinity of YBaCo4O7+δ-based layered cobaltites with protonic conductors of cerate-zirconate family. Ceram. Int. 2017, 43, 15418–15423. [Google Scholar] [CrossRef]
- Kinyanjui, F.G.; Norberg, S.T.; Knee, C.S.; Eriksson, S.G. Proton conduction in oxygen deficient Ba3In1.4Y0.3M0.3ZrO8 (M = Ga3+ or Gd3+) perovskites. J. Alloys Compd. 2014, 605, 56–62. [Google Scholar] [CrossRef]
- Yahia, H.B.; Mauvy, F.; Grenier, J.C. Ca3−xLaxCo4O9+δ (x = 0, 0.3): New cobaltite materials as cathodes for proton conducting solid oxide fuel cell. J. Solid State Chem. 2010, 183, 527–531. [Google Scholar] [CrossRef]
- Macias, M.A.; Sandoval, M.V.; Martinez, N.G.; Vázquez-Cuadriello, S.; Suescun, L.; Roussel, P.; Świerczek, K.; Gauthier, G.H. Synthesis and preliminary study of La4BaCu5O13+δ and La6.4Sr1.6Cu8O20±δ ordered perovskites as SOFC/PCFC electrode materials. Solid State Ion. 2016, 288, 68–75. [Google Scholar] [CrossRef]
- Lin, B.; Ding, H.; Dong, Y.; Wang, S.; Zhang, X.; Fang, D.; Meng, G. Intermediate-to-low temperature protonic ceramic membrane fuel cells with Ba0.5Sr0.5Co0.8Fe0.2O3−δ-BaZr0.1Ce0.7Y0.2O3−δ composite cathode. J. Power Sources 2009, 186, 58–61. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Lou, X.; Liu, M. Electrical conductivity and electrochemical performance of cobalt-doped BaZr0.1Ce0.7Y0.2O3−δ cathode. Int. J. Hydrog. Energy 2011, 36, 2266–2270. [Google Scholar] [CrossRef]
- Sun, W.; Yan, L.; Lin, B.; Zhang, S.; Liu, W. High performance proton-conducting solid oxide fuel cells with a stable Sm0.5Sr0.5Co3−δ–Ce0.8Sm0.2O2−δ composite cathode. J. Power Sources 2010, 195, 3155–3158. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, Z.; Jiang, Y.; Shi, Z.; Yan, L.; Liu, W. Optimization of BaZr0.1Ce0.7Y0.2O3−δ-based proton-conducting solid oxide fuel cells with a cobalt-free proton-blocking La0.7Sr0.3FeO3−δ–Ce0.8Sm0.2O2−δ composite cathode. Int. J. Hydrog. Energy 2011, 36, 9956–9966. [Google Scholar] [CrossRef]
- Fabbri, E.; Licoccia, S.; Traversa, E.; Wachsman, E.D. Composite cathodes for proton conducting electrolytes. Fuel Cells 2009, 9, 128–138. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Q. A functionally graded cathode for proton-conducting solid oxide fuel cells. J. Power Sources 2012, 212, 186–191. [Google Scholar] [CrossRef]
- Fabbri, E.; Bi, L.; Pergolesi, D.; Traversa, E. High-performance composite cathodes with tailored mixed conductivity for intermediate temperature solid oxide fuel cells using proton conducting electrolytes. Energy Environ. Sci. 2011, 4, 4984–4993. [Google Scholar] [CrossRef]
- Vert, V.B.; Solís, C.; Serra, J.M. Electrochemical properties of PSFC-BCYb composites as cathodes for proton conducting solid oxide fuel cells. Fuel Cells 2011, 11, 81–90. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Zhao, H.; Shen, Y.; Du, Z.; Zhang, C. Electrochemical properties of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ-Nd1.95NiO4+δ composite cathode for protonic ceramic fuel cells. Int. J. Hydrog. Energy 2015, 40, 2800–2807. [Google Scholar] [CrossRef]
- Dailly, J.; Taillades, G.; Ancelin, M.; Pers, P.; Marrony, M. High performing BaCe0.8Zr0.1Y0.1O3−δ-Sm0.5Sr0.5CoO3−δ based protonic ceramic fuel cell. J. Power Sources 2017, 361, 221–226. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Ling, Y.; He, B.; Xu, J.; Zhao, L. Probing novel triple phase conducting composite cathode for high performance protonic ceramic fuel cells. Int. J. Hydrog. Energy 2016, 41, 5074–5083. [Google Scholar] [CrossRef]
- Bausá, N.; Solís, C.; Strandbakke, R.; Serra, J.M. Development of composite steam electrodes for electrolyzers based on barium zirconate. Solid State Ion. 2017, 306, 62–68. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Chen, S.; Wu, Y.; Xie, K. Composite manganate oxygen electrode enhanced with iron oxide nanocatalyst for high temperature steam electrolysis in a proton-conducting solid oxide electrolyzer. Int. J. Hydrog. Energy 2015, 40, 7920–7931. [Google Scholar] [CrossRef]
- Ding, H.; Sullivan, N.P.; Ricote, S. Double perovskite Ba2FeMoO6−δ as fuel electrode for protonic-ceramic membranes. Solid State Ion. 2017, 306, 97–103. [Google Scholar] [CrossRef]
- Robinson, S.; Manerbino, A.; Coors, W.G. Galvanic hydrogen pumping in the protonic ceramic perovskite BaCe0.2Zr0.7Y0.1O3−δ. J. Membr. Sci. 2013, 446, 99–105. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Manerbino, A.; Coors, W.G.; Stoukides, M. Methane steam reforming at low temperatures in a BaZr0.7Ce0.2Y0.1O2.9 proton conducting membrane reactor. Appl. Catal. B Environ. 2016, 186, 1–9. [Google Scholar] [CrossRef]
- Shen, C.T.; Lee, Y.H.; Xie, K.; Yen, C.P.; Jhuang, J.W.; Lee, K.R.; Lee, S.W.; Tseng, C.J. Correlation between microstructure and catalytic and mechanical properties during redox cycling for Ni-BCY and Ni-BCZY composites. Ceram. Int. 2017, 43, S671–S674. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Antunes, I.; Perez, J.; Fagg, D.P. Electrochemical behaviour of Ni-BZO and Ni-BZY cermet anodes for Protonic Ceramic Fuel Cells (PCFCs)—A comparative study. Electrochim. Acta 2015, 154, 387–396. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Brandão, A.D.; Yaremchenko, A.A.; Fagg, D.P. The impact of porosity, pH2 and pH2O on the polarisation resistance of Ni–BaZr0.85Y0.15O3−δ cermet anodes for Protonic Ceramic Fuel Cells (PCFCs). Int. J. Hydrog. Energy 2014, 39, 21231–21241. [Google Scholar] [CrossRef]
- Pikalova, E.; Medvedev, D. Effect of anode gas mixture humidification on the electrochemical performance of the BaCeO3-based protonic ceramic fuel cell. Int. J. Hydrog. Energy 2016, 41, 4016–4025. [Google Scholar] [CrossRef]
- Park, Y.E.; Ji, H.I.; Kim, B.K.; Lee, J.H.; Lee, H.W.; Park, J.S. Pore structure improvement in cermet for anode-supported protonic ceramic fuel cells. Ceram. Int. 2013, 39, 2581–2587. [Google Scholar] [CrossRef]
- Taillades, G.; Pers, P.; Mao, V.; Taillades, M. High performance anode-supported proton ceramic fuel cell elaborated by wet powder spraying. Int. J. Hydrog. Energy 2016, 41, 12330–12336. [Google Scholar] [CrossRef]
- Li, G.; Jin, H.; Cui, Y.; Gui, L.; He, B.; Zhao, L. Application of a novel (Pr0.9La0.1)2(Ni0.74Cu0.21Nb0.05)O4+δ-infiltrated BaZr0.1Ce0.7Y0.2O3−δ cathode for high performance protonic ceramic fuel cells. J. Power Sources 2017, 341, 192–198. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Lenrick, F.; Wallenberg, R. LaCoO3: Promising cathode material for protonic ceramic fuel cells based on BaCe0.2Zr0.7Y0.1O3-delta electrolyte. J. Power Sources 2012, 218, 313–319. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Ricote, S.; Sullivan, N.P. Characterization of ionic transport through BaCe0.2Zr0.7Y0.1O3−δ membranes in galvanic and electrolytic operation. Int. J. Hydrog. Energy 2015, 40, 9278–9286. [Google Scholar] [CrossRef]
- Strandbakke, R.; Vøllestad, E.; Robinson, S.A.; Fontaine, M.L.; Norby, T. Ba0.5Gd0.8La0.7Co2O6−δ Infiltrated in Porous BaZr0.7Ce0.2Y0.1O3 Backbones as Electrode Material for Proton Ceramic Electrolytes. J. Electrochem. Soc. 2017, 164, F196–F202. [Google Scholar] [CrossRef]
- Song, S.H.; Yoon, S.E.; Choi, J.; Kim, B.K.; Park, J.S. A high-performance ceramic composite anode for protonic ceramic fuel cells based on lanthanum strontium vanadate. Int. J. Hydrog. Energy 2014, 39, 16534–16540. [Google Scholar] [CrossRef]
- Lapina, A.; Chatzichristodoulou, C.; Holtappels, P.; Mogensen, M. Composite Fe-BaCe0.2Zr0.6Y0.2O2.9 Anodes for Proton Conductor Fuel Cells. J. Electrochem. Soc. 2014, 161, F833–F837. [Google Scholar] [CrossRef]
- Miyazaki, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Development of Ni–Ba(Zr,Y)O3 cermet anodes for direct ammonia-fueled solid oxide fuel cells. J. Power Sources 2017, 365, 148–154. [Google Scholar] [CrossRef]
- Rioja-Monllor, L. In Situ Exsolution Synthesis of Composite Cathodes for Protonic Ceramic Fuel Cells. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2018. [Google Scholar]
- Lee, K.T.; Manthiram, A. Comparison of Ln0.6Sr0.4CoO3−δ (Ln = La, Pr, Nd, Sm, and Gd) as Cathode Materials for Intermediate Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2006, 153, A794–A798. [Google Scholar] [CrossRef]
- Taguchi, H.; Komatsu, T.; Chiba, R.; Nozawa, K.; Orui, H.; Arai, H. Characterization of LaNixCoyFe1−x−yO3 as a cathode material for solid oxide fuel cells. Solid State Ion. 2011, 182, 127–132. [Google Scholar] [CrossRef]
- Tietz, F.; Arul Raj, I.; Zahid, M.; Stöver, D. Electrical conductivity and thermal expansion of La0.8Sr0.2(Mn,Fe,Co)O3−δ perovskites. Solid State Ion. 2006, 177, 1753–1756. [Google Scholar] [CrossRef]
- Petric, A.; Huang, P.; Tietz, F. Evaluation of La–Sr–Co–Fe–O perovskites for solid oxide fuel cells and gas separation membranes. Solid State Ion. 2000, 135, 719–725. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1−xSrxCo1−yFeyO3. Part 1. The system La0.8Sr0.2Co1−yFeyO3. Solid State Ion. 1995, 76, 259–271. [Google Scholar] [CrossRef]
- Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature Solid Oxide Fuel Cells: A brief review. J. Power Sources 2015, 298, 46–67. [Google Scholar] [CrossRef]
- Rath, M.K.; Lee, K.T. Investigation of aliovalent transition metal doped La0.7Ca0.3Cr0.8X0.2O3−δ (X = Ti, Mn, Fe, Co, and Ni) as electrode materials for symmetric solid oxide fuel cells. Ceram. Int. 2015, 41, 10878–10890. [Google Scholar] [CrossRef]
- Wei, B.; Lü, Z.; Jia, D.; Huang, X.; Zhang, Y.; Su, W. Thermal expansion and electrochemical properties of Ni-doped GdBaCo2O5+δ double-perovskite type oxides. Int. J. Hydrog. Energy 2010, 35, 3775–3782. [Google Scholar] [CrossRef]
- Kharton, V.; Naumovich, E.; Kovalevsky, A.; Viskup, A.; Figueiredo, F.; Bashmakov, I.; Marques, F.M. Mixed electronic and ionic conductivity of LaCo(M)O3 (M = Ga, Cr, Fe or Ni): IV. Effect of preparation method on oxygen transport in LaCoO3−δ. Solid State Ion. 2000, 138, 135–148. [Google Scholar] [CrossRef]
- Radaelli, P.G.; Cheong, S.W. Structural phenomena associated with the spin-state transition in LaCoO3. Phys. Rev. B 2002, 66, 094408. [Google Scholar] [CrossRef]
- Zobel, C.; Kriener, M.; Bruns, D.; Baier, J.; Grüninger, M.; Lorenz, T.; Reutler, P.; Revcolevschi, A. Evidence for a low-spin to intermediate-spin state transition in (formula presented). Phys. Rev. B Condens. Matter Mater. Phys. 2002, 66, 1–4. [Google Scholar] [CrossRef]
- Ullmann, H.; Trofimenko, N.; Tietz, F.; Stöver, D.; Ahmad-Khanlou, A. Correlation between thermal expansion and oxide ion transport in mixed conducting perovskite-type oxides for SOFC cathodes. Solid State Ion. 2000, 138, 79–90. [Google Scholar] [CrossRef]
- Thommy, L.; Joubert, O.; Hamon, J.; Caldes, M.T. Impregnation versus exsolution: Using metal catalysts to improve electrocatalytic properties of LSCM-based anodes operating at 600 °C. Int. J. Hydrog. Energy 2016, 41, 14207–14216. [Google Scholar] [CrossRef]
- Jiang, Z.; Xia, C.; Chen, F. Nano-structured composite cathodes for intermediate-temperature solid oxide fuel cells via an infiltration/impregnation technique. Electrochim. Acta 2010, 55, 3595–3605. [Google Scholar] [CrossRef]
- Li, G.; He, B.; Ling, Y.; Xu, J.; Zhao, L. Highly active YSB infiltrated LSCF cathode for proton conducting solid oxide fuel cells. Int. J. Hydrog. Energy 2015, 40, 13576–13582. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. LnBaCo2O5+δ Oxides as Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2008, 155, B385–B390. [Google Scholar] [CrossRef]
- Riza, F.; Ftikos, C.; Tietz, F.; Fischer, W. Preparation and characterization of Ln0.8Sr0.2Fe0.8Co0.2O3−δ (Ln = La, Pr, Nd, Sm, Eu, Gd). J. Eur. Ceram. Soc. 2001, 21, 1769–1773. [Google Scholar] [CrossRef]
- Klyndyuk, A.I. Thermal and chemical expansion of LnBaCuFeO5+δ (Ln = La, Pr, Gd) ferrocuprates and LaBa0.75Sr0.25CuFeO5+δ solid solution. Russ. J. Inorg. Chem. 2007, 52, 1343–1349. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A. Properties of perovskite-like phases LnBaCuFeO5+δ (Ln = La, Pr). Glass Phys. Chem. 2008, 34, 313–318. [Google Scholar] [CrossRef]
- Jin, F.; Xu, H.; Long, W.; Shen, Y.; He, T. Characterization and evaluation of double perovskites LnBaCoFeO5+δ (Ln = Pr and Nd) as intermediate-temperature solid oxide fuel cell cathodes. J. Power Sources 2013, 243, 10–18. [Google Scholar] [CrossRef]
- Che, X.; Shen, Y.; Li, H.; He, T. Assessment of LnBaCo1.6Ni0.4O5+δ (Ln = Pr, Nd, and Sm) double-perovskites as cathodes for intermediate-temperature solid-oxide fuel cells. J. Power Sources 2013, 222, 288–293. [Google Scholar] [CrossRef]
- Mori, M.; Hiei, Y.; Sammes, N.M.; Tompsett, G.A. Thermal-Expansion Behaviors and Mechanisms for Ca- or Sr-Doped Lanthanum Manganite Perovskites under Oxidizing Atmospheres. J. Electrochem. Soc. 2000, 147, 1295–1302. [Google Scholar] [CrossRef]
- Shao, Z.; Yang, W.; Cong, Y.; Dong, H.; Tong, J.; Xiong, G. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen membrane. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- Shao, Z.; Dong, H.; Xiong, G.; Cong, Y.; Yang, W. Performance of a mixed-conducting ceramic membrane reactor with high oxygen permeability for methane conversion. J. Membr. Sci. 2001, 183, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 2004, 431, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ran, R.; Zheng, Y.; Shao, Z.; Jin, W.; Xu, N.; Ahn, J. Evaluation of Ba0.5Sr0.5Co0.8Fe0.2O3−δ as a potential cathode for an anode-supported proton-conducting solid-oxide fuel cell. J. Power Sources 2008, 180, 15–22. [Google Scholar] [CrossRef]
- Patra, H.; Rout, S.K.; Pratihar, S.K.; Bhattacharya, S. Thermal, electrical and electrochemical characteristics of Ba1−xSrxCo0.8Fe0.2O3−δ cathode material for intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2011, 36, 11904–11913. [Google Scholar] [CrossRef]
- Wei, B.; Lü, Z.; Huang, X.; Miao, J.; Sha, X.; Xin, X.; Su, W. Crystal structure, thermal expansion and electrical conductivity of perovskite oxides BaxSr1−xCo0.8Fe0.2O3−δ (0.3 ≤ x ≤ 0.7). J. Eur. Ceram. Soc. 2006, 26, 2827–2832. [Google Scholar] [CrossRef]
- McIntosh, S.; Vente, J.F.; Haije, W.G.; Blank, D.H.A.; Bouwmeester, H.J.M. Oxygen Stoichiometry and Chemical Expansion of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Measured by in Situ Neutron Diffraction. Chem. Mater. 2006, 18, 2187–2193. [Google Scholar] [CrossRef]
- Kriegel, R.; Kircheisen, R.; Töpfer, J. Oxygen stoichiometry and expansion behavior of Ba0.5Sr0.5Co0.8Fe0.2O3−δ. Solid State Ion. 2010, 181, 64–70. [Google Scholar] [CrossRef]
- Zhu, Q.; Jin, T.; Wang, Y. Thermal expansion behavior and chemical compatibility of BaxSr1−xCo1−yFeyO3−δ with 8YSZ and 20GDC. Solid State Ion. 2006, 177, 1199–1204. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Feldhoff, A.; Caro, J. Investigation of phase structure, sintering, and permeability of perovskite-type Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. J. Membr. Sci. 2005, 262, 20–26. [Google Scholar] [CrossRef]
- Hwang, H.J.; Moon, J.W.; Lee, S.; Lee, E.A. Electrochemical performance of LSCF-based composite cathodes for intermediate temperature SOFCs. J. Power Sources 2005, 145, 243–248. [Google Scholar] [CrossRef]
- Richter, J.; Holtappels, P.; Graule, T.; Nakamura, T.; Gauckler, L.J. Materials design for perovskite SOFC cathodes. Monatshefte für Chemie 2009, 140, 985–999. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1−xSrxCo1−yFeyO3. Part 2. The system La1−xSrxCo0.2Fe0.8O3. Solid State Ion. 1995, 76, 273–283. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, D.; Zhang, F.; Chen, W.; Chen, M.; Liu, H. Structure, electrical conducting and thermal expansion properties of La0.6Sr0.4Co0.8Fe0.2O3−δ–Ce0.8Sm0.2O2−δ composite cathodes. J. Alloys Compd. 2008, 454, 460–465. [Google Scholar] [CrossRef]
- Fan, B.; Yan, J.; Yan, X. The ionic conductivity, thermal expansion behavior, and chemical compatibility of La0.54Sr0.44Co0.2Fe0.8O3−δ as SOFC cathode material. Solid State Sci. 2011, 13, 1835–1839. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.H.; Lee, K.S.; Yu, J.H.; Seong, Y.H.; Han, I.S. Mechanical properties of LSCF (La0.6Sr0.4Co0.2Fe0.8O3−δ)–GDC (Ce0.9Gd0.1O2−δ) for oxygen transport membranes. Ceram. Int. 2017, 43, 1916–1921. [Google Scholar] [CrossRef]
- Kostogloudis, G.C.; Ftikos, C. Properties of A-site-deficient La0.6Sr0.4Co0.2Fe0.8O3−δ-based perovskite oxides. Solid State Ion. 1999, 126, 143–151. [Google Scholar] [CrossRef]
- Kharton, V.V.; Yaremchenko, A.A.; Patrakeev, M.V.; Naumovich, E.N.; Marques, F.M.B. Thermal and chemical induced expansion of La0.3Sr0.7(Fe,Ga)O3−δ ceramics. J. Eur. Ceram. Soc. 2003, 23, 1417–1426. [Google Scholar] [CrossRef]
- Wang, S.; Katsuki, M.; Dokiya, M.; Hashimoto, T. High temperature properties of La0.6Sr0.4Co0.8Fe0.2O3−δ phase structure and electrical conductivity. Solid State Ion. 2003, 159, 71–78. [Google Scholar] [CrossRef]
- Duan, C.; Hook, D.; Chen, Y.; Tong, J.; O’Hayre, R. Zr and Y co-doped perovskite as a stable, high performance cathode for solid oxide fuel cells operating below 500 °C. Energy Environ. Sci. 2017, 10, 176–182. [Google Scholar] [CrossRef]
- Wang, F.; Yan, D.; Zhang, W.; Chi, B.; Pu, J.; Jian, L. LaCo0.6Ni0.4O3−δ as cathode contact material for intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2013, 38, 646–651. [Google Scholar] [CrossRef]
- Kharton, V.; Viskup, A.P.; Bochkoc, D.M.; Naumovich, E.N.; Reut, O.P. Mixed electronic and ionic conductivity of LaCo(M)O3 (M = Ga, Cr, Fe or Ni) III. Diffusion of oxygen through LaCo1−x−yFexNiyO3 ceramics. Solid State Ion. 1998, 110, 61–68. [Google Scholar] [CrossRef]
- Chiba, R.; Yoshimura, F.; Sakurai, Y. An investigation of LaNi1−xFexO3 as a cathode material for solid oxide fuel cells. Solid State Ion. 1999, 124, 281–288. [Google Scholar] [CrossRef]
- Grande, T.; Tolchard, J.R.; Selbach, S.M. Anisotropic Thermal and Chemical Expansion in Sr-Substituted LaMnO3+δ: Implications for Chemical Strain Relaxation. Chem. Mater. 2012, 24, 338–345. [Google Scholar] [CrossRef]
- Huang, Y.; Dass, R.; Xing, Z.; Goodenough, J. Double perovskites as anode materials for solid-oxide fuel cells. Science 2006, 312, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, Q.; Huang, Y.H.; Goodenough, J.B. Cobalt-based double-perovskite symmetrical electrodes with low thermal expansion for solid oxidefuel cells. J. Mater. Chem. 2012, 22, 225–231. [Google Scholar] [CrossRef]
- Cherepanov, V.A.; Aksenova, T.V.; Gavrilova, L.Y.; Mikhaleva, K.N. Structure, nonstoichiometry and thermal expansion of the NdBa(Co,Fe)2O5+δ layered perovskite. Solid State Ion. 2011, 188, 53–57. [Google Scholar] [CrossRef]
- West, M.; Manthiram, A. Layered LnBa1−xSrxCoCuO5+δ (Ln = Nd and Gd) perovskite cathodes for intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2013, 38, 3364–3372. [Google Scholar] [CrossRef]
- Kim, J.H.; Irvine, J.T.S. Characterization of layered perovskite oxides NdBa1−xSrxCo2O5+δ (x = 0 and 0.5) as cathode materials for IT-SOFC. Int. J. Hydrog. Energy 2012, 37, 5920–5929. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Xu, H.; Xu, Q.; Jiang, L.; Shi, Y.; Zhang, Q. Scandium-doped PrBaCo2−xScxO6−δ oxides as cathode material for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2013, 38, 12035–12042. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Park, S.; Kim, C.; Shin, J.; Kim, G. Effect of Mn on the electrochemical properties of a layered perovskite NdBa0.5Sr0.5Co2−xMnxO5+δ (x = 0, 0.25, and 0.5) for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2013, 112, 712–718. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Lü, S.; Meng, X.; Zhang, Y.; Yu, B.; Li, X.; Sui, Y.; Yang, J.; Fu, C.; et al. Synthesis and characterization of SmSrCo2−xMnxO5+δ (x = 0.0, 0.2, 0.4, 0.6, 0.8, 1.0) cathode materials for intermediate-temperature solid-oxide fuel cells. Ceram. Int. 2014, 40, 11343–11350. [Google Scholar] [CrossRef]
- Phillipps, M.B.; Sammes, N.M.; Yamamoto, O. Gd1−xAxCo1−yMnyO3 (A = Sr, Ca) as a cathode for the SOFC. Solid State Ion. 1999, 123, 131–138. [Google Scholar] [CrossRef]
- Liu, L.; Guo, R.; Wang, S.; Yang, Y.; Yin, D. Synthesis and characterization of PrBa0.5Sr0.5Co2−xNixO5+δ (x = 0.1, 0.2 and 0.3) cathodes for intermediate temperature SOFCs. Ceram. Int. 2014, 40, 16393–16398. [Google Scholar] [CrossRef]
- Jo, S.H.; Muralidharan, P.; Kim, D.K. Enhancement of electrochemical performance and thermal compatibility of GdBaCo2/3Fe2/3Cu2/3O5+δ cathode on Ce1.9Gd0.1O1.95 electrolyte for IT-SOFCs. Electrochem. Commun. 2009, 11, 2085–2088. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Lü, S.; Meng, X.; Zhao, X.; Ji, Y.; Wang, Y.; Fu, C.; Liu, X.; Li, X.; et al. Effect of Cu doping on YBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2014, 134, 107–115. [Google Scholar] [CrossRef]
- Jin, F.; Shen, Y.; Wang, R.; He, T. Double-perovskite PrBaCo2/3Fe2/3Cu2/3O5+δ as cathode material for intermediate-temperature solid-oxide fuel cells. J. Power Sources 2013, 234, 244–251. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, T.; Zeng, R.; Zhang, W.X.; Huang, Y.H. Thermal and electrochemical properties of PrBa0.5Sr0.5Co2−xFexO5+δ (x = 0.5, 1.0, 1.5) cathode materials for solid-oxide fuel cells. J. Power Sources 2013, 232, 279–285. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, J.; He, B.; Chen, F.; Xia, C. Synthesis, characterization and evaluation of PrBaCo2−xFexO5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2011, 36, 3658–3665. [Google Scholar] [CrossRef]
- Ni, D.W.; Charlas, B.; Kwok, K.; Molla, T.T.; Hendriksen, P.V.; Frandsen, H.L. Influence of temperature and atmosphere on the strength and elastic modulus of solid oxide fuel cell anode supports. J. Power Sources 2016, 311, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.N.; Kim, J.H.; Manthiram, A. Effect of Fe substitution on the structure and properties of LnBaCo2−xFexO5+δ (Ln = Nd and Gd) cathodes. J. Power Sources 2010, 195, 6411–6419. [Google Scholar] [CrossRef]
- Volkova, N.E.; Gavrilova, L.Y.; Cherepanov, V.A.; Aksenova, T.V.; Kolotygin, V.A.; Kharton, V.V. Synthesis, crystal structure and properties of SmBaCo2−xFexO5+δ. J. Solid State Chem. 2013, 204, 219–223. [Google Scholar] [CrossRef]
- Xue, J.; Shen, Y.; He, T. Double-perovskites YBaCo2−xFexO5+δ cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2011, 196, 3729–3735. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, W.C.J.; Guo, Y.; Jia, D. LaSrMnCoO5+δ as cathode for intermediate-temperature solid oxide fuel cells. Electrochem. Commun. 2012, 19, 36–38. [Google Scholar] [CrossRef]
- Aksenova, T.V.; Gavrilova, L.Y.; Yaremchenko, A.A.; Cherepanov, V.A.; Kharton, V.V. Oxygen nonstoichiometry, thermal expansion and high-temperature electrical properties of layered NdBaCo2O5+δ and SmBaCo2O5+δ. Mater. Res. Bull. 2010, 45, 1288–1292. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, Y.; Yang, C.; Shen, Y.; Du, Z.; Świerczek, K. Electrochemical performance of Pr1−xYxBaCo2O5+δ layered perovskites as cathode materials for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2013, 38, 16365–16372. [Google Scholar] [CrossRef]
- Zhao, L.; Nian, Q.; He, B.; Lin, B.; Ding, H.; Wang, S.; Peng, R.; Meng, G.; Liu, X. Novel layered perovskite oxide PrBaCuCoO5+δ as a potential cathode for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 453–456. [Google Scholar] [CrossRef]
- Mogni, L.; Prado, F.; Jiménez, C.; Caneiro, A. Oxygen order-disorder phase transition in layered GdBaCo2O5+δ perovskite: Thermodynamic and transport properties. Solid State Ion. 2013, 240, 19–28. [Google Scholar] [CrossRef]
- Kuroda, C.; Zheng, K.; Świerczek, K. Characterization of novel GdBa0.5Sr0.5Co2−xFexO5+δ perovskites for application in IT-SOFC cells. Int. J. Hydrog. Energy 2013, 38, 1027–1038. [Google Scholar] [CrossRef]
- Tarancón, A.; Burriel, M.; Santiso, J.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799–3813. [Google Scholar] [CrossRef]
- Hu, Y.; Bouffanais, Y.; Almar, L.; Morata, A.; Tarancon, A.; Dezanneau, G. La2−xSrxCoO4−δ (x = 0.9, 1.0, 1.1) Ruddlesden-Popper-type layered cobaltites as cathode materials for IT-SOFC application. Int. J. Hydrog. Energy 2013, 38, 3064–3072. [Google Scholar] [CrossRef]
- Prado, F.; Mogni, L.; Cuello, G.J.; Caneiro, A. Neutron powder diffraction study at high temperature of the Ruddlesden-Popper phase Sr3Fe2O6+δ. Solid State Ion. 2007, 178, 77–82. [Google Scholar] [CrossRef]
- Flura, A.; Dru, S.; Nicollet, C.; Vibhu, V.; Fourcade, S.; Lebraud, E.; Rougier, A.; Bassat, J.M.; Grenier, J.C. Chemical and structural changes in Ln2NiO4+δ (Ln = La, Pr or Nd) lanthanide nickelates as a function of oxygen partial pressure at high temperature. J. Solid State Chem. 2015, 228, 189–198. [Google Scholar] [CrossRef]
- Amow, G.; Skinner, S.J. Recent developments in Ruddlesden-Popper nickelate systems for solid oxide fuel cell cathodes. J. Solid State Electrochem. 2006, 10, 538–546. [Google Scholar] [CrossRef]
- Amow, G.; Davidson, I.J.; Skinner, S.J. A comparative study of the Ruddlesden-Popper series, Lan+1NinO3n+1 (n = 1, 2 and 3), for solid-oxide fuel-cell cathode applications. Solid State Ion. 2006, 177, 1205–1210. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen diffusion and surface exchange in La2−xSrxNiO4+δ. Solid State Ion. 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Steil, M.C.; Dordor, P.; Mauvy, F.; Grenier, J.C. Oxygen transport properties of La2Ni1−xCuxO4+δ mixed conducting oxides. Solid State Sci. 2003, 5, 973–981. [Google Scholar] [CrossRef]
- Vibhu, V.; Rougier, A.; Nicollet, C.; Flura, A.; Grenier, J.C.; Bassat, J.M. La2−xPrxNiO4+δ as suitable cathodes for metal supported SOFCs. Solid State Ion. 2015, 278, 32–37. [Google Scholar] [CrossRef]
- Kharton, V.V.; Kovalevsky, A.V.; Avdeev, M.; Tsipis, E.V.; Patrakeev, M.V.; Yaremchenko, A.A.; Naumovich, E.N.; Frade, J.R. Chemically induced expansion of La2NiO4+δ-based materials. Chem. Mater. 2007, 19, 2027–2033. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2−xNiO4+δ oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Jang, Y.H.; Bae, J. Fabrication of solid oxide fuel cells (SOFCs) by solvent-controlled co-tape casting technique. Int. J. Hydrog. Energy 2017, 42, 1648–1660. [Google Scholar] [CrossRef]
- Dailly, J.; Ancelin, M.; Marrony, M. Long term testing of BCZY-based protonic ceramic fuel cell PCFC: Micro-generation profile and reversible production of hydrogen and electricity. Solid State Ion. 2017, 306, 69–75. [Google Scholar] [CrossRef]
- Mahata, T.; Nair, S.R.; Lenka, R.K.; Sinha, P.K. Fabrication of Ni-YSZ anode supported tubular SOFC through iso-pressing and co-firing route. Int. J. Hydrog. Energy 2012, 37, 3874–3882. [Google Scholar] [CrossRef]
- Ramousse, S.; Menon, M.; Brodersen, K.; Knudsen, J.; Rahbek, U.; Larsen, P.H. Manufacturing of anode-supported SOFC’s: Processing parameters and their influence. ECS Trans. 2007, 7, 317–327. [Google Scholar] [CrossRef]
- Majumdar, S.; Claar, T.; Flandermeyer, B. Stress and Fracture Behavior of Monolithic Fuel Cell Tapes. J. Am. Ceram. Soc. 1986, 69, 628–633. [Google Scholar] [CrossRef]
- Prakash, B.S.; Kumar, S.S.; Aruna, S.T. Properties and development of Ni/YSZ as an anode material in solid oxide fuel cell: A review. Renew. Sustain. Energy Rev. 2014, 36, 149–179. [Google Scholar] [CrossRef]
- Nasani, N.; Wang, Z.J.; Willinger, M.G.; Yaremchenko, A.A.; Fagg, D.P. In-situ redox cycling behaviour of NieBaZr0.85Y0.15O3−δ cermet anodes for Protonic Ceramic Fuel Cells. Int. J. Hydrog. Energy 2014, 39, 19780–19788. [Google Scholar] [CrossRef]
- Onishi, T.; Han, D.; Noda, Y.; Hatada, N.; Majima, M.; Uda, T. Evaluation of performance and durability of Ni-BZY cermet electrodes with BZY electrolyte. Solid State Ion. 2018, 317, 127–135. [Google Scholar] [CrossRef]
- Sažinas, R.; Einarsrud, M.A.; Grande, T. Toughening of Y-doped BaZrO3 proton conducting electrolytes by hydration. J. Mater. Chem. A 2017, 5, 5846–5857. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C. A durability model for solid oxide fuel cell electrodes in thermal cycle processes. J. Power Sources 2010, 195, 6611–6618. [Google Scholar] [CrossRef]
- Lee, K.T.; Vito, N.J.; Wachsman, E.D. Comprehensive quantification of Ni-Gd0.1Ce0.9O1.95 anode functional layer microstructures by three-dimensional reconstruction using a FIB/SEM dual beam system. J. Power Sources 2013, 228, 220–228. [Google Scholar] [CrossRef]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.Z.; Deevi, S.C. A review on the status of anode materials for solid oxide fuel cells. Mater. Sci. Eng. A 2003, 362, 228–239. [Google Scholar] [CrossRef]
- Lee, S.; Park, I.; Lee, H.; Shin, D. Continuously gradient anode functional layer for BCZY based proton-conducting fuel cells. Int. J. Hydrog. Energy 2014, 39, 14342–14348. [Google Scholar] [CrossRef]
- Anandakumar, G.; Li, N.; Verma, A.; Singh, P.; Kim, J.H. Thermal stress and probability of failure analyses of functionally graded solid oxide fuel cells. J. Power Sources 2010, 195, 6659–6670. [Google Scholar] [CrossRef]
- Dippon, M.; Babiniec, S.M.; Ding, H.; Ricote, S.; Sullivan, N.P. Exploring electronic conduction through BaCexZr0.9−xY0.1O3-d proton-conducting ceramics. Solid State Ion. 2016, 286. [Google Scholar] [CrossRef]
- Biswas, S.; Nithyanantham, T.; Nambiappan Thangavel, S.; Bandopadhyay, S. High-temperature mechanical properties of reduced NiO–8YSZ anode-supported bi-layer SOFC structures in ambient air and reducing environments. Ceram. Int. 2013, 39, 3103–3111. [Google Scholar] [CrossRef]
- Patki, N.S.; Manerbino, A.; Way, J.D.; Ricote, S. Galvanic hydrogen pumping performance of copper electrodes fabricated by electroless plating on a BaZr0.9−xCexY0.1O3−δ proton-conducting ceramic membrane. Solid State Ion. 2018, 317, 256–262. [Google Scholar] [CrossRef]
- Stange, M.; Stefan, E.; Denonville, C.; Larring, Y.; Rørvik, P.M.; Haugsrud, R. Development of novel metal-supported proton ceramic electrolyser cell with thin film BZY15–Ni electrode and BZY15 electrolyte. Int. J. Hydrog. Energy 2017, 42, 13454–13462. [Google Scholar] [CrossRef]
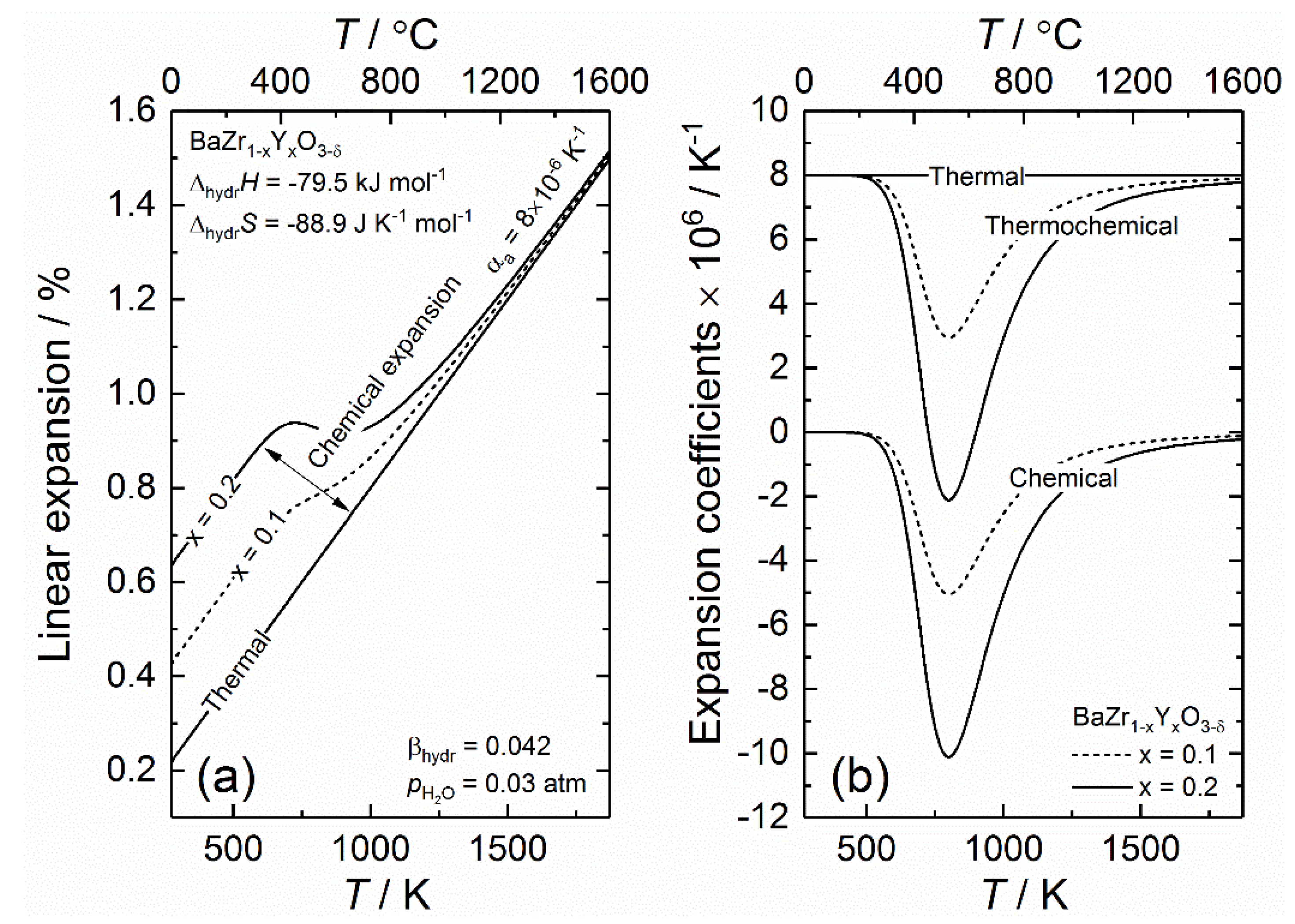










| Cooling Rate K∙min−1 | Temperature Range °C | <α> 10−6 K−1 |
|---|---|---|
| 1 | 750–1000 | 9.3(2) |
| 3 | 750–980 | 9.3(2) |
| 5 | 750–960 | 9.3(2) |
| 10 | 750–935 | 9.2(2) |
| 1 | 100–750 | 8.5(3) |
| 3 | 110–750 | 8.8(3) |
| 5 | 120–750 | 8.7(2) |
| 10 | 170–750 | 8.5(2) |
| Compounds | <α> (10−6 K−1) | Comments and References |
|---|---|---|
| Undoped and Y-doped BaZrO3 | ||
| BaZrO3 | 4.7 1) 6.87 2) 7.13 3) 7.5(2) 4) 8.02 5) 7.89 6) 8.65 7) | 1) Neutron scattering, 100–300 K, gas composition not specified [145] 2) HT-XRD, estimated by αV/3 (αV = 20.6 × 10−6 K−1), RT–600 °C, gas composition not specified [118] 3) Dilatometry, RT–1000 °C, reducing conditions—not specified [146] 4) HT-XRD, 298–1675 K, air [147] 5) HT-XRD, 30–1000 °C, dry Ar [78] 6) Dilatometry, temperature range used for <α> not clear, sample made by citrate method [148] 7) Dilatometry, temperature range used for <α> not clear, sample made by solid state synthesis [148] |
| BaZr0.98Y0.02O3−δ | 8.47 1) | 1) HT-XRD, 30–1000 °C, dry Ar [78] |
| BaZr0.95Y0.05O3−δ | 8.62 1) 8.75 2) | 1) HT-XRD, 30–1000 °C, dry Ar [78] 2) HT-XRD, 373–1123 K, vacuum conditions (0.1 mbar) [77] |
| BaZr0.9Y0.1O3−δ | 6.19 1) 8.45 2) 8.78 3) 8.80 4) 13 5) | 1) HT-ND, 300–773 K, dry N2 [149] 2) HT-XRD, 650–1000 °C, dry 4% H2 in Ar [119] 3) HT-XRD, 30–1000 °C, dry Ar [78] 4) HT-XRD, 373–1123 K, vacuum conditions (0.1 mbar) [77] 5) Dilatometry, 30–1000 °C, air [150] |
| BaZr0.85Y0.15O3−δ | 8 1) 8.7 2) 9.25 3) | 1) Dilatometry, dry and wet ( = 0.023 bar) give same result [80] 2) Dilatometry, Estimate made from thermal expansion LT (RT–320 °C), air [120] 3) HT-XRD, 30–1000 °C, dry Ar [78] |
| BaZr0.8Y0.2O3−δ | 8.2 1) 9.65 2) 10.1 3) | 1) HT-XRD, 100–900 °C, air [151] 2) HT-XRD, 373–1123 K, vacuum conditions (0.1 mbar) [77] 3) HT-XRD, 30–1000 °C, dry Ar [78] |
| BaZr0.75Y0.25O3−δ | 10.2 1) | 1) HT-XRD, 30–1000 °C, dry Ar [78] |
| BaZr0.7Y0.3O3−δ | 10.0 1) | 1) HT-XRD, 30–1000 °C, dry Ar [78] |
| Acceptor doped BaZrO3 (other elements than Y) | ||
| BaZr0.8Sc0.2O3−δ | 9.4 1) | 1) HT-XRD, Estimate made for lattice expansion at HT (600–1000 °C) under dry conditions [75] |
| BaZr0.8Sm0.2O3−δ | 9.2 1) | 1) HT-XRD, Estimate made for lattice expansion at HT (600–1000 °C) under dry conditions [75] |
| BaZr0.8Eu0.2O3−δ | 9.9 1) | 1) HT-XRD, Estimate made for lattice expansion at HT (600–1000 °C) under dry conditions [75] |
| BaZr0.8Dy0.2O3−δ | 10.5 1) | 1) HT-XRD, Estimate made for lattice expansion at HT (600–1000 °C) under dry conditions [75] |
| Undoped and acceptor doped BaCeO3 | ||
| BaCeO3 | 11.2 1) 12.2 2) | 1) Dilatometry, RT–1000 °C, reducing conditions—not specified [146] 2) DFT, QHA, 300 K, conditions not applicable [86] |
| BaCe0.8Y0.2O3−δ | 11.6 1) 9.94 2) 10.5 3) 11.6 4) | 1) HT-XRD, 100–900 °C, air [151] 2) HT-XRD, 373–1123 K, vacuum conditions (0.1 mbar) [77] 3) HT-XRD, RT–1000 °C, dry O2, estimate made based on changes in pseudo-cubic volume [152] 4) HT-ND, 30–800 °C, air, estimate made based on changes in pseudo-cubic volume [153] |
| Y:BaZrO3-BaCeO3 solid solutions (in order of increasing Zr-content) | ||
| BaZr0.1Ce0.7Y0.2O3−δ | 11.3 1) 12.1 2) | 1) HT-XRD, 600–900 °C, air [151] 2) Dilatometry, 50–900 °C, air, <α> extracted for 50–600 °C where the expansion is linear [154] |
| BaZr0.1Ce0.7Y0.1Yb0.1O3−δ | 9.07 1) 11.60 2) | 1) Dilatometry, 720–1100 °C, Ar, not indicated [155] 2) Dilatometry, 800–1100 °C, air, not indicated [155] |
| BaZr0.2Ce0.6Y0.2O3−δ | 11.3 1) | 1) HT-XRD, 600–900 °C, air [151] |
| BaZr0.3Ce0.5Y0.2O3−δ | 10.8 1) | 1) HT-XRD, 600–900 °C, air [151] |
| BaZr0.4Ce0.4Y0.2O3−δ | 10.9 1) | 1) HT-XRD, 600–900 °C, air [151] |
| BaZr0.5Ce0.3Y0.2O3−δ | 9.3 1) | 1) HT-XRD, 600–900 °C, air [151] |
| BaZr0.6Ce0.2Y0.2O3−δ | 9.1 1) | 1) HT-XRD, 600–900 °C, air [151] |
| BaZr0.7Ce0.1Y0.2O3−δ | 8.4 1) | 1) HT-XRD, 600–900 °C, air [151] |
| BaZr0.7Ce0.2Y0.1O3−δ | 12.7 1) 10.2 2) 9.3 3) | 1) HT-ND, pre-hydrated sample, estimated to be 0.001 atm based on thermodynamic modelling [126] 2) HT-XRD, 650–1000 °C, dry 4% H2 in Ar [119] 3) Dilatometry, this work, dry Ar, see Section 3.6 for more details |
| BaZr0.8075Ce0.0425Y0.15O3−δ | 8 1) | 1) Dilatometry, dry and wet ( = 0.023 bar) give same result [80] |
| Compounds | Conductivity at 600 °C (10−3 S cm−1) | <α> (10−6 K−1) | Comments and References |
|---|---|---|---|
| Ca- and Sr-based perovskites | |||
| CaZrO3 | 0.3 in air 1) | 8.02 + 0.005 × T 2) 8.92 + 0.003 × T 3) T: temperature | 1) for 3 mol% Sc-doped specimen [156] 2) Dilatometry, dry ( = 0.0004 atm) air, nonlinear thermal expansion [156] 3) Dilatometry, moist ( = 0.025 atm) air, nonlinear thermal expansion [156] |
| SrZrO3 | 0.8 in H2 1) | 9.69 2) | 1) for 5 mol% Y-doped specimen [157,158] 2) Dilatometry, gas conditions not specified, undoped sample [159] |
| SrCeO3 | 2 in H2 1) | 11.1 2) | 1) for 5 mol% Yb-doped specimen [27,160] 2) Dilatometry and HT-XRD under reducing conditions and helium, respectively, undoped sample [161] |
| SrMoO3 | N.A. | 7.98 1) | 1) Dilatometry and HT-XRD under reducing conditions and helium, respectively, undoped sample [161] |
| SrHfO3 | N.A. | 11.3 1) | 1) Dilatometry and HT-XRD under reducing conditions and helium, respectively, undoped sample [161] |
| SrRuO3 | N.A. | 10.3 1) | 1) Dilatometry and HT-XRD under reducing conditions and helium, respectively, undoped sample [161] |
| Ba-based perovskites | |||
| BaSnO3 | 0.05 in air 1) | 9.3 2) | 1) tOH ≈ 0.75, doped with 10 mol% Lu, measured at 450 °C [162] 2) Dilatometry, gas composition not specified, undoped sample [163] |
| BaHfO3 | 0.05 in air 1) | 6.93 2) | 1) tOH unknown, doped with 10 mol% Y, measured at 450 °C [164] 2) Dilatometry, gas composition not specified, undoped sample [165] |
| BaThO3 | 6 in Ar 1) | 11.09 2) | 1) tOH ≈ 0.55, doped with 5 mol% Nd [166] 2) Dilatometry, gas composition not specified, undoped sample [167] |
| BaTbO3 | 6 in Ar 1) | 9 2) | 1) tOH not specified, doped with 5 mol% Yb [166] 2) Estimated from HT-XRD data for temperature range 280–800 K, undoped sample [168] |
| BaMoO3 | N.A. | 9.46 1) | 1) Dilatometry and HT-XRD under reducing conditions and helium, respectively, undoped sample [161] |
| BaUO3 | N.A. | 11.0 1) | 1) Dilatometry and HT-XRD under reducing conditions and helium, respectively, undoped sample [161] |
| LaREO3 series | |||
| LaScO3 | 4 in H2 1) | 8 2) | 1) tOH ≈ 1, doped with 10 mol% Sr [169] 2) Estimated from dilatometry plot, gas composition not specified, sample doped with 10 mol% Sr [170] |
| LaYO3 | 0.8 in air 1) | 11.1(1) 2) 11.4(3) 3) | 1) tOH ≈ 1, doped with 10 mol% Sr [171] 2) Dilatometry, dry ( = 0.001 atm) air, sample doped with 10 mol% Sr [172] 3) Dilatometry, moist ( = 0.025 atm) air, sample doped with 10 mol% Sr [172] |
| LaYbO3 | 0.8 in air 1) | 8.95 2) | 1) tOH ≈ 1, doped with 10 mol% Sr [171] 2) Dilatometry, gas composition not specified, undoped sample [173] |
| LaLuO3 | 0.7 in H2 1) | 4.5 2) | 1) tOH ≈ 1, doped with 10 mol% Sr [169] 2) Estimation of αV/3, undoped LaLuO3 single crystal measured by HT XRD camera in temperature range from RT–1000 °C [174] |
| LaInO3 | 3 in air 1) | 6–9 2) | 1) tOH ≈ 0.9, for 10 mol% Sr-doped specimen [171] 2) The range of true coefficient calculated from dilatometry in the temperature range 250–873 K, gas composition not specified, undoped sample [175] |
| Compound | Conductivity at 800 °C (10−3 S cm−1) | Temp. Range (°C) | Structure Type | <α> (10−6 K−1) | Comments and References |
|---|---|---|---|---|---|
| Acceptor doped LaNbO4 (maximum 2–3 mol% dopant) | |||||
| undoped | 0.02 in H2 1) | RT to ~500 | fergusonite | 14 2)/15.3 3) 17.1 4)/17.3 5) | 1) tOH not specified [211] 2) Dilatometry, measured upon cooling, gas conditions not specified [200] 3) Dilatometry, measured upon cooling in moist Ar [103] 4) Expansion of the unit cell measured by ND [103] 5) Expansion of the unit cell measured or HT-XRD [63] |
| ~500–1000 | scheelite | 8.4 2)/8.8 3) 4.2 4)/7.1 5) | |||
| Ca-doped | 0.8 in O2 1) 0.7 in H2 1) | RT to ~500 | fergusonite | 12 2) | 1) tOH ≈ 1; doped with 1 mol% Ca [65] 2) Dilatometry, measured upon cooling, gas conditions not specified, sample doped with 2 mol% Ca [200] |
| ~500–1000 | scheelite | 8.3 2) | |||
| Ba- or Sr-doped | 0.4 in H2 1) | RT to ~500 | fergusonite | 14 2) | 1) tOH ≈ 1; for 2 mol% Sr [211] 2) Dilatometry, averaged for all compositions, measurement upon cooling, gas conditions not specified [200] |
| ~500–1000 | scheelite | 8.6(5) 2) | |||
| Mg-doped | 2 in H2 1) | RT to 490 | fergusonite | 12 2)/17.7 3) | 1) at 720 °C, tOH not specified, for 2 mol% Mg [202] 2) Dilatometry, gas conditions not specified [102] 3) Expansion of the unit cell measured by HT-XRD [102] |
| 490–1000 | scheelite | 8 2)/10.8 3) | |||
| Ti-doped (Nb-site) | 0.1 in N2 and O2 1) | RT to ~500 | fergusonite | 13 2) | 1) tOH not specified, 2–3 mol% Ti [203] 2) Dilatometry, gas conditions not specified [203] |
| ~500–1000 | scheelite | 7 2) | |||
| Acceptor doped LaNb1−xMx O4 (M = V, Ta or Sb)—M and Nb are isovalent | |||||
| LaNb1−xVxO4 | 1) tOH estimated to 0.7–0.9, sample acceptor doped with 2 mol% Sr [220] 2) Dilatometry, gas composition not specified [220] | ||||
| x = 0.3 | 0.1 in O2 1) | RT–1000 | scheelite | 8 2) | |
| LaNb1−xTaxO4 | 1) tOH not specified, acceptor doped with 1 mol% Ca [221] 2) Expansion of the unit cell measured by HT-XRD, sample without acceptor dopants [63] | ||||
| x = 0.2 | 0.3 in Ar 1) | RT–670 | fergusonite | 15.7(3) 2) | |
| 670–1000 | scheelite | 9.1(1) 2) | |||
| x = 0.4 | 0.08 in Ar 1) | RT–800 | fergusonite | 14.0(2) 2) | |
| 800–1000 | scheelite | 11.5(1) 2) | |||
| LaNb1−xSbxO4 | 1) tOH ≈ 1, sample acceptor doped with 2 mol% Ca [214] 2) Dilatometry, dry Ar, measured on sample without acceptors [64] | ||||
| x = 0.1 | 0.3 in air 1) | RT–351 | fergusonite | 16.7(4) 2) | |
| 351–1000 | scheelite | 9.1(7) 2) | |||
| x = 0.3 | 0.08 in air 1) | RT–1000 | scheelite | 8.1(3) 2) | |
| LaNb1−xAsxO4 | |||||
| x = 0.1 | N/A | RT–340 | fergusonite | 13.9(1) 1) | 1) Dilatometry, dry Ar, measured on sample without acceptors [222] |
| 340–1000 | scheelite | 8.6(2) 1) | |||
| x = 0.3 | N/A | RT–1000 | scheelite | 8.3(1) 1) | |
| Compounds | Conductivity at 800 °C (10−3 S cm−1) | Structure Type | <α> (10−6 K−1) | Comments and References |
|---|---|---|---|---|
| Rare earth orthophosphates | ||||
| YPO4 | 0.09 in O2 1) | xenotime | 6.2 2)/5.4 3) | 1) tOH ≈ 1, doped with 3 mol% Ca [199] 2) Dilatometry, gas conditions not specified, undoped sample [226] 3) Expansion of the unit cell measured by HT-XRD [227] |
| LaPO4 | 0.03 in air 1) | monazite | 10.0 2) | 1) tOH ≈ 1, doped with 4 mol% Sr [196] 2) Dilatometry, gas conditions not specified, undoped sample [228] |
| Lanthanide orthoniobates (La is excluded) | ||||
| NdNbO4 | 0.5 in H2 1) | fergusonite | 10.3 2) | 1) tOH ≈ 1, doped with 1 mol% Ca [194] 2) Dilatometry, gas composition not specified, undoped sample [229] |
| ErNbO4 | 0.04 in H2 1) | fergusonite | 12.0 2) | 1) tOH ≈ 1, doped with 1 mol% Ca [194] 2) Expansion of the unit cell measured by HT-XRD, undoped sample [230] |
| Lanthanide orthotantalates | ||||
| LaTaO4 | 0.2 in H2 1) | layered perovskite | 5.33 2) | 1) tOH ≈ 1, doped with 1 mol% Ca [193] 2) Dilatometry, gas composition not specified, undoped sample [231] |
| NdTaO4 | 0.07 in H2 1) | fergusonite | 9.87 2) | 1) tOH ≈ 1, doped with 1 mol% Ca [193] 2) Dilatometry, gas composition not specified, undoped sample [231] |
| GdTaO4 | 0.03 in H2 1) | fergusonite | a 6.17 2) b 12.12 2) c 13.40 2) average 10.6 3) | 1) tOH ≈ 0.7, doped with 1 mol% Ca [193] 2) Dilatometry, gas conditions not specified, measured for an undoped single crystal along a, b and c axis [232] 3) Calculated for comparative reasons |
| Others | ||||
| LaAsO4 | 0.03 in O2 1) | monazite | 7.7 2) | 1) tOH ≈ 1, doped with 1 mol% Sr [197] 2) Calculated from the chemical bond theory of dielectric description, undoped material [233] |
| LaVO4 | 0.3 in O2 1) | monazite | 6.1 2) | 1) tOH ≈ 0.15, doped with 1 mol% Ca, at this temperature predominantly oxygen ion conductor, protonic conductivity dominates below 500 °C [234] 2) Calculated from the chemical bond theory of dielectric description, undoped material [235] |
| Composition | <α> 10−6 K−1 | Reference |
|---|---|---|
| La2Ce2O7 | 10–12 | [243] |
| La2Zr2O7 | 8.7–9.6 | [247] |
| La1.8Nd0.2Zr2O7 La1.6Nd0.4Zr2O7 La1.4Nd0.6Zr2O7 La1.2Nd0.8Zr2O7 | 8.75–9.25 8.25–9.25 9–9.25 9.2–9.75 | [247] [247] [247] [247] |
| La2Zr2O7 | 8.5–9 | [248] |
| Nd2Zr2O7 | 9.2–9.5 | [248] |
| Eu2Zr2O7 | 10.5 | [248] |
| Gd2Zr2O7 | 9–10.5 | [248] |
| La1.4Nd0.6Zr2O7 | 8.5–8.7 | [248] |
| La1.4Eu0.6Zr2O7 | 9–9.2 | [248] |
| La1.4Gd0.6Zr2O7 La1.7Dy0.3Zr2O7 | 9.2–9.3 8.5–9 | [248] [248] |
| Type | Composition | Reference | Application |
|---|---|---|---|
| Single phase | Materials with perovskite structure | ||
| BaCoO3−δ | [150] | PCFC cathode | |
| Ba0.5Sr0.5CoO3−δ | |||
| Ba0.5La0.5CoO3−δ | [150,262] | ||
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | [263,264,265] | ||
| BaCo0.4Fe0.4Zr0.2O3−δ | [266] | ||
| BaCo0.4Fe0.4Zr0.1Y0.1O3−δ | [7] | ||
| BaCo0.4Fe0.4Zr0.2O3−δ | [261] | ||
| BaCexFe1−xO3−δ | [267] | ||
| Ba0.5Sr0.5Fe0.8Zn0.2O3−δ | [268,269] | ||
| BaPr0.8Gd0.2O3−δ | [270] | ||
| BaCe0.4Pr0.4Y0.2O3−δ | [271] | ||
| BaCe0.5Bi0.5O3−δ | [272] | ||
| SrCoO3−δ | [150] | ||
| Sr0.5Sm0.5CoO3−δ | [273] | ||
| Sr0.5La0.5CoO3−δ | [150] | ||
| LaCoO3−δ | |||
| La0.6Sr0.4Co0.2Fe0.8O3−δ | [264,265] | ||
| La0.58Sr0.4Co0.2Fe0.8O3−δ | [96] | ||
| La0.8Sr0.2MnO3−δ | [265] | ||
| La0.6Sr0.4Fe0.8Ni0.2O3−δ | [265,274] | ||
| SrFe0.75Mo0.25O3−δ | [16] | PCFC cathode/PCFC anode | |
| Sr0.5Sm0.5CoO3−δ | [17] | PCEC anode | |
| (La0.75Sr0.25)0.97Cr0.5Mn0.5O3−δ | [275] | ||
| Layered perovskites and perovskite-related structures | |||
| PrBaCo2O6−δ | [264] | PCFC cathode | |
| PrBa0.5Sr0.5Co1.5Fe0.5O6−δ | [26] | ||
| SmBaCo2O6−δ | [276] | ||
| NdBa0.5Sr0.5Co1.5Fe0.5O6−δ | [277] | ||
| GdBaCo2O6−δ | [278,279] | ||
| LaBaCo2O6−δ | [262] | ||
| PrBaCo2O6−δ | [280] | ||
| SmBa0.5Sr0.5Co2O6−δ | [281] | ||
| SmBaCuCoO6−δ | [282] | ||
| PrBaCuFeO6−δ | [283] | ||
| SmBaCuFeO6−δ | [282] | ||
| PrBaCuFeO6−δ | [283] | ||
| BaPrCo2O6−δ | [260] | PCFC cathode/PCEC anode | |
| BaGd0.8La0.2Co2O6−δ | |||
| BaGdCo1.8Fe0.2O6−δ | |||
| BaPrCo1.4Fe0.6O6−δ | |||
| Ruddlesden-Popper structure A2NiO4+δ | |||
| Pr2NiO4+δ | [264,265,284,285,286] | PCFC cathode | |
| La2NiO4+δ | [265,287] | ||
| Nd2NiO4+δ | |||
| LaSrNiO4±δ | [265] | ||
| LaSrCo0.5Fe0.5O4−δ | [288] | ||
| Other structures | |||
| BaGa2O4 | [289] | PCFC cathode | |
| Ba3Co2O6(CO3)0.6 | |||
| YBaCo3.5Zn0.5O7+δ | [290] | ||
| Ba3In1.4Y0.3M0.3ZrO8 (M = Gd3+/Ga3+) | [291] | ||
| Ca3−xLaxCo4O9+δ (x = 0, 0.3) | [292] | ||
| La4BaCu5O13+δ | [293] | ||
| La6.4Sr1.6Cu8O20±δ | |||
| Composite by mixing | Cercer composites | ||
| Ba0.5Sr0.5Co0.5Fe0.5O3−δ /BZCY (3:2) | [294] | PCFC cathode | |
| Sm0.5Sr0.5CoO3−δ/BZCY (7:3) | [295] | ||
| Sm0.5Sr0.5CoO3−δ /Ce0.8Sm0.2O2−δ (6:4) | [296] | ||
| La0.7Sr0.3FeO3−δ /Ce0.8Sm0.2O2−δ | [297] | ||
| SrCe0.9Yb0.1O3–δ or BaCe0.9Yb0.1O3–δ/La0.6Sr0.4Co0.2Fe0.8O3−δ | [298] | ||
| 50% PrBaCo2O6−δ/50% BZCY and 75–25 graded | [299] | ||
| Ba(Zr0.1Ce0.7Y0.2)O3−δ and La0.6Sr0.4Co0.2Fe0.8O3−δ | [271] | ||
| La0.6Sr0.4Co0.2Fe0.8O3−δ/BaZr0.5Pr0.3Y0.2O3−δ | [300] | ||
| Pr0.58Sr0.4Fe0.8Co0.2O3–δ/BaCe0.9Yb0.1O3–δ | [301] | ||
| Sm0.5Sr0.5CoO3−δ/BaCe0.8Sm0.2O3−δ | [257] | ||
| BaZr0.1Ce0.7Y0.1Yb0.1O3−δ/Nd1.95NiO4+δ | [302] | ||
| BaCe0.8Zr0.1Y0.1O3−δ/Sm0.5Sr0.5CoO3−δ | [303] | ||
| Gd0.1Ce0.9O2−δ infiltrated PrBaCo2O6−δ and BaZr0.1Ce0.7Y0.2O3−δ | [304] | ||
| La0.6Sr0.4Co0.2Fe0.8O3−δ/BZCY72 | [305] | PCEC anode | |
| La2NiO4+δ/BZCY72 | |||
| La0.87Sr0.13CrO4/BZCY72 | |||
| La0.8Sr0.2MnO3−δ/BZCY72 | |||
| La0.8Sr0.2MnO3−δ/BaCe0.5Zr0.3Y0.16Zn0.04O3−δ with Fe2O3 nanoparticles | [306] | ||
| Ba2FeMoO6−δ/BaZr0.1Ce0.7Y0.1Yb0.1O3−δ for methane dehydro-aromatization | [307] | Electrodes for membrane reactors | |
| Cermet composites | |||
| 65 wt.% NiO–35 wt.% BZCY72 | [308] | PCFC anode/PCEC cathode | |
| 65 wt.% NiO–35 wt.% BZCY72 | [309] | ||
| BaCe0.8Y0.2O3−δ and BaCe0.6Zr0.2Y0.2O3−δ with NiO 50:50 | [310] | ||
| BZCY72-Ni | [8] | ||
| NiO-BZO and NiO-BZY15 (40 vol% Ni) | [311] | ||
| 65 wt.% NiO–35 wt.% BZCY72 | [28] | ||
| NiO-BZY cermet anodes (40 vol% Ni) | [312] | ||
| 60 wt% NiO + 40 wt% BCGCu with functional layer 45 wt% NiO + 55 wt% BCGCu and BCGCu electrolyte BaCe0.89Gd0.1Cu0.01O3–δ | [313] | ||
| Ba(Zr0.85Yb0.15)O3−δ and NiO the volume ratio of Ni to the total bulk volume in the reduced composite was designed to be around 40 vol% | [314] | ||
| NiO-BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (60:40 wt.%) | [315] | ||
| Composite by infiltration | Cercer composites | ||
| (Pr0.9La0.1)2(Ni0.74Cu0.21Nb0.05)O4+δinfiltrated in BaZr0.1Ce0.7Y0.2O3−δ | [316] | PCFC cathode | |
| La0.58Sr0.4Co0.2Fe0.8O3−δ infiltrated in BZCY72 | [96] | ||
| LaCoO3 infiltrated in BZCY72 | [317] | ||
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ infiltrated in BZCY72 | [305] | PCEC anode | |
| La2NiO4+δ infiltrated in BZCY72 | [318] | ||
| Ba0.5Gd0.8La0.7Co2O6−δ infiltrated in BZCY72 | [319] | ||
| (La0.7Sr0.3)V0.90O3−δ infiltrated in Ba(Ce0.51Zr0.30Y0.15Zn0.04)O3−δ | [320] | PCFC anode/PCEC cathode | |
| Cermet composites | |||
| Fe infiltrated in BaCe0.2Zr0.6Y0.2O2.9 | [321] | PCFC anode/PCEC cathode | |
| Ni infiltrated in BaCe0.9Y0.1O3−δ and BaZr1−xYxO3−δ (x = 0.10, 0.20) | [322] | ||
| Exsolution | Cercer composites | ||
| La0.5Ba0.5Co1/3Mn1/3Fe1/3O3−δ/BaZr1−zYzO3−δ | [323] | PCFC cathode | |
| x | Thermal Expansion Coefficient/10−6 K−1 | Temperature Range | References |
|---|---|---|---|
| 0.3 | 12.4 1) | RT–300 °C | 1) Patra et al. [351] |
| 20.4 2) | 50–1000 °C | 2) Wei et al. [352] | |
| 0.4 | 14.6 1) | RT–300 °C | 1) Patra et al. [351] |
| 20.1 2) | 50–1000 °C | 2) Wei et al. [352] | |
| 19.6 3) | RT–850 °C | 3) Zhu et al. [355] | |
| 0.5 | 14.2 1) | RT–300 °C | 1) Patra et al. [351] |
| 20.0 2) | 50–1000 °C | 2) Wei et al. [352] | |
| 19.0–20.8 3) * | 50–1000 °C | 3) McIntosh et al. [353] | |
| 18.5–24.4 4) * | 600–900 °C | 4) Kriegel et al. [354] | |
| 19.2 5) | RT–1000 °C | 5) Zhu et al. [355] | |
| 11.5 6) | RT–850 °C | 6) Wang et al. [356] | |
| 0.6 | 13.0 1) | RT–300 °C | 1) Patra et al. [351] |
| 20.2 2) | 50–1000 °C | 2) Wei et al. [352] | |
| 19.1 3) | RT–850 °C | 3) Zhu et al. [355] | |
| 0.7 | 15.4 1) | RT–300 °C | 1) Patra et al. [351] |
| 20.3 2) | 50–1000 °C | 2) Wei et al. [352] | |
| 19.7 3) | RT–850 °C | 3) Zhu et al. [355] | |
| 0.8 | 12.4 1) | RT–300 °C | 1) Patra et al. [351] |
| 22.0 2) | RT–850 °C | 2) Zhu et al. [355] | |
| 0.9 | 22.1 1) | RT–850 °C | 1) Zhu et al. [355] |
| 1.0 | 19.6 1) | RT–850 °C | 1) Zhu et al. [355] |
| x | Thermal Expansion Coefficient/10−6 K−1 | Temperature Range | References |
|---|---|---|---|
| 0 | 13.1 1) | 100–500 °C | 1) Tai et al. [359] |
| 0.1 | 16.0 1) | 300–900 °C | 1) Tai et al. [359] |
| 0.2 | 15.4 1) | 100–800 °C | 1) Tai et al. [359] |
| 14.8 2) | 30–1000 °C | 2) Petric et al. [327] | |
| 0.3 | 16.0 1) | 30–1000 °C | 1) Petric et al. [327] |
| 0.4 | 15.3 1) | 100–600 °C | 1) Tai et al. [359] |
| 17.5 2) | 30–1000 °C | 2) Petric et al. [327] | |
| 16.6 3) | 100–650 °C | 3) Xu et al. [360] | |
| 11–15 4) | 100–700 °C | 4) Fan et al. [361] | |
| 13–14 5) * | RT–950 °C | 5) Bishop et al. [99] | |
| 15.4 6) * | 100–600 °C | 6) Adler [47] | |
| 16.1 7) | Not given | 7) Kim et al. [362] | |
| 15.3 8) | 20–700 °C | 8) Kostogloudis and Ftikos [363] | |
| 0.6 | 16.8 1) | 100–400 °C | 1) Tai et al. [359] |
| y | Composition | Linear Chemical Expansion Coefficient | Temperature | References |
|---|---|---|---|---|
| 0 | La0.5Sr0.5FeO3−δ La0.3Sr0.7FeO3−δ | 0.059 1) 0.017–0.047 2) | 800 °C 650–875 °C | 1) Fossdal et al. [123] 2) Kharton et al. [364] |
| 0.2 | La0.6Sr0.4Co0.2Fe0.8O3−δ | 0.031 1) 0.032 2) | 700–900 °C 700–890 °C | 1) Bishop et al. [99] 2) Adler [47] |
| 0.5 | La0.5Sr0.5Co0.5Fe0.5O3−δ | 0.036–0.039 1) | 800–1000 °C | 1) Lein et al. [121] |
| 0.8 | La0.6Sr0.4Co0.8Fe0.2O3−δ | 0.022 1) | 800 °C | 1) Wang et al. [365] |
| 1 | La1−xSrxCoO3−δ (x = 0.2–0.7) La0.5Sr0.5CoO3−δ | 0.023–0.024 1) 0.035 2) | 600–900 °C 800 °C | 1) Chen et al. [122] 2) Lein et al. [121] |
| Composition | Thermal Expansion Coefficient/10−6 K−1 | Temperature Range | References |
|---|---|---|---|
| BaCo0.4Fe0.4Zr0.2O3−δ | 21.9 | 300–700 °C | Duan et al. [366] |
| BaCo0.4Fe0.4Zr0.1Y0.1O3−δ | 21.6 | 300–700 °C | Duan et al. [366] |
| LaNi0.4Co0.2Fe0.4O3−δ LaNi0.3Co0.3Fe0.4O3−δ LaNi0.2Co0.4Fe0.4O3−δ | 14.3 15.5 17.2 | RT–900 °C | Taguchi et al. [325] |
| LaCo0.6Ni0.4O3−δ | 17.2 | RT–900 °C | Wang et al. [367] |
| LaCo0.8Fe0.1Ni0.1O3−δ LaCo0.7Fe0.1Ni0.2O3−δ LaCo0.6Fe0.2Ni0.2O3−δ LaCo0.5Fe0.2Ni0.3O3−δ | 20.8 18.6 19.2 18 | RT–827 °C | Kharton et al. [368] |
| LaNiO3−δ LaNi0.8Fe0.2O3−δ LaNi0.6Fe0.4O3−δ LaNi0.4Fe0.6O3−δ LaNi0.2Fe0.8O3−δ LaFeO3−δ | 12.3 11.5 11 9.9 9.4 9.7 | RT–800 °C | Chiba et al. [369] |
| La0.8Sr0.2MnO3−δ | 11.8 | 30–1000 °C | Tietz et al. [326] |
| La0.8Sr0.2MnO3+δ La0.7Sr0.3MnO3+δ | 14.2 * 14.2 * | RT–400 °C RT–500 °C | Grande et al. [370] |
| Composition | Thermal Expansion Coefficient/10−6 K−1 | Temperature Range | References |
|---|---|---|---|
| Thermal only | |||
| LaSrMnCoO6−δ | 15.8 | RT–1000 °C | Zhou et al. [390] |
| BaSmCo1.4Fe0.6O6−δ BaSmCo1.2Fe0.8O6−δ | 21.1 21.0 | RT–1100 °C | Volkova et al. [388] |
| BaSmCo2O6−δ | 21.0 | RT–1100 °C | Aksenova et al. [391] |
| BaPr0.7Y0.3Co2O6−δ BaPr0.5Y0.5Co2O6−δ | 17.6 17.2 | 50–800 °C | Zhao et al. [392] |
| BaPrCoCuO6−δ | 15.2 | RT–1000 °C | Zhao et al. [393] |
| LT/HT | |||
| BaLaCo2O6−δ | 24.3 29.8 | 80–900 °C 500–900 °C | Kim et al. [340] |
| BaNdCo2O6−δ | 19.1 21.1 | 80–900 °C 500–900 °C | |
| BaSmCo2O6−δ | 17.1 18.7 | 80–900 °C 500–900 °C | |
| BaGdCo2O6−δ | 16.6 16.8 | 80–900 °C 500–900 °C | |
| BaYCo2O6−δ | 15.8 14.9 | 80–900 °C 500–900 °C | |
| BaPrCo1.95Sc0.05O6−δ | 17.0 27.0 | 30–300 °C 300–1000 °C | Li et al. [376] |
| BaPrCo1.9Sc0.10O6−δ | 16.0 26.0 | 30–300 °C 300–1000 °C | |
| BaPrCo1.8Sc0.20O6−δ | 15.8 25.8 | 30–300 °C 300–1000 °C | |
| BaSmCo1.6Fe0.4O6−δ | 19.0 20.8 | 30–400 °C 400–1100 °C | Volkova et al. [388] |
| BaSmCo1.8Fe0.2O6−δ | 18.4 20.4 | 30–470 °C 470–1100 °C | |
| BaSmCo1.9Fe0.1O6−δ | 18.7 21.1 | 30–500 °C 500–1100 °C | |
| BaGdCo2O6−δ | 18.0 19.6 | 100–350 °C 600–900 °C | Jo et al. [381] |
| BaGdCo2/3Fe2/3Ni2/3O6−δ | 15.2 16.8 | 100–350 °C 600–900 °C | |
| BaGdCo2/3Fe2/3Cu2/3O6−δ | 13.6 15.7 | 100–350 °C 600–900 °C | |
| BaGdCoCuO6−δ | 14.0 12.8 | 100–350 °C 600–900 °C | |
| BaGdCo2O6−δ | 17.6 19.0 | RT–450 °C 500–900 °C | Mogni et al. [394] |
| Ba0.5Sr0.5GdCo1.5Fe0.5O6−δ | 15.4 21.8 | RT–200 °C 300–800 °C | Kuroda et al. [395] |
| BaNdCo2O6−δ | 17.9 22.9 | RT–300 °C RT–900 °C | Kim et al. [375] |
| Ba1.5Sr0.5NdCo2O6−δ | 17.2 26.3 | RT–300 °C RT–900 °C | |
| BaNdCo2O6−δ | 18.3 23.8 | RT–250 °C 350–1000 °C | Cherepanov et al. [373] |
| BaNdCo1.8Fe0.2O6−δ | 18.8 21.9 | RT–250 °C 350–1000 °C | |
| BaNdCo1.6Fe0.4O6−δ | 18.9 21.9 | RT–250 °C 350–1000 °C | |
| BaNdCo1.4Fe0.6O6−δ | 18.3 22.1 | RT–250 °C 350–1000 °C | |
| BaNdCo1.2Fe0.8O6−δ | 18.4 21.9 | RT–250 °C 350–1000 °C | |
| Average over whole T range | |||
| BaPrCo2/3Fe2/3Cu2/3O6−δ | 16.6 | 30–850 °C | Jin et al. [383] |
| BaPrCo1.6Ni0.4O6−δ BaNdCo1.6Ni0.4O6−δ BaSmCo1.6Ni0.4O6−δ | 20.6 19.4 16.6 | 30–1000 °C | Che et al. [345] |
| BaPrCo2O6−δ | 21.5 | 50–800 °C | Zhao et al. [392] |
| BaPrCuCoO6−δ | 24.1 | 30–1000 °C | Zhao et al. [393] |
| Ba0.5Sr0.5PrCo1.5Fe0.5O6−δ Ba0.5Sr0.5PrCo0.5Fe1.5O6−δ | 21.3 19.2 | RT–900 °C | Jiang et al. [384] |
| BaPrCo2O6−δ BaPrCo1.5Fe0.5O6−δ BaPrCoFeO6−δ BaPrCo0.5Fe1.5O6−δ BaPrFe2O6−δ | 24.6 26.0 25.0 19.1 17.2 | 20–800 °C | Zhao et al. [385] |
| Ba0.5Sr0.5PrCo1.9Ni0.1O6−δ Ba0.5Sr0.5PrCo1.7Ni0.3O6−δ | 21.9 19.7 | 30–900 °C | Liu et al. [380] |
| BaPrCoFeO6−δ BaNdCoFeO6−δ | 21.0 19.5 | 30–1000 °C | Jin et al. [344] |
| BaNdCo2O6−δ | 23.0 | RT–1100 °C | Aksenova et al. [391] |
| Ba0.5Sr0.5NdCo2O6−δ Ba0.5Sr0.5NdCo1.75Mn0.25O6−δ Ba0.5Sr0.5NdCo1.5Mn0.5O6−δ | 20.3 16.4 14.3 | RT–900 °C | Kim et al. [377] |
| BaNdCo2O6−δ BaNdFe2O6−δ BaGdCo2O6−δ BaGdFe2O6−δ | 21.5 18.3 19.9 18.8 | 80–900 °C | Kim et al. [387] |
| BaGdCoCuO6−δ Ba0.25Sr0.75GdCoCuO6−δ Ba0.25Sr0.75NdCoCuO6−δ | 16.3 16.0 17.0 | RT–900 °C | West et al. [374] |
| BaGdCo1.9Ni0.1O6−δ BaGdCo1.7Ni0.3O6−δ | 18.9 15.5 | 30–900 °C | Wei et al. [331] |
| BaYCo2O6−δ BaYCo1.8Cu0.2O6−δ BaYCo1.6Cu0.4O6−δ BaYCo1.4Cu0.6O6−δ BaYCo1.2Cu0.8O6−δ | 17.8 16.7 15.7 14.7 13.4 | 30–850 °C | Zhang et al. [382] |
| BaYCo2O6−δ BaYCo1.6Fe0.4O6−δ | 16.3 18.0 | 30–900 °C | Xue et al. [389] |
| SrSmCo2O6−δ SrSmCo1.8Mn0.2O6−δ SrSmCo1.6Mn0.4O6−δ SrSmCo1.4Mn0.6O6−δ SrSmCo1.2Mn0.8O6−δ SrSmCoMnO6−δ | 22.6 21.0 20.8 18.0 17.5 13.7 | RT–850 °C | Wang et al. [378] |
| Composition | Thermal Expansion Coefficient/10−6 K−1 | Temperature Range | References | |
|---|---|---|---|---|
| LaSrCoO4−δ La0.9Sr1.1CoO4−δ La1.1Sr0.9CoO4−δ | 25.3 24.3 25.4 | 300–700 °C | Hu et al. [397] | |
| Sr3Fe2O6+δ | 20 | RT–900 °C | Prado et al. [398] | |
| Pr2NiO4+δ La2NiO4+δ Nd2NiO4+δ | 13.4 13 12.4 | 400–1000 °C | Flura et al. [399] | |
| La2NiO4+δ Pr2NiO4+δ Nd2NiO4+δ | 13 13.6 12.7 | RT–950 °C | Boehm et al. [406] | |
| La2NiO4+δ | 13.7 | RT–900 °C | Skinner et al. [402] | |
| La2Ni0.5Cu0.5O4+δ La2NiCuO4+δ | 12.8 8.6 13.6 | RT–950 °C RT–250 °C 250–900 °C | Boehm et al. [403] | |
| Lan+1NinO3n+1 | n = 1 n = 2 n = 3 | 13.8 13.2 13.2 | RT–900 °C | Amow et al. [400,401] |
| La2−xPrxNiO4+δ 0≤ x ≤ 0.2 | 13–13.5 | RT–1000 °C | Vibhu et al. [404] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Løken, A.; Ricote, S.; Wachowski, S. Thermal and Chemical Expansion in Proton Ceramic Electrolytes and Compatible Electrodes. Crystals 2018, 8, 365. https://doi.org/10.3390/cryst8090365
Løken A, Ricote S, Wachowski S. Thermal and Chemical Expansion in Proton Ceramic Electrolytes and Compatible Electrodes. Crystals. 2018; 8(9):365. https://doi.org/10.3390/cryst8090365
Chicago/Turabian StyleLøken, Andreas, Sandrine Ricote, and Sebastian Wachowski. 2018. "Thermal and Chemical Expansion in Proton Ceramic Electrolytes and Compatible Electrodes" Crystals 8, no. 9: 365. https://doi.org/10.3390/cryst8090365





