Molecular Dynamics Investigation of Graphene Nanoplate Diffusion Behavior in Poly-α-Olefin Lubricating Oil
Abstract
:1. Introduction
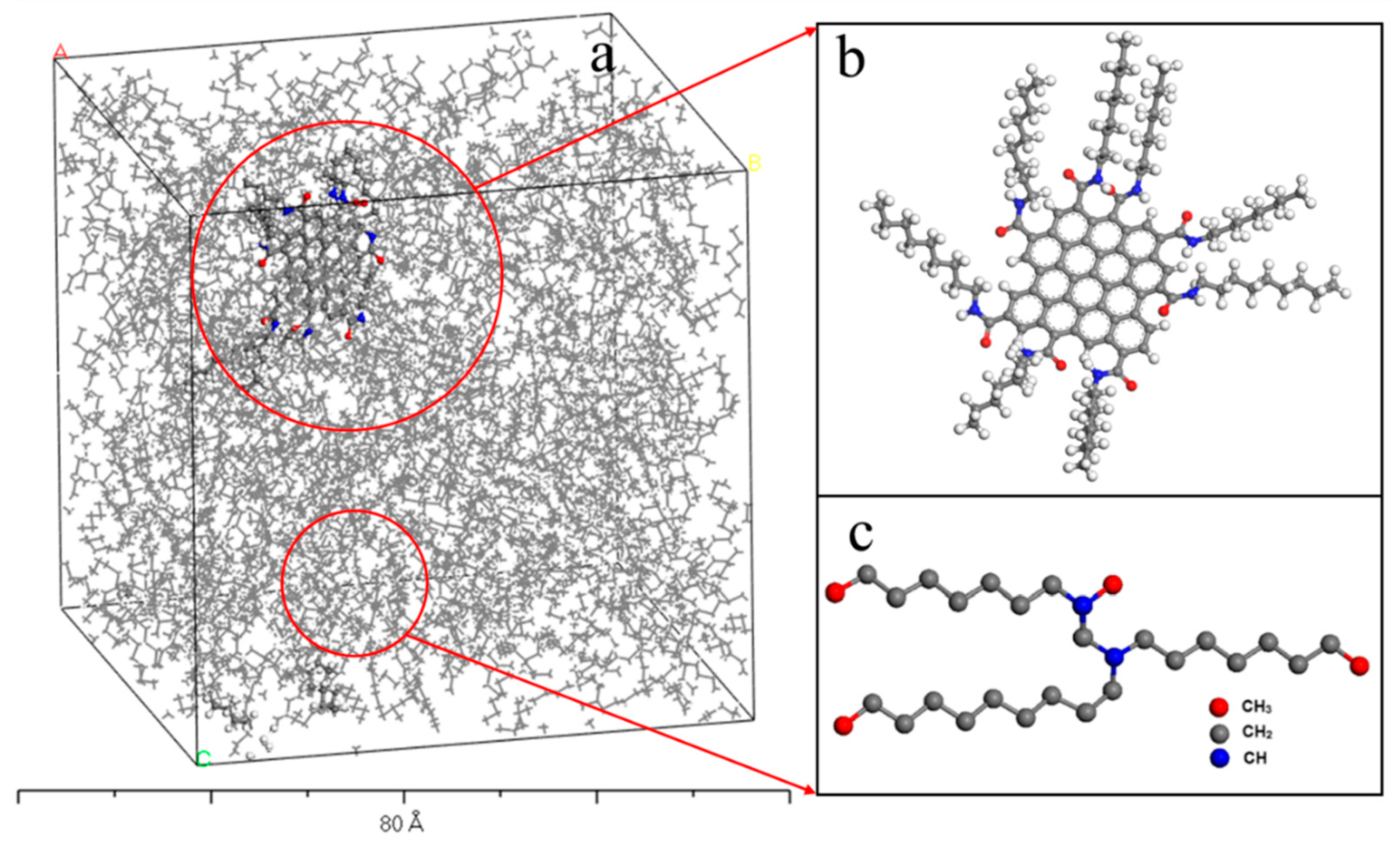
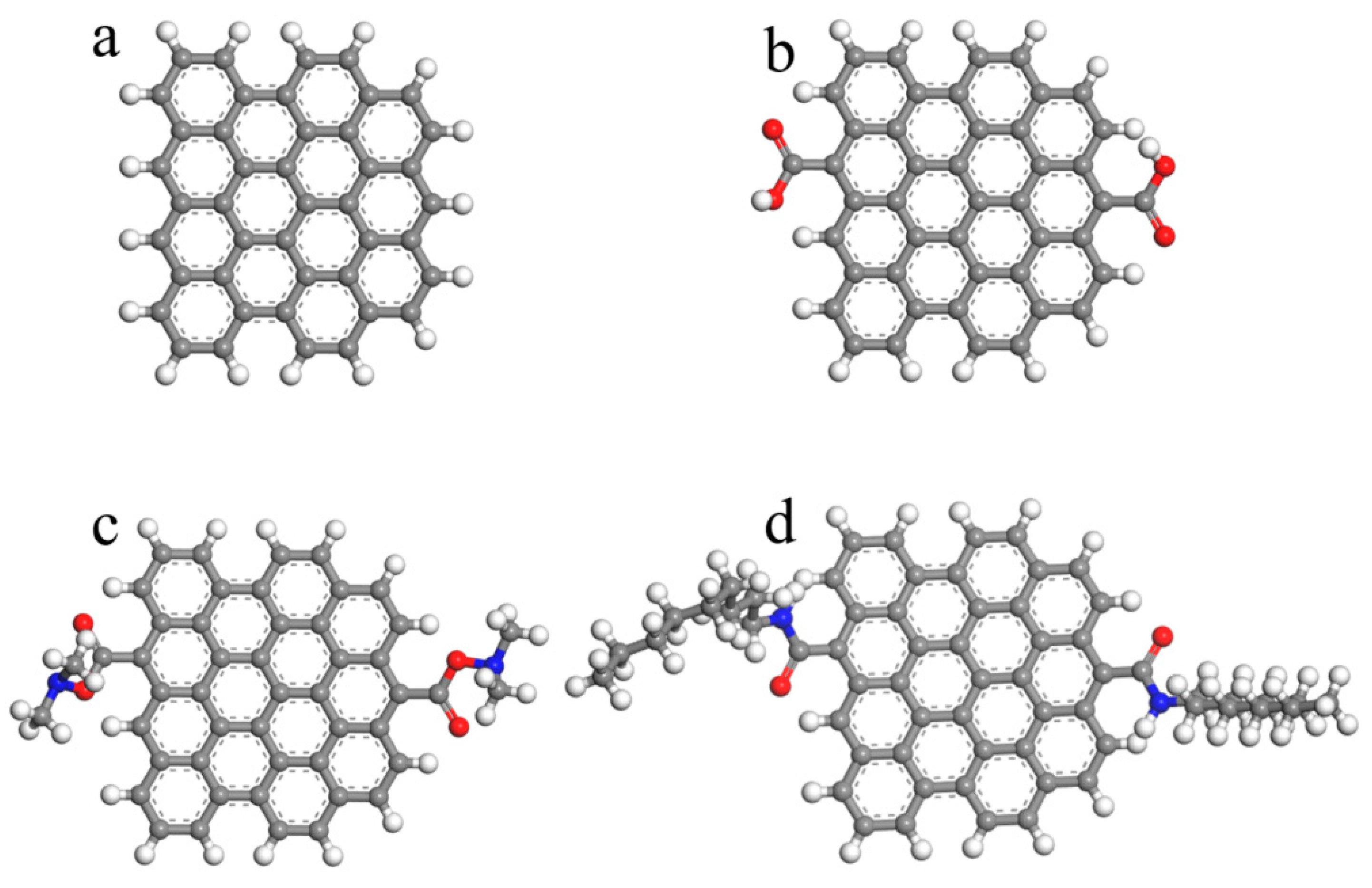
2. Materials and Simulation Method
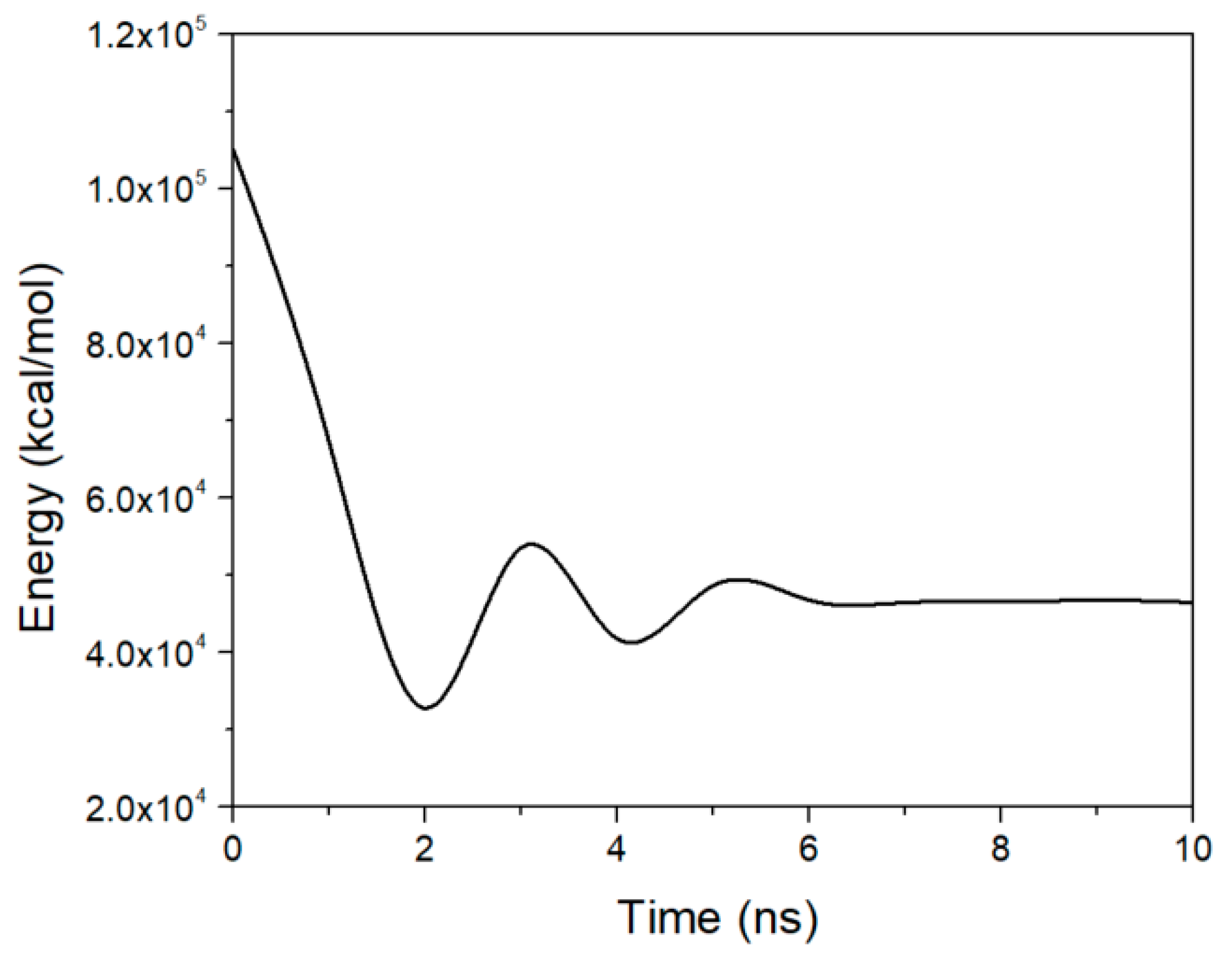
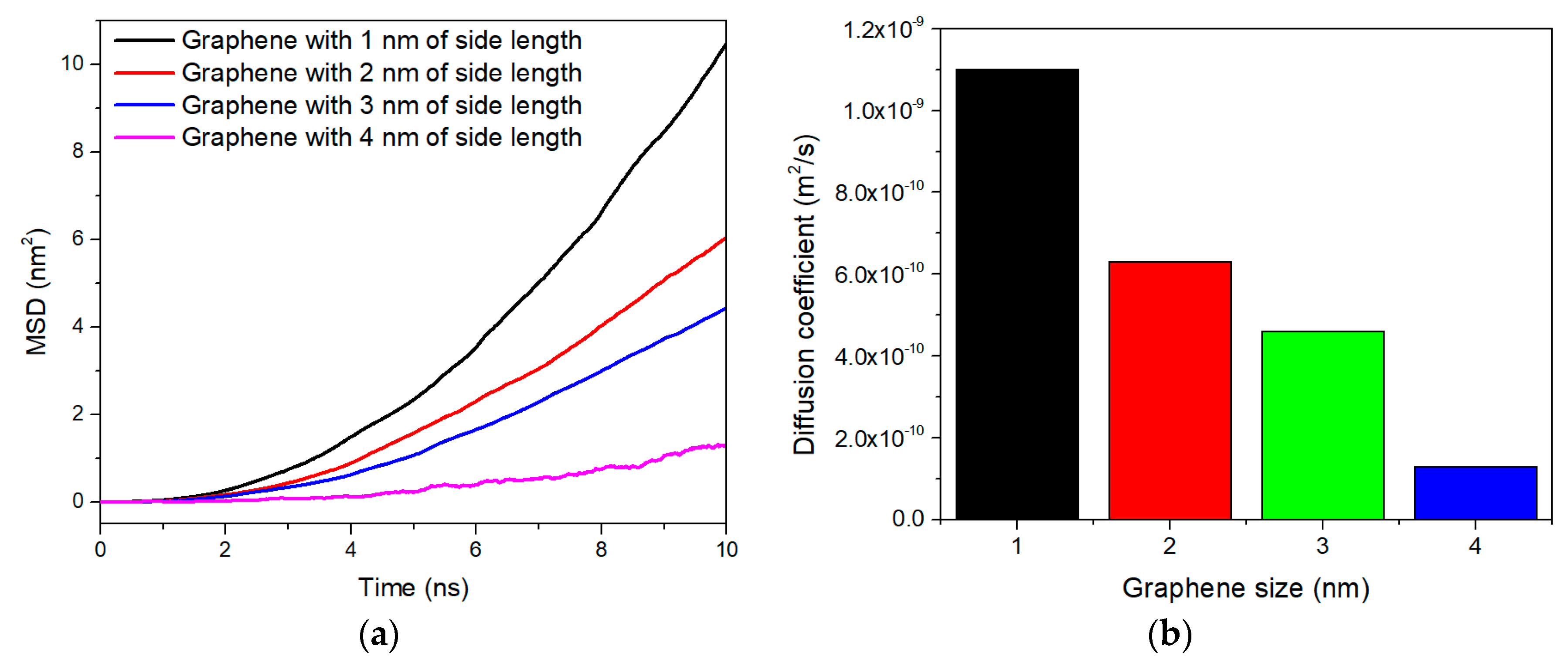
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jost, H.P. Tribology—Origin and future. Wear 1990, 136, 1–17. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef] [Green Version]
- Jost, H.P.; Schofield, J. The practical aspects of friction, lubrication and wear. Proc. Inst. Mech. Eng. 1981, 195, 151–173. [Google Scholar] [CrossRef]
- Jiao, D.; Zheng, S.; Wang, Y.; Guan, R.; Cao, B. The tribology properties of alumina/silica composite nanoparticles as lubricant additives. Appl. Surf. Sci. 2011, 257, 5720–5725. [Google Scholar] [CrossRef]
- Etemadi, H.; Shojaei, A.; Jahanmard, P. Effect of alumina nanoparticle on tribological performance of automotive brake friction materials. J. Reinf. Plast. Compos. 2014, 33, 166–178. [Google Scholar] [CrossRef]
- Wu, H.X.; Qin, L.G.; Dong, G.N.; Hua, M.; Yang, S.C.; Zhang, J.F. An investigation on the lubrication mechanism of MoS2 nano sheet in point contact: The manner of particle entering the contact area. Tribol. Int. 2017, 107, 48–55. [Google Scholar] [CrossRef]
- Aralihalli, S.; Biswas, S.K. Grafting of dispersants on MoS2 in base oil lubrication of steel. Tribol. Lett. 2013, 49, 61–76. [Google Scholar] [CrossRef]
- Pottuz, L.J.; Dassenoy, F.; Belin, M.; Vacher, B.; Martin, J.; Fleischer, N. Ultralow-friction and wear properties of IF-WS 2 under boundary lubrication. Tribol. Lett. 2005, 18, 477–485. [Google Scholar] [CrossRef]
- Aldana, P.U.; Dassenoy, F.; Vacher, B.; Mogne, T.L.; Thiebaut, B. WS2 nanoparticles anti-wear and friction reducing properties on rough surfaces in the presence of ZDDP additive. Tribol. Int. 2016, 102, 213–221. [Google Scholar] [CrossRef]
- Sharma, V.; Timmons, R.; Erdemir, A.; Aswath, P.B. Plasma-functionalized polytetrafluoroethylene nanoparticles for improved wear in lubricated contact. ACS Appl. Mater. Interfaces 2017, 9, 25631–25641. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Wu, H.X.; Desanker, M.; Pickens, D.; Chung, Y.W.; Wang, Q.J. Direct formation of lubricious and wear-protective carbon films from phosphorus- and sulfur-free oil-soluble additives. Tribol. Lett. 2018, 66, 2. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Ren, R.R.; Song, H.J.; Jia, X.H. Improved tribological properties of the synthesized copper/carbon nanotube nanocomposites for rapeseed oil-based additives. Appl. Surf. Sci. 2018, 428, 630–639. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Etacheri, V.; Dysart, A.D.; Stacke, L.E.; Pol, V.G.; Sadeghi, F. ultrasmooth submicrometer carbon spheres as lubricant additives for friction and wear reduction. ACS Appl. Mater. Interfaces 2015, 7, 5514–5521. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.Z.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Shahnazar, S.; Bagheri, S.; Hamid, S.B.A. Enhancing lubricant properties by nanoparticle Additives. Int. J. Hydrogen Energy 2016, 41, 3153–3170. [Google Scholar] [CrossRef]
- Yu, W.M.; David, M.; Routbort, J.L.; Choi, S. Review and comparison of nanofluid thermal conductivity and heat transfer enhancements. Heat. Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Rasheed, A.K.; Khalid, M.; Rashmi, W.; Guupta, T.C.S.M.; Chan, A. Graphene based nanofluids and nanolubricants—Review of recent developments. Renew. Sustain. Energy Rev. 2016, 63, 346–362. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Graphene-based engine oil nanofluids for tribological applications. ACS Appl. Mater. Interfaces 2011, 3, 4221–4227. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Gu, L.; Xie, Z.J.; Zhang, C.W.; Song, B.Y. Improved tribological properties of Si3N4/GCr15 sliding pairs with few layer graphene as oil additives. Ceram. Int. 2017, 43, 14218–14224. [Google Scholar] [CrossRef]
- Mao, J.Y.; Zhao, J.; Wang, W.; He, Y.Y.; Luo, J.B. Influence of the micromorphology of reduced graphene oxide sheets on lubrication properties as a lubrication additive. Tribol. Int. 2018, 117, 614–621. [Google Scholar] [CrossRef]
- Zheng, D.; Cai, Z.B.; Shen, M.X.; Li, Z.Y.; Zhu, M.H. Investigation of the tribology behaviour of the graphene nanosheets as oil additives on textured alloy cast iron Surface. Appl. Surf. Sci. 2016, 387, 66–75. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.F.; Song, H.J.; Chen, B.B.; Zhang, Z.Z. Synthesis of the liquid-like graphene with excellent tribological properties. Tribol. Int. 2017, 105, 118–124. [Google Scholar] [CrossRef]
- Lin, J.S.; Wang, L.W.; Chen, G.H. Modification of graphene platelets and their tribological properties as a lubricant additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Wu, X.H.; Gong, K.L.; Zhao, G.Q.; Lou, W.J.; Wang, X.B.; Liu, W.M. Mechanical synthesis of chemically bonded phosphorus-graphene hybrid as high-temperature lubricating oil additive. RSC Adv. 2018, 8, 4595–4603. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Feng, S.W.; Zhang, L.; Zhang, L.; Jiao, Z.L.; Zhan, Y.Q.; Wang, Y.J. Preparation and tribological properties of novel boehmite/graphene oxide nano-hybrid. Ceram. Int. 2016, 42, 6178–6186. [Google Scholar] [CrossRef]
- Moghaddam, M.B.; Goharshadi, E.K.; Moosavi, F. Structural and transport properties and solubility parameter of graphene/glycerol nanofluids: A molecular dynamics simulation study. J. Mol. Liq. 2016, 222, 82–87. [Google Scholar] [CrossRef]
- Goyenola, C.; Gueorguiev, G.K.; Stafstrom, S.; Hultman, L. Fullerene-like CSx: A first-principles study of synthetic growth. Chem. Phys. Lett. 2011, 506, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Sun, S.Q.; Li, C.L.; Pittman, C.U.; Lacy, T.E.; Hu, S.Q.; Gwaltney, S.R. Behavior of protruding lateral plane graphene sheets in liquid dodecane: Molecular dynamics simulations. J. Nanopart. Res. 2016, 18, 317–329. [Google Scholar] [CrossRef]
- Loya, A.; Stair, J.L.; Jafri, A.R.; Yang, K.; Ren, G.G. A molecular dynamic investigation of viscosity and diffusion coefficient of nanoclusters in hydrocarbon fluids. Comp. Mater. Sci. 2015, 99, 242–246. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, Y.; Yang, X.N.; Liu, D.M.; Shan, S.J.; Cui, F.Y. Understanding the roles of solution chemistries and functionalization on the aggregation of graphene-based nanomaterials using molecular dynamic simulations. J. Phys. Chem. C 2017, 121, 13888–13897. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.N.; Li, Y.P. Molecular simulation perspective of liquid-phase exfoliation, dispersion, and stabilization for graphene. Curr. Opin. Colloid Interface Sci. 2015, 20, 339–345. [Google Scholar] [CrossRef]
- Sangiovanni, D.G.; Gueorguiev, G.K.; Kakanakova-Georgieva, A. Ab initio molecular dynamics of atomic-scale surface reactions: Insights into metal organic chemical vapor deposition of AlN on graphene. PCCP 2015, 20, 17751–17761. [Google Scholar] [CrossRef] [PubMed]
- Saathoff, J.D.; Clancy, P. Effect of edge-functionalization on the ease of graphene nanoribbon aggregation in solvent. Carbon 2017, 115, 154–161. [Google Scholar] [CrossRef]
- Konatham, D.; Striolo, A. Molecular design of stable graphene nanosheets dispersions. Nano Lett. 2008, 8, 4630–4641. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Sun, S.Q.; Li, C.L.; Pittman, C.U.; Lacy, T.E.; Hu, S.Q.; Gwaltney, S.R. Molecular dynamics simulations of the graphene sheet aggregation in dodecane. Comput. Mater. Sci. 2017, 19, 195–206. [Google Scholar] [CrossRef]
- Cha, J.; Kyoung, W. Molecular dynamics simulation of the effects of affinity of functional groups and particle-size on the behavior of a graphene sheet in nanofluid. Comput. Mater. Sci. 2017, 139, 202–208. [Google Scholar] [CrossRef]
- Liu, P.Z.; Lu, J.; Yu, H.L.; Ren, N.; Lockwood, F.E.; Wang, Q.J. Lubricant shear thinning behavior correlated with variation of radius of gyration via molecular dynamics simulations. J. Chem. Phys. 2017, 147, 084904. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Shi, G.Q. Assembly of chemically modified graphene: Methods and applications. J. Mater. Chem. 2011, 21, 3311–3323. [Google Scholar] [CrossRef]
- Ai, K.L.; Liu, Y.L.; Lu, L.H.; Cheng, X.L.; Huo, L.H. A novel strategy for making soluble reduced graphene oxide sheets cheaply by adopting an endogenous reducing agent. J. Mater. Chem. 2011, 21, 3365–3370. [Google Scholar] [CrossRef]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications. J. Mater. Chem. 2012, 22, 21032–21039. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Khakan, H.; Yeganegi, S. Molecular dynamics simulations of amide functionalized imidazolium bis (trifluoromethanesulfonyl) imide dicationic ionic liquids. J. Phys. Chem. B 2017, 121, 7455–7463. [Google Scholar] [CrossRef] [PubMed]
- Michalis, V.K.; Moultos, O.A.; Tsimpanogianis, I.N.; Economou, I.G. Molecular dynamics simulations of the diffusion coefficients of light n-alkanes in water over a wide range of temperature and pressure. Fluid Phase Equilib. 2016, 407, 236–242. [Google Scholar] [CrossRef]
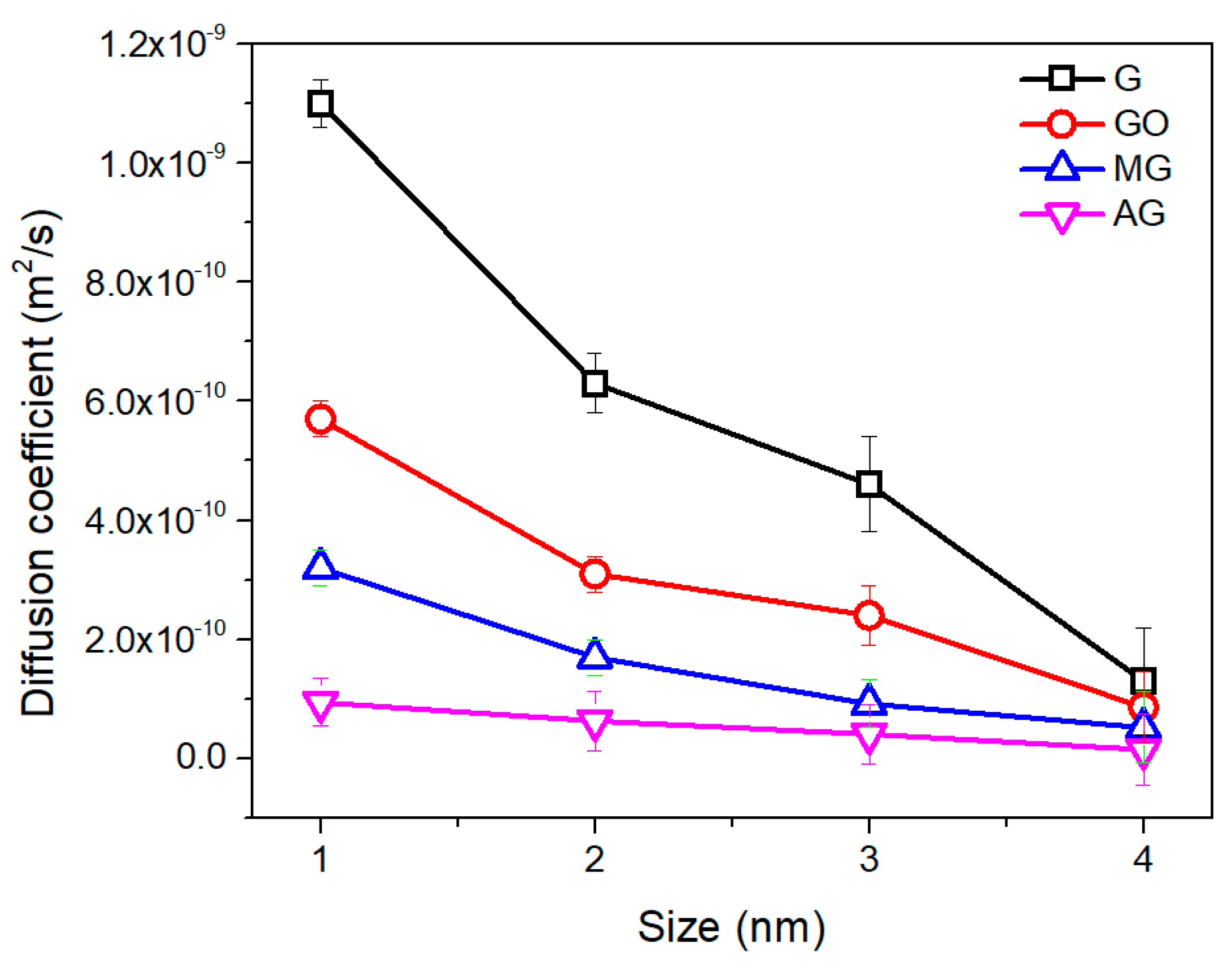
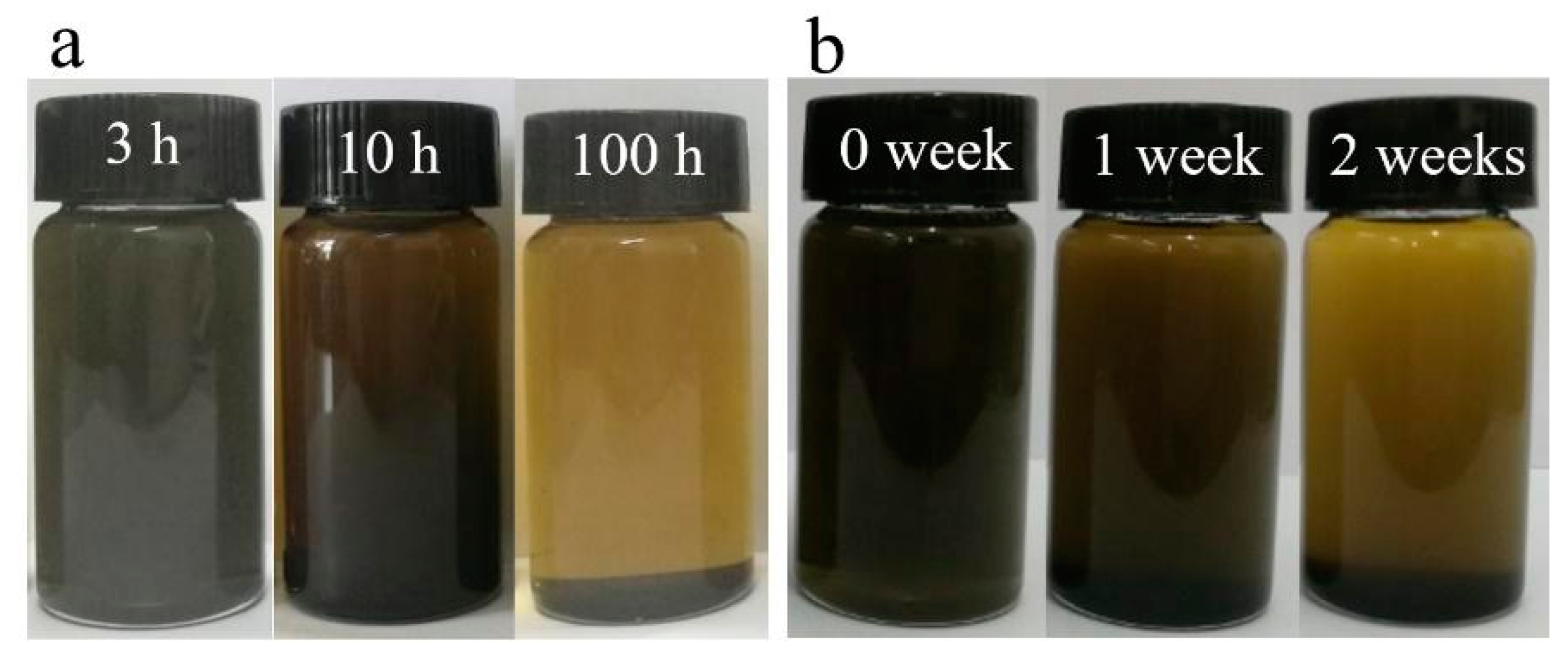

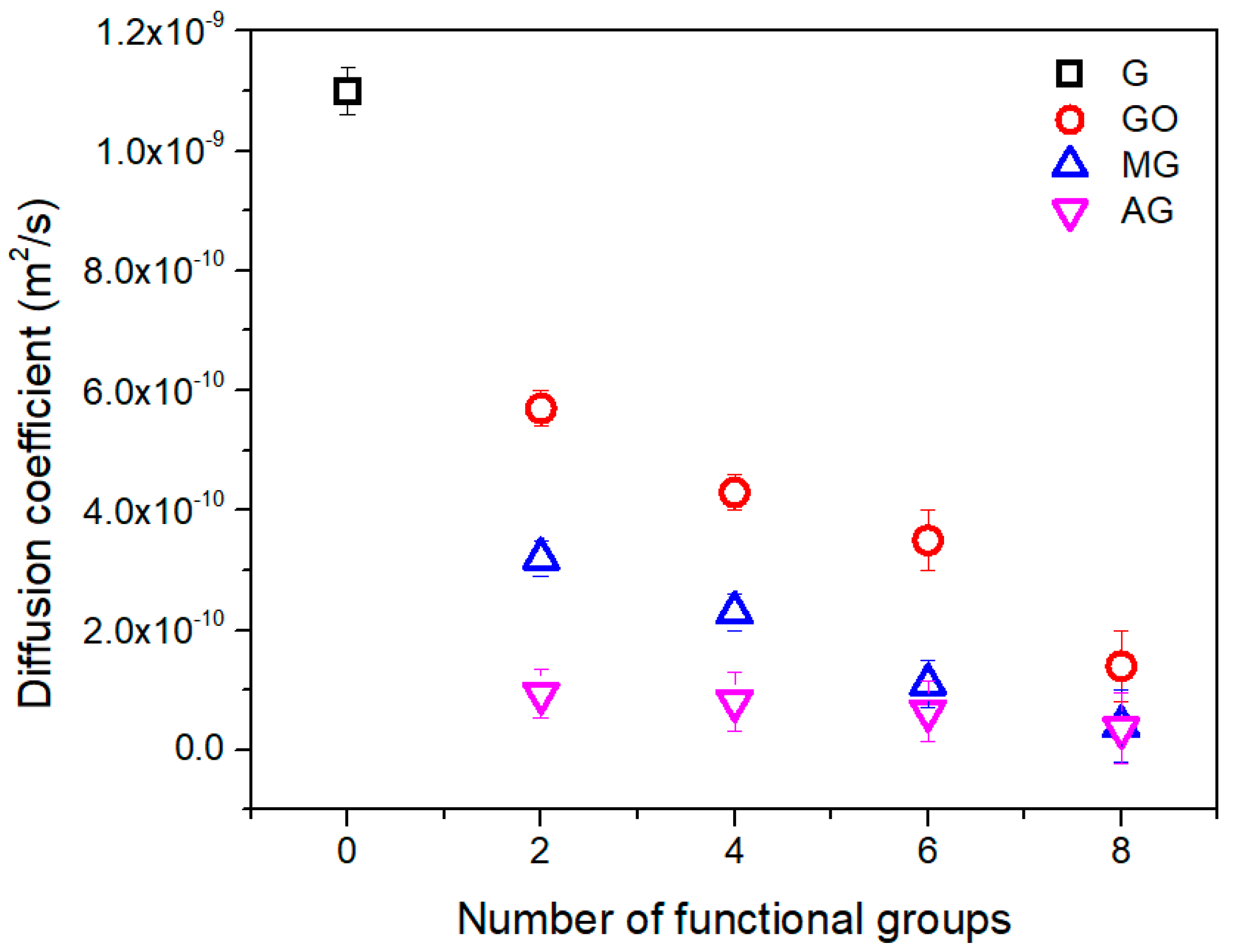
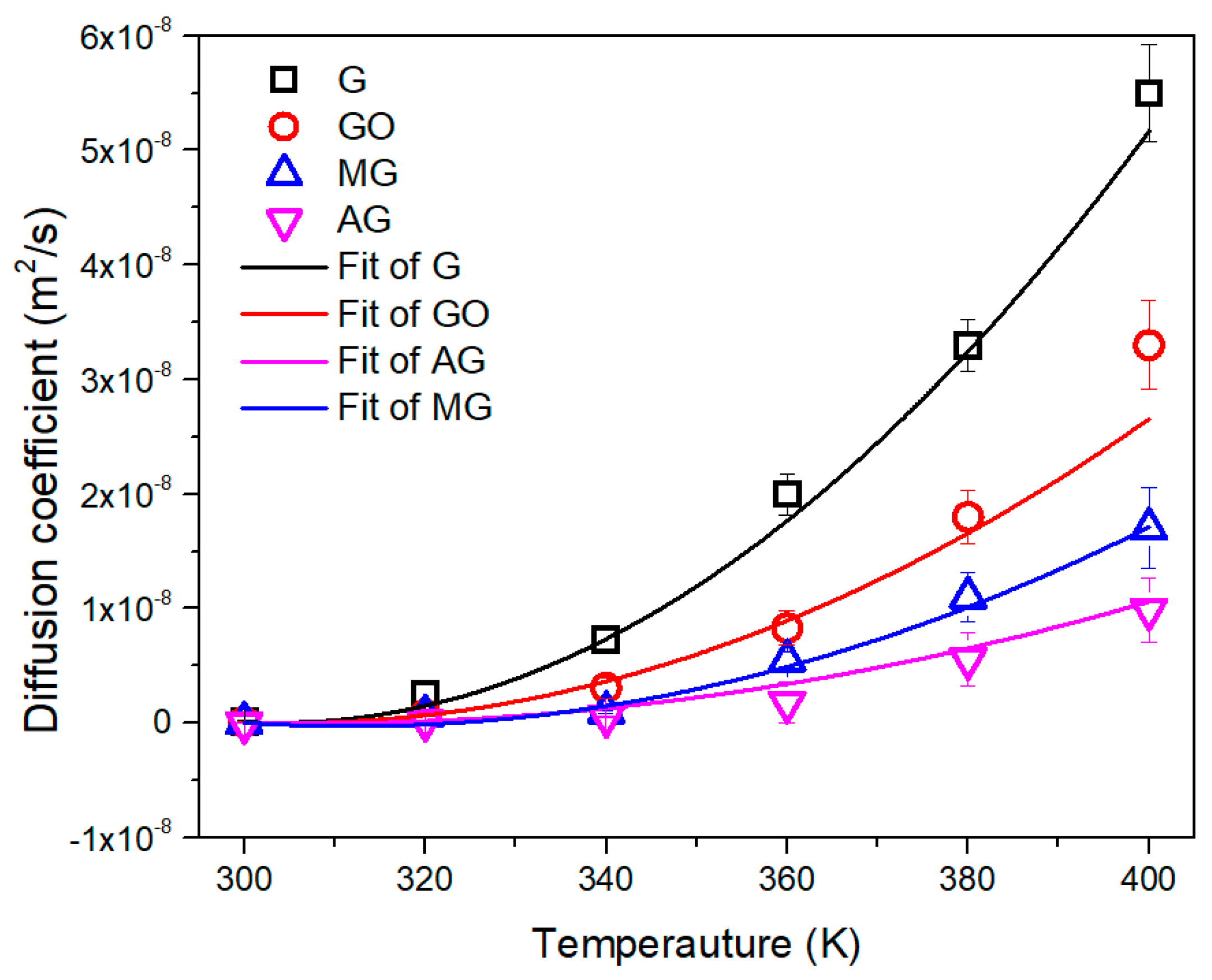

| Number | 2 | 4 | 6 | 8 |
|---|---|---|---|---|
| GO | 48.18 | 60.91 | 68.18 | 87.27 |
| MG | 70.91 | 79.09 | 90.0 | 96.36 |
| AG | 91.45 | 92.64 | 94.09 | 96.73 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Song, B.; Keer, L.M.; Gu, L. Molecular Dynamics Investigation of Graphene Nanoplate Diffusion Behavior in Poly-α-Olefin Lubricating Oil. Crystals 2018, 8, 361. https://doi.org/10.3390/cryst8090361
Wu L, Song B, Keer LM, Gu L. Molecular Dynamics Investigation of Graphene Nanoplate Diffusion Behavior in Poly-α-Olefin Lubricating Oil. Crystals. 2018; 8(9):361. https://doi.org/10.3390/cryst8090361
Chicago/Turabian StyleWu, Lupeng, Baoyu Song, Leon M. Keer, and Le Gu. 2018. "Molecular Dynamics Investigation of Graphene Nanoplate Diffusion Behavior in Poly-α-Olefin Lubricating Oil" Crystals 8, no. 9: 361. https://doi.org/10.3390/cryst8090361





