New Topologically Unique Metal-Organic Architectures Driven by a Pyridine-Tricarboxylate Building Block
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Physical Measurements
2.2. Synthesis of {[Pb3(µ5-cpta)(µ6-cpta)(phen)2]·2H2O}n (1)
2.3. Synthesis of {[Zn2(µ4-cpta)(µ-OH)(4,4′-bipy)]·6H2O}n (2)
2.4. X-ray Crystallography
3. Results and Discussion
3.1. Hydrothermal Self-Assembly Synthesis
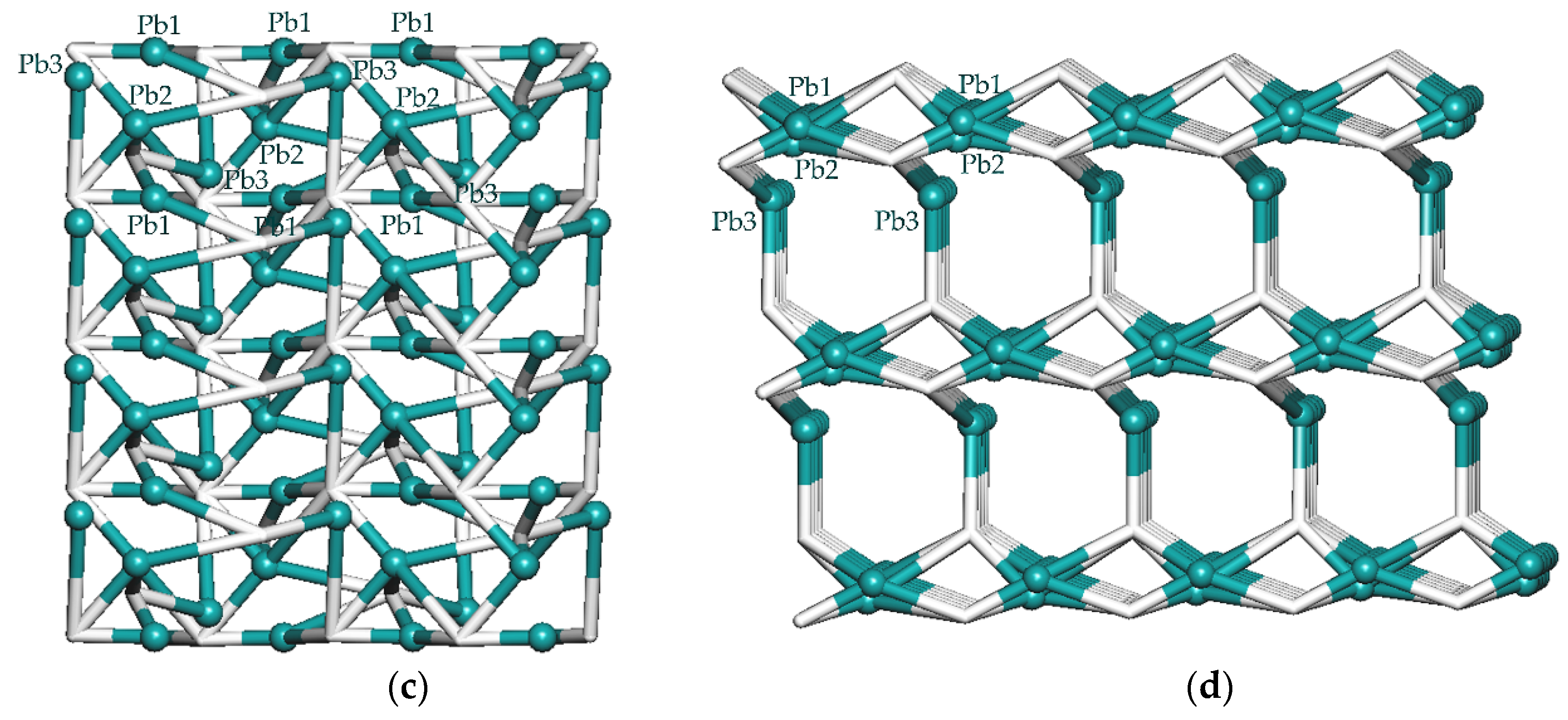
3.2. Crystal Structure of {[Pb3(µ5-cpta)(µ6-cpta)(phen)2]·2H2O}n (1)
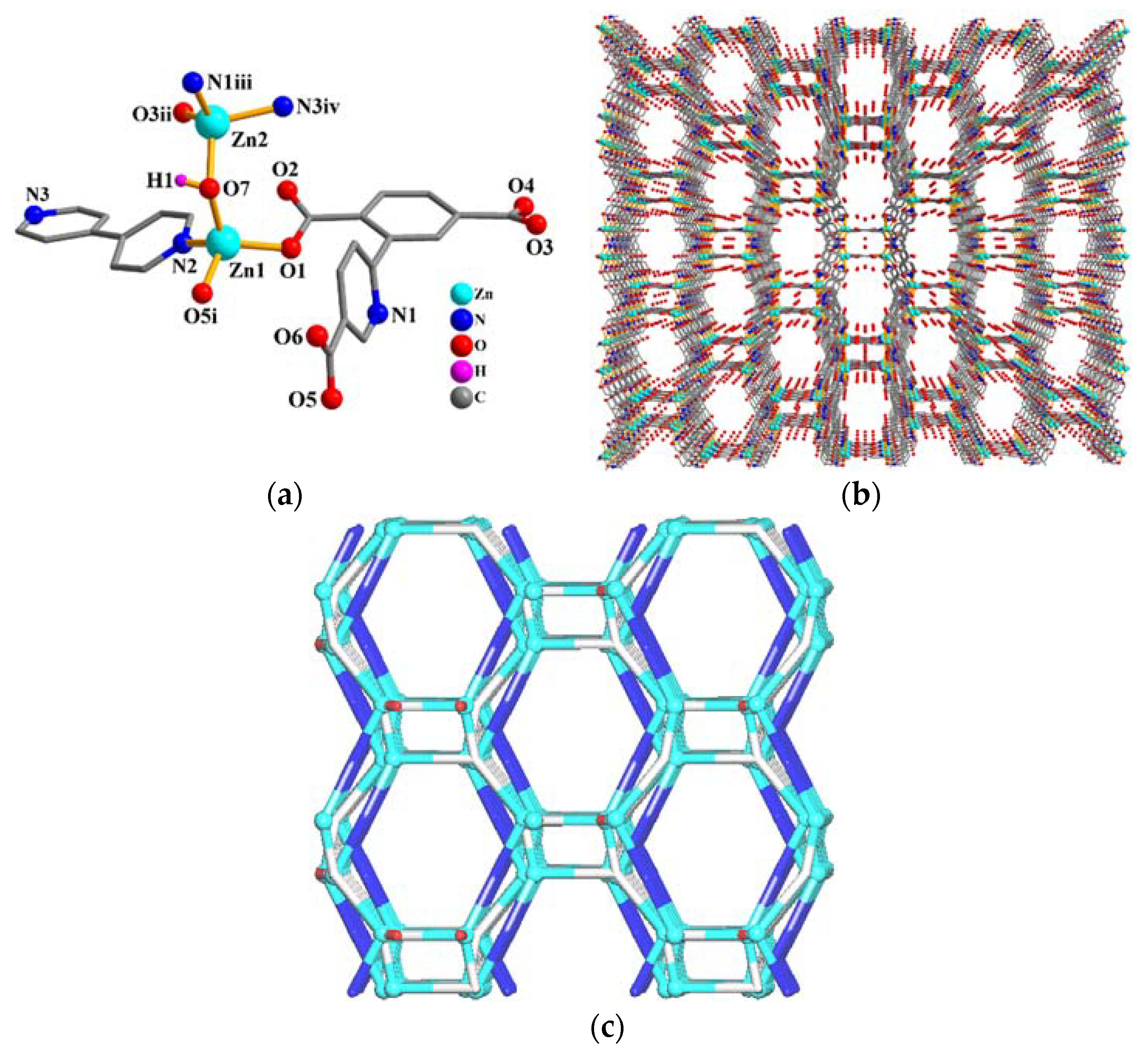
3.3. Crystal Structure of {[Zn2(µ4-cpta)(µ-OH)(µ-4,4′-bipy)]·6H2O}n (2)
3.4. Thermogravimetric and Powder X-ray Diffraction Analysis
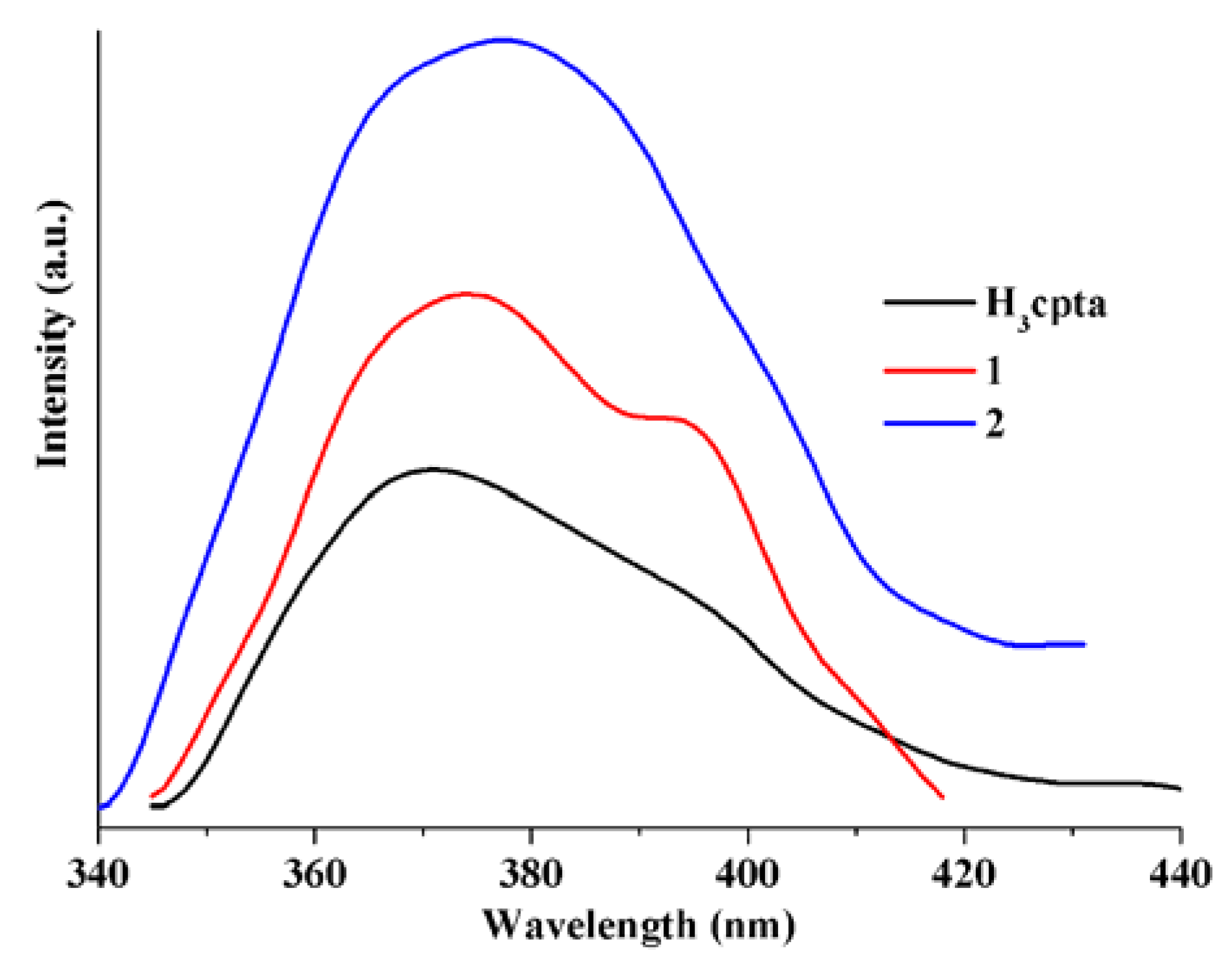
3.5. Luminescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Q.L.; Xu, Q. Metal-organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef] [PubMed]
- Papazoi, E.; Douvali, A.; Rapti, S.; Skliri, E.; Armatas, G.S.; Papaefstathiou, G.S.; Wang, X.; Huang, Z.F.; Kaziannis, S.; Kosmidis, C. A microporous Mg2+ MOF with cation exchange properties in a single-crystal-to-single-crystal fashion. Inorg. Chem. Front. 2017, 4, 530–536. [Google Scholar] [CrossRef]
- Cui, Y.J.; Yue, Y.F.; Qian, G.D.; Chen, B.L. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.H.; Yin, Z.; Tan, Y.X.; Zhang, W.X.; He, Y.P.; Kurmoo, M. Nanoporous cobalt(II) MOF exhibiting four magnetic ground states and changes in gas sorption upon post-synthetic modification. J. Am. Chem. Soc. 2014, 136, 4680–4688. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.X.; Zhang, X.J.; Bing, Y.M.; Xu, N.; Shi, W.; Cheng, P. In situ generation of NiO nanoparticles in a magnetic metal-organic framework exhibiting three-dimensional magnetic ordering. Inorg. Chem. 2016, 55, 12938–12943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Wu, M.C.; Zeng, M.H.; Liang, H. Magneto-structural correlation in a metamagnetic cobalt(II)-based pillared trilayer motif constructed by mixed pyridyl-type carboxylate ligands. Inorg. Chem. 2009, 48, 10146–10150. [Google Scholar] [CrossRef] [PubMed]
- Fidelli, A.M.; Armakola, E.; Demadis, K.D.; Kessler, V.G.; Escue, A.; Papaefstathiou, G.S. Cu-II frameworks from di-2-pyridyl ketone and benzene-1,3,5-triphosphonic acid. Eur. J. Inorg. Chem. 2018, 91–98. [Google Scholar] [CrossRef]
- Murray, L.J.; Dincă, M.; Long, L.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Kourtellaris, A.; Moushi, E.E.; Spanopoulos, I.; Tampaxis, C.; Steriotis, T.A.; Papaefstathiou, G.S.; Trikalitis, P.N.; Tasiopoulos, A.J. A microporous Cu2+ MOF based on a pyridyl isophthalic acid Schiff base ligand with high CO2 uptake. Inorg. Chem. Front. 2016, 3, 1527–1535. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhan, Z.Y.; Liang, X.Y.; Chen, C.; Liu, X.L.; Jia, Y.J.; Hu, M. Lanthanide-MOFs constructed from mixed dicarboxylate ligands as selective multi-responsive luminescent sensors. Dalton Trans. 2018, 47, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wang, X.S.; Li, L.; Huang, Y.B.; Cao, R. Highly selective sensing of Fe3+ by an anionic metal-organic framework containing uncoordinated nitrogen and carboxylate oxygen sites. Dalton Trans. 2018, 47, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Z.; Liang, X.X.; Cui, Y.H.; Wu, J.; Shi, Z.F.; Kirillov, A.M. Introducing 2-(2-carboxyphenoxy)terephthalic acid as a new versatile building block for design of diverse coordination polymers: Synthesis, structural features, luminescence sensing, and magnetism. CrystEngComm 2017, 19, 2570–2588. [Google Scholar] [CrossRef]
- Gu, J.Z.; Cai, Y.; Liang, X.X.; Wu, J.; Shi, Z.F.; Kirillov, A.M. Bringing 5-(3,4-dicarboxylphenyl) picolinic acid to crystal engineering research: Hydrothermal assembly, structural features, and photocatalytic activity of Mn, Ni, Cu, and Zn coordination polymers. CrystEngComm 2018, 20, 906–916. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Santos, C.I.M.; André, V.; Kłak, J.; Kirillova, M.V.; Kirillov, M.V. Copper(II) coordination polymers self-assembled from aminoalcohols and pyromellitic acid: Highly active precatalysts for the mild water-promoted oxidation of alkanes. Inorg. Chem. 2016, 55, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sotnik, S.A.; Polunin, R.A.; Kiskin, M.A.; Kirillov, A.M.; Dorofeeva, V.N.; Gavrilenko, K.S.; Eremenko, I.L.; Novotortsev, V.M.; Kolotilov, S.V. Heterometallic coordination polymers assembled from trigonal trinuclear Fe2Ni-pivalate blocks and polypyridine spacers: Topological diversity, sorption, and catalytic properties. Inorg. Chem. 2015, 54, 5169–5181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L.; Kirillov, A.M. Alkali metal directed assembly of heterometallic V.-v/M. (M. = Na, K., Cs) coordination polymers: Structures, topological analysis, and oxidation catalytic properties. Inorg. Chem. 2013, 52, 8601–8611. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Z.; Kirillov, A.M.; Wu, J.; Lv, D.Y.; Tang, Y.; Wu, J.C. Synthesis, structural versatility, luminescent and magnetic properties of a series of coordination polymers constructed from biphenyl-2,4,4′-tricarboxylate and different N-donor ligands. CrystEngComm 2013, 15, 10287–10303. [Google Scholar] [CrossRef]
- Shao, Y.L.; Cui, Y.H.; Gu, J.Z.; Wu, J.; Wang, Y.W.; Kirillov, A.M. Exploring biphenyl-2,4,4′-tricarboxylic acid as a flexible building block for the hydrothermal self-assembly of diverse metal-organic and supramolecular networks. CrystEngComm 2016, 18, 765–778. [Google Scholar] [CrossRef]
- Jia, J.H.; Athwal, H.S.; Blake, A.J.; Champness, N.R.; Hubberstey, P.; Schroder, M. Increasing nuclearity of secondary building units in porous cobalt(II) metal-organic frameworks: Variation in structure and H2 adsorption. Dalton Trans. 2011, 40, 12342–12349. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Z.; Liang, X.X.; Cui, Y.H.; Wu, J.; Kirillov, A.M. Exploring 4-(3-carboxyphenyl)picolinic acid as a semirigid building block for the hydrothermal self-assembly of diverse metal-organic and supramolecular networks. CrystEngComm 2017, 19, 117–128. [Google Scholar] [CrossRef]
- Gu, J.Z.; Cui, Y.H.; Liang, X.X.; Wu, J.; Lv, D.Y.; Kirillov, A.M. Structurally distinct metal-organicand H-bonded networks derived from 5-(6-carboxypyridin-3-yl)isophthalic acid: Coordination and template effect of 4,4 ′-bipyridine. Cryst. Growth Des. 2016, 16, 4658–4670. [Google Scholar] [CrossRef]
- Gu, J.Z.; Gao, Z.Q.; Tang, Y. pH and auxiliary ligand influence on the structural variations of 5(2′-carboxylphenyl) nicotate coordination polymers. Cryst. Gorwth Des. 2012, 12, 3312–3323. [Google Scholar] [CrossRef]
- Gu, J.Z.; Wen, M.; Liang, X.X.; Shi, Z.; Kirillova, M.V.; Kirillov, A.M. Multifunctional aromatic carboxylic acids as versatile building blocks for hydrothermal design of coordination polymers. Crystals 2018, 8, 83. [Google Scholar] [CrossRef]
- Yan, W.; Han, L.J.; Jia, H.L.; Shen, K.; Wang, T.; Zheng, H.G. Three highly stable cobalt MOFs based on “Y”-shaped carboxylic acid: Synthesis and absorption of anionic dyes. Inorg. Chem. 2016, 55, 8816–8821. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Wang, L.; Yang, G.P.; Wu, Y.L.; Bai, N.N.; Zhang, W.Y.; Wang, Y.Y. Four new lanthanide-organic frameworks: Selective luminescent sensing and magnetic properties. Dalton Trans. 2016, 45, 12800–12806. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Han, L.W.; Wu, D.S.; Huang, Y.B.; Cao, R. Structure versatility of coordination polymers constructed from a semirigid tetracarboxylate ligand: Syntheses, structures, and photoluminescent properties. Cryst. Growth Des. 2013, 13, 255–263. [Google Scholar] [CrossRef]
- Xu, W.; Si, Z.X.; Xie, M.; Zhou, L.X.; Zheng, Y.Q. Experimental and theoretical approaches to three uranyl coordination polymers constructed by phthalic acid and N,N′-donor bridging ligands: Crystal structures, luminescence, and photocatalytic degradation of tetracycline hydrochloride. Cryst. Growth Des. 2017, 17, 2147–2157. [Google Scholar] [CrossRef]
- Wu, Y.P.; Wu, X.Q.; Wang, J.F.; Zhao, J.; Dong, W.W.; Li, D.S.; Zhang, Q.C. Assembly of two novel Cd3/(Cd3 + Cd5)-cluster-based metal-organic frameworks: Structures, luminescence, and photocatalytic degradation of organic dyes. Cryst. Growth Des. 2016, 16, 2309–2316. [Google Scholar] [CrossRef]
- Shao, Y.L.; Cui, Y.H.; Gu, J.Z.; Kirillov, A.M.; Wu, J.; Wang, Y.W. A variety of metal-organic and supramolecular networks constructed from a new flexible multifunctional building block bearing picolinate and terephthalate functionalities: Hydrothermal self-assembly, structural features, magnetic and luminescent properties. RSC Adv. 2015, 5, 87484–87495. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Qiu, W.D.; You, A.; Zou, X.Z.; Gu, J.Z.; Chen, B. Mn(II) and Co(II) coordination polymers constructed from pyridine-tricarboxylate ligand. Chin. J. Struct. Chem. 2017, 36, 661–670. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for X-ray Crystal Structure Determination; University of Gottingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for X-ray Crystal Structure Refinement; University of Gottingen: Göttingen, Germany, 1997. [Google Scholar]
- Van de Sluis, P.; Spek, A.L. Bypass-an effective merhve method for the refinement of crystal-structures containing disordered solvent regions. Acta Crystallogr. Sect. A 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. IUCr CompComm Newsl. 2006, 7, 4–38. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package topospro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Gu, J.Z.; Liang, X.X.; Cai, Y.; Wu, J.; Shi, Z.F.; Kirillov, A.M. Hydrothermal assembly, structures, topologies, luminescence, and magnetism of a novel series of coordination polymers driven by a trifunctional nicotinic acid building block. Dalton Trans. 2017, 46, 10908–10925. [Google Scholar] [CrossRef] [PubMed]

| Compound | 1 | 2 |
|---|---|---|
| Chemical formula | C52H32Pb3N6O14 | C24H27Zn2N3O13 |
| Molecular weight | 1586.40 | 696.18 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | Pn | C2/c |
| a/Å | 16.1620(4) | 24.2264(8) |
| b/Å | 8.61606(17) | 19.3903(9) |
| c/Å | 18.3591(4) | 4.0603(6) |
| α/(°) | 90 | 90 |
| β/(°) | 109.333(3) | 98.645(4) |
| γ/(°) | 90 | 90 |
| V/Å 3 | 2412.40(10) | 6529.9(5) |
| Z | 2 | 8 |
| F(000) | 1488 | 2608 |
| Crystal size/mm | 0.29 × 0.26 × 0.25 | 0.21 × 0.18 × 0.16 |
| θ range for data collection | 3.332–25.050 | 3.265–25.050 |
| Limiting indices | −19 ≤ h ≤ 11, −9 ≤ k ≤ 10, −17 ≤ l ≤ 21 | −28 ≤ h ≤ 19, −11 ≤ k ≤ 23, −15 ≤ l ≤ 16 |
| Reflections collected/unique (Rint) | 8845/5582 (0.0385) | 12181/5789 (0.0660) |
| Dc/(Mg·cm−3) | 2.184 | 1.306 |
| μ/mm−1 | 10.520 | 1.518 |
| Data/restraints/parameters | 5582/61/677 | 5789/0/343 |
| Goodness-of-fit on F2 | 1.020 | 0.975 |
| Final R indices[(I ≥ 2σ(I))] R1, wR2 | 0.0374, 0.0450 | 0.0707, 0.1809 |
| R indices (all data) R1, wR2 | 0.0688, 0.0742 | 0.1320, 0.2111 |
| Largest diff. peak and hole/(e·Å−3) | 1.345 and −0.931 | 0.932 and −0.490 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Cai, Y.; Wen, M.; Ge, Z.; Kirillov, A.M. New Topologically Unique Metal-Organic Architectures Driven by a Pyridine-Tricarboxylate Building Block. Crystals 2018, 8, 353. https://doi.org/10.3390/cryst8090353
Gu J, Cai Y, Wen M, Ge Z, Kirillov AM. New Topologically Unique Metal-Organic Architectures Driven by a Pyridine-Tricarboxylate Building Block. Crystals. 2018; 8(9):353. https://doi.org/10.3390/cryst8090353
Chicago/Turabian StyleGu, Jinzhong, Yan Cai, Min Wen, Zhijun Ge, and Alexander M. Kirillov. 2018. "New Topologically Unique Metal-Organic Architectures Driven by a Pyridine-Tricarboxylate Building Block" Crystals 8, no. 9: 353. https://doi.org/10.3390/cryst8090353






