Investigation of Approaches to Control the Compositions of Zn(Se,OH) Buffers Prepared by Chemical Bath Deposition Process for Cu(In,Ga)Se2 (CIGS) Solar Cells
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
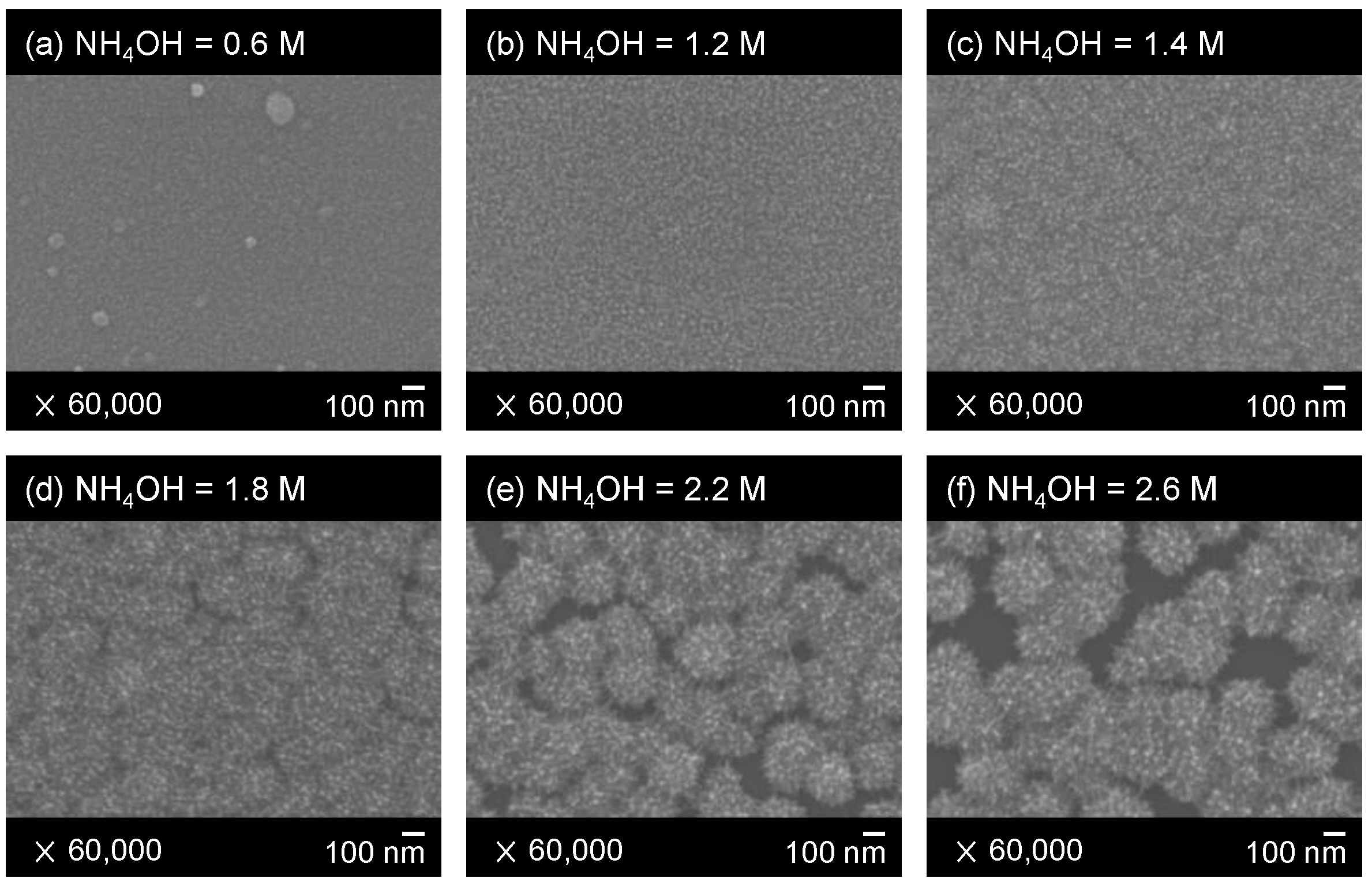
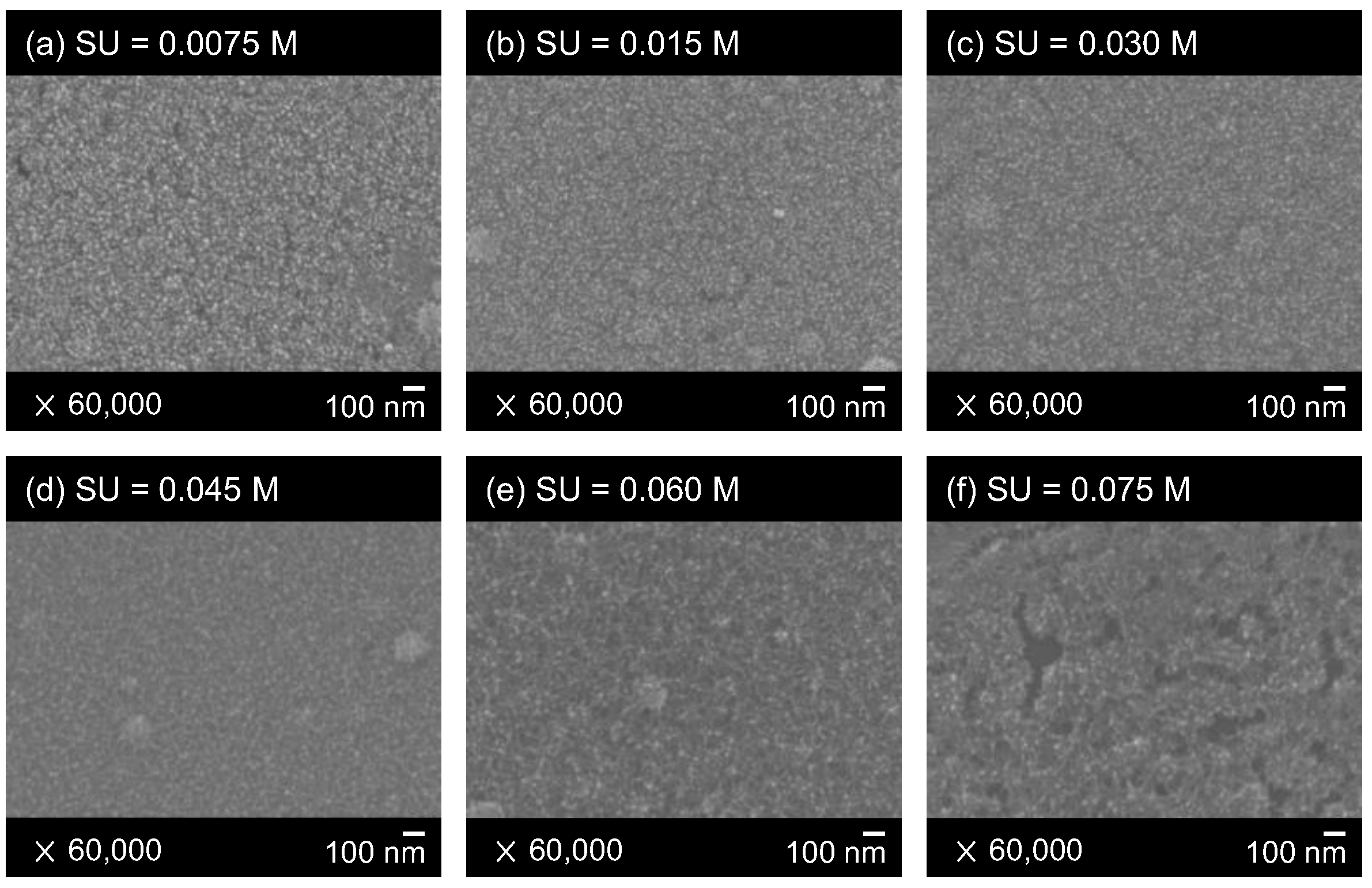
3.1. Surface Morphologies
3.2. Crystal Structures
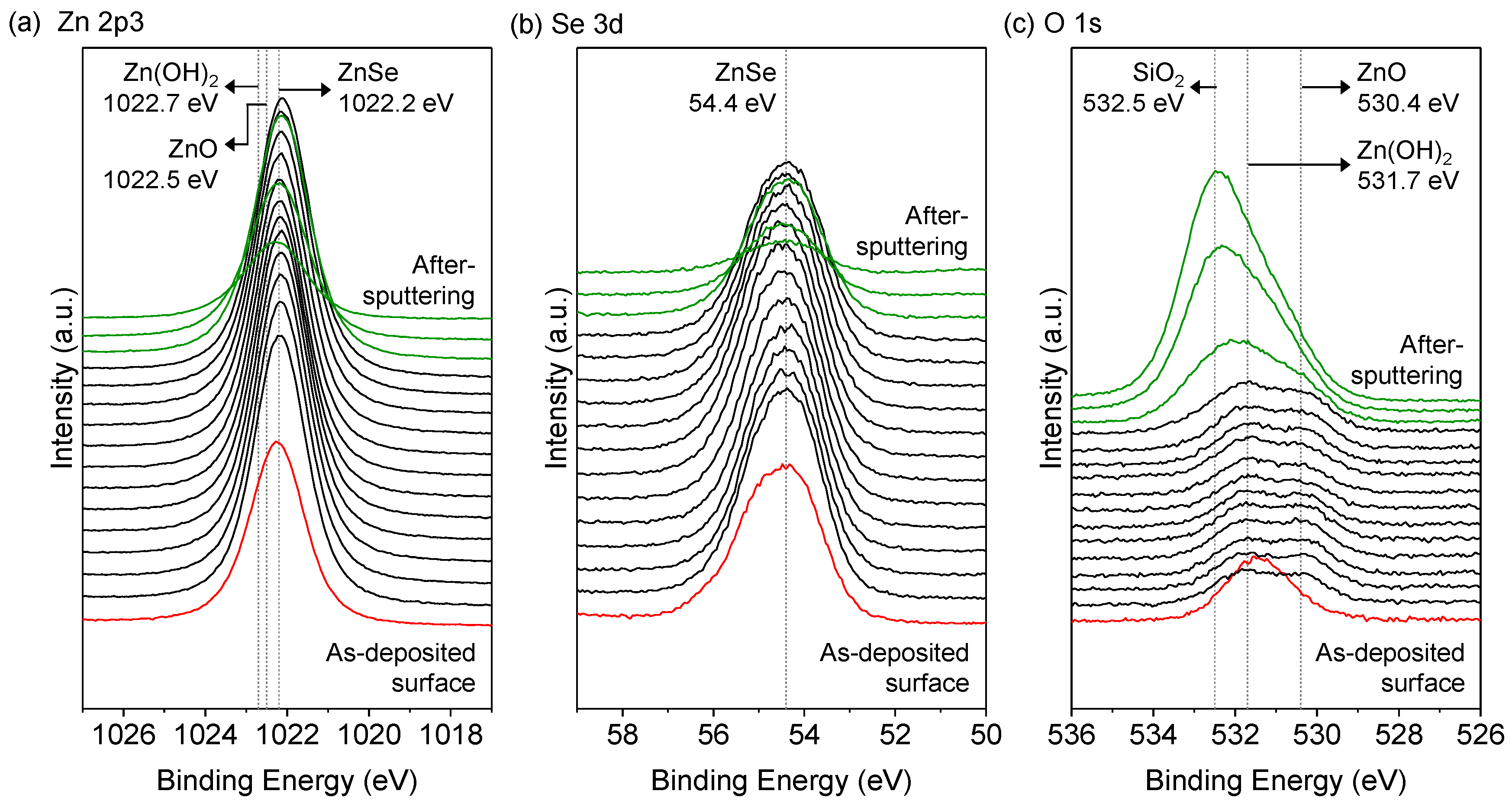
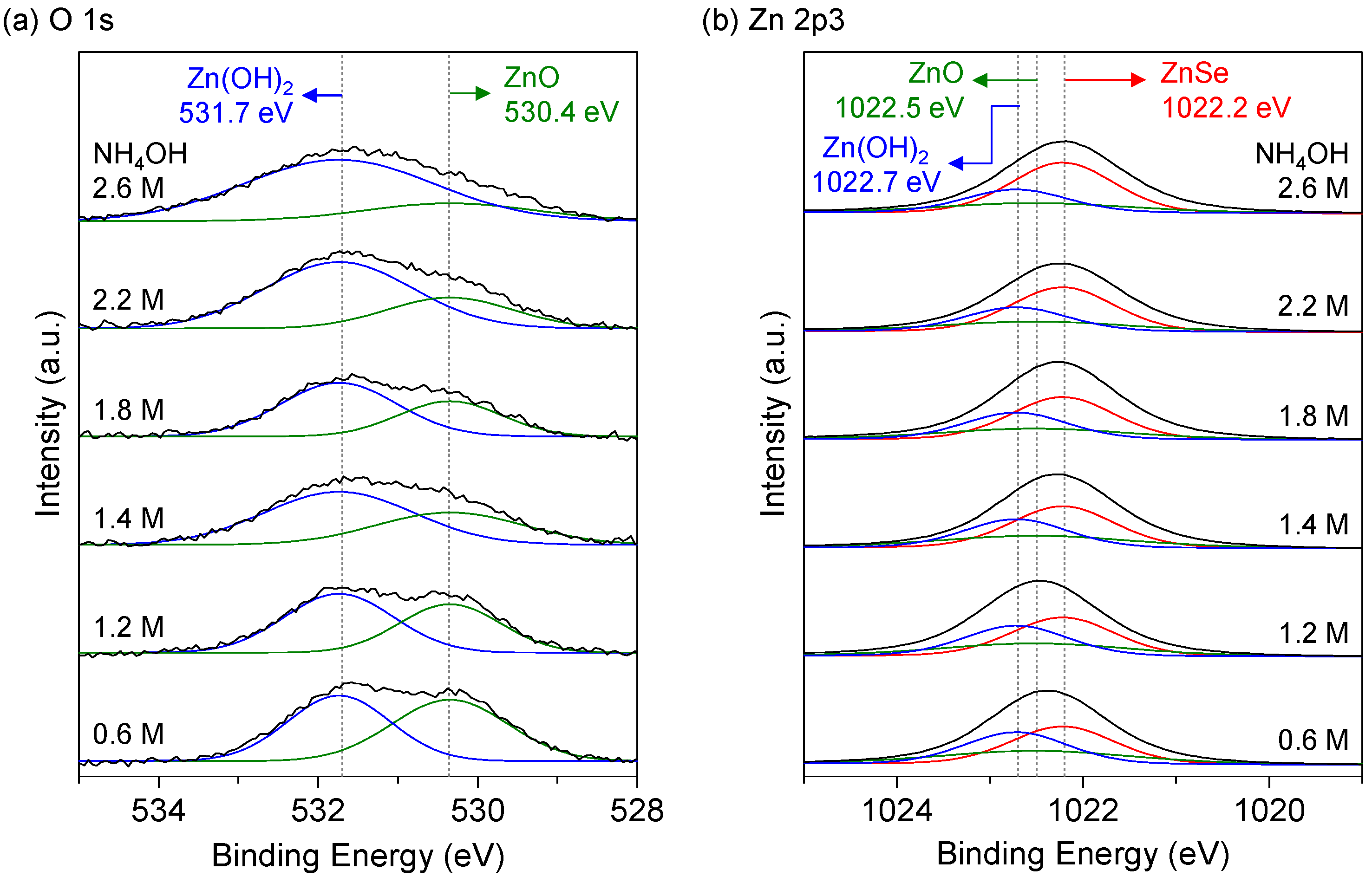
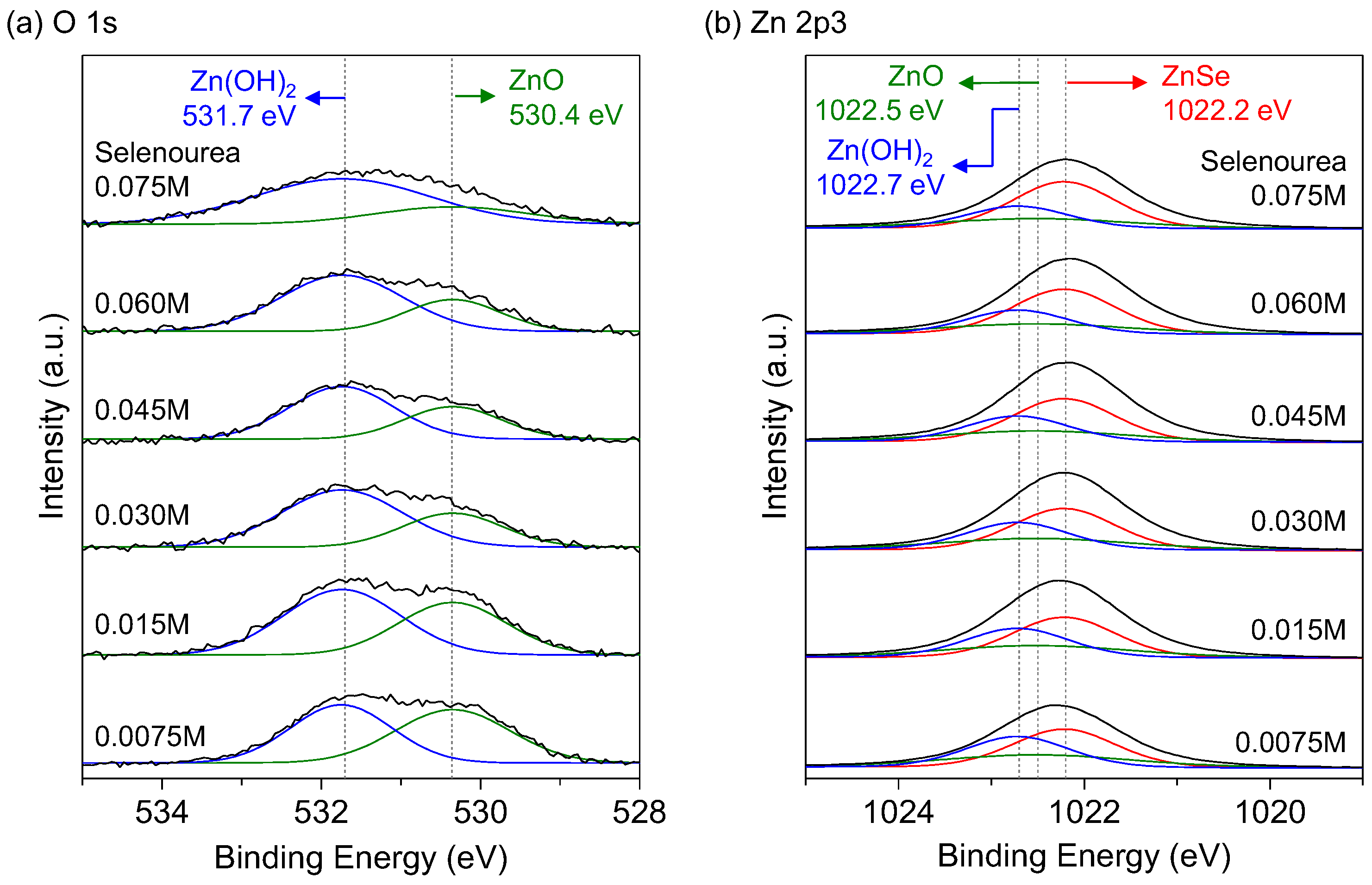
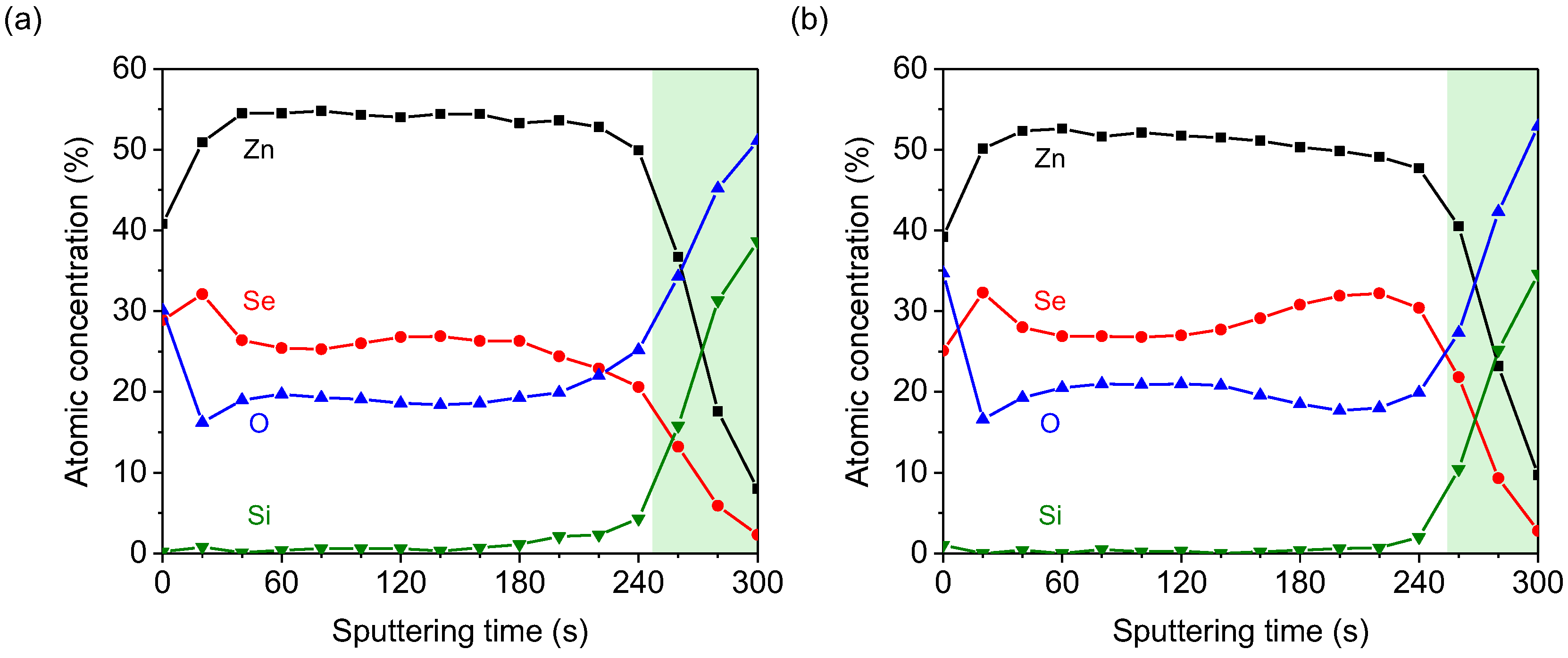
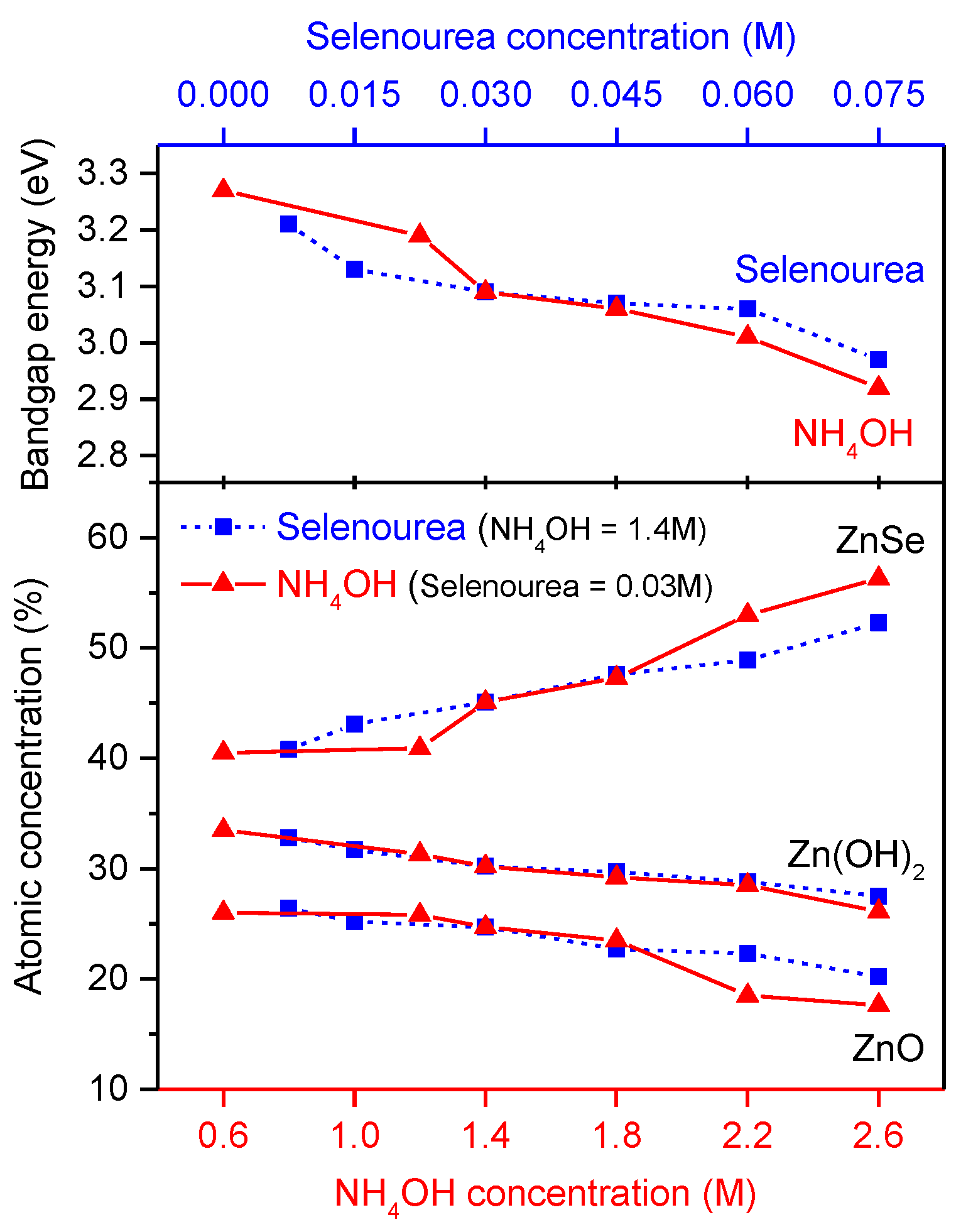
3.3. Compositions
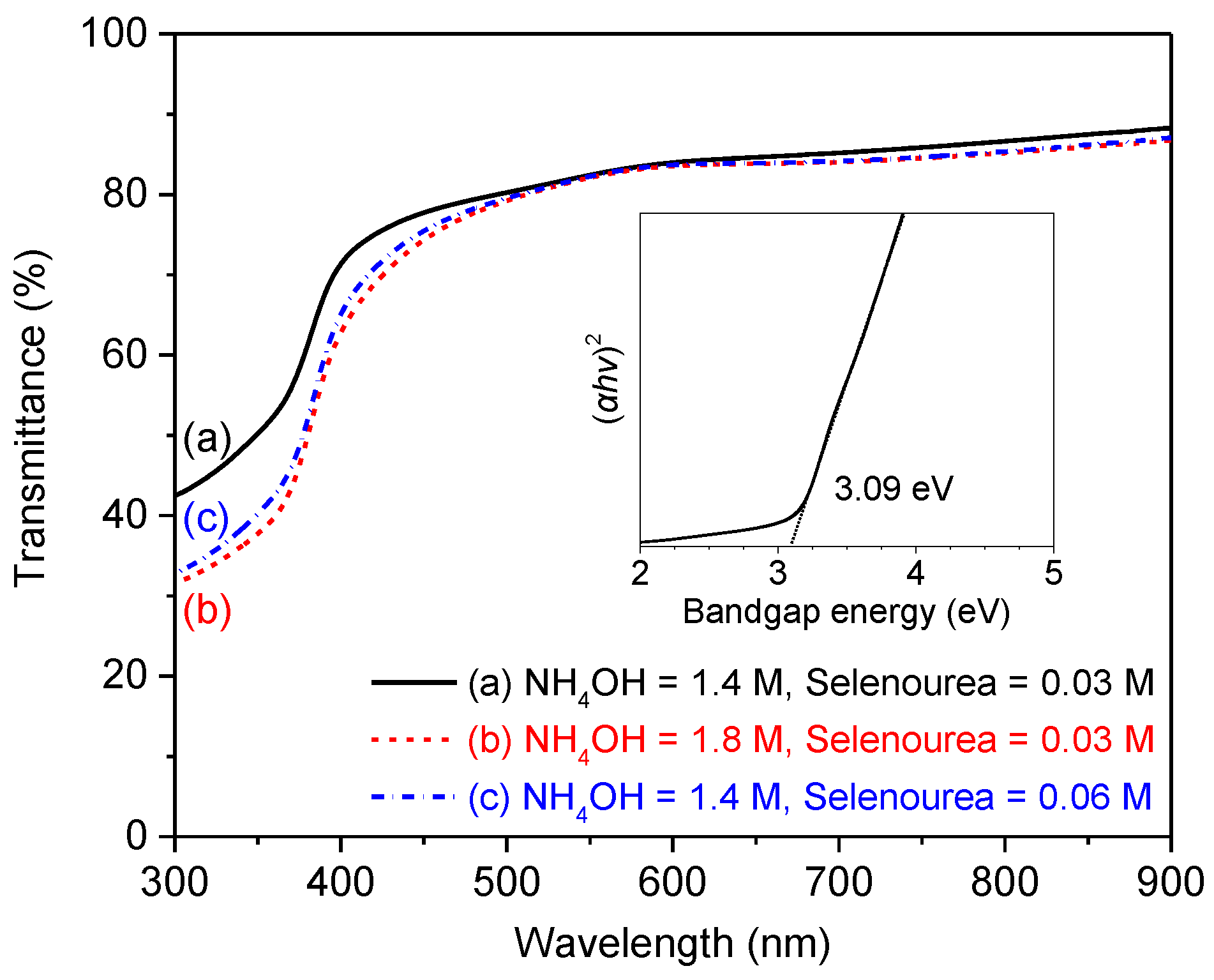
3.4. Optical Properties
3.5. Bandgap Energies
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of heavy alkali elements in Cu(In,Ga)Se2 solar cells with efficiencies up to 22.6%. Phys. Status Solidi-Rapid Res. Lett. 2016, 10, 583–586. [Google Scholar] [CrossRef]
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (version 52). Prog. Photovolt. Res. Appl. 2018, 26, 427–436. [Google Scholar] [CrossRef]
- Mughal, M.A.; Engelken, R.; Sharma, R. Progress in indium (III) sulfide (In2S3) buffer layer deposition techniques for CIS, CIGS, and CdTe-based thin film solar cells. Sol. Energy 2015, 120, 131–146. [Google Scholar] [CrossRef]
- Pawar, S.M.; Pawar, B.S.; Kim, J.H.; Joo, O.-S.; Lokhande, C.D. Recent status of chemical bath deposited metal chalcogenide and metal oxide thin films. Curr. Appl. Phys. 2011, 11, 117–161. [Google Scholar] [CrossRef]
- Naghavi, N.; Abou-Ras, D.; Allsop, N.; Barreau, N.; Bücheler, S.; Ennaoui, A.; Fischer, C.H.; Guillen, C.; Hariskos, D.; Herrero, J.; et al. Buffer layers and transparent conducting oxides for chalcopyrite Cu(In,Ga)(S,Se)2 based thin film photovoltaics: Present status and current developments. Prog. Photovolt. Res. Appl. 2010, 18, 411–433. [Google Scholar] [CrossRef]
- Kato, T. Cu(In,Ga)(Se,S)2 solar cell research in Solar Frontier: Progress and current status. Jpn. J. Appl. Phys. 2017, 56, 04CA02. [Google Scholar] [CrossRef]
- Hariskos, D.; Ruckh, M.; Rühle, U.; Walter, T.; Schock, H.W.; Hedström, J.; Stolt, L. A novel cadmium free buffer layer for Cu(In,Ga)Se2 based solar cells. Sol. Energy Mater. Sol. Cell 1996, 41–42, 345–353. [Google Scholar] [CrossRef]
- Ennaoui, A.; Blieske, U.; Lux-Steiner, M.C. 13.7%-efficient Zn(Se,OH)x/Cu(In,Ga)(S,Se)2 thin-film solar cell. Prog. Photovolt. Res. Appl. 1998, 6, 447–451. [Google Scholar] [CrossRef]
- Ennaoui, A.; Weber, M.; Saad, M.; Harneit, W.; Lux-Steiner, M.C.; Karg, F. Chemical bath deposited Zn(Se,OH)x on Cu(In,Ga)(S,Se)2 for high efficiency thin film solar cells: Growth kinetics, electronic properties, device performance and loss analysis. Thin Solid Films 2000, 361, 450–453. [Google Scholar] [CrossRef]
- Ennaoui, A.; Siebentritt, S.; Lux-Steiner, M.C.; Riedl, W.; Karg, F. High-efficiency Cd-free CIGSS thin-film solar cells with solution grown zinc compound buffer layers. Sol. Energy Mater. Sol. Cell 2001, 67, 31–40. [Google Scholar] [CrossRef]
- Eisele, W.; Ennaoui, A.; Schubert-Bischoff, P.; Giersig, M.; Pettenkofer, C.; Krauser, J.; Lux-Steiner, M.; Zweigart, S.; Karg, F. XPS, TEM and NRA investigations of Zn(Se,OH)/Zn(OH)2 films on Cu(In,Ga)(S,Se)2 substrates for highly efficient solar cells. Sol. Energy Mater. Sol. Cell 2003, 75, 17–26. [Google Scholar] [CrossRef]
- Ennaoui, A.; Eisele, W.; Lux-Steiner, M.; Niesen, T.P.; Karg, F. Highly efficient Cu(Ga,In)(S,Se)2 thin film solar cells with zinc-compound buffer layers. Thin Solid Films 2003, 431–432, 335–339. [Google Scholar] [CrossRef]
- Chaparro, A.; Maffiotte, C.; Gutiérrez, M.; Herrero, J.; Klaer, J.; Siemer, K.; Bräunig, D. Characterisation of CuInS2/ZnSe junctions by XPS and electroreflectance. Thin Solid Films 2001, 387, 104–107. [Google Scholar] [CrossRef]
- Chaparro, A.M.; Gutiérrez, M.T.; Herrero, J.; Klaer, J.; Romero, M.J.; Al-Jassim, M.M. Characterisation of CuInS2/Zn(Se,O)/ZnO solar cells as a function of Zn(Se,O) buffer deposition kinetics in a chemical bath. Prog. Photovolt. Res. Appl. 2002, 10, 465–480. [Google Scholar] [CrossRef]
- Kanevce, A.; Ramanathan, K.; Contreras, M. Impact of buffer and absorber properties in the vicinity of the interface on wide-gap Cu(In,Ga)Se2 solar cell performance. In Proceedings of the 40th IEEE Photovoltaic Specialist Conference, Denver, CO, USA, 8–13 June 2014; pp. 382–386. [Google Scholar]
- Niemegeers, A.; Burgelman, M.; De Vos, A. On the CdS/CuInSe2 conduction band discontinuity. Appl. Phys. Lett. 1995, 67, 843–845. [Google Scholar] [CrossRef]
- Klenk, R. Characterisation and modelling of chalcopyrite solar cells. Thin Solid Films 2001, 387, 135–140. [Google Scholar] [CrossRef]
- Gloeckler, M.; Sites, J.R. Efficiency limitations for wide-band-gap chalcopyrite solar cells. Thin Solid Films 2005, 480–481, 241–245. [Google Scholar] [CrossRef]
- Song, T.; Kanevce, A.; Sites, J.R. Emitter/absorber interface of CdTe solar cells. J. Appl. Phys. 2016, 119, 233104. [Google Scholar] [CrossRef] [Green Version]
- Li, J.V.; Grover, S.; Contreras, M.A.; Ramanathan, K.; Kuciauskas, D.; Noufi, R. A recombination analysis of Cu(In,Ga)Se2 solar cells with low and high ga compositions. Sol. Energy Mater. Sol. Cell 2014, 124, 143–149. [Google Scholar] [CrossRef]
- Paul, S.; Grover, S.; Repins, I.L.; Keyes, B.M.; Contreras, M.A.; Ramanathan, K.; Noufi, R.; Zhao, Z.; Liao, F.; Li, J.V. Analysis of back-contact interface recombination in thin-film solar cells. IEEE J. Photovolt. 2018, 8, 871–878. [Google Scholar] [CrossRef]
- Agawane, G.L.; Shin, S.W.; Suryawanshi, M.P.; Gurav, K.V.; Moholkar, A.V.; Lee, J.Y.; Patil, P.S.; Yun, J.H.; Kim, J.H. Novel reduced toxic route synthesis and characterization of chemical bath deposited ZnSe thin films. Ceram. Int. 2014, 40, 367–374. [Google Scholar] [CrossRef]
- Agawane, G.L.; Wook Shin, S.; Suryawanshi, M.P.; Gurav, K.V.; Moholkar, A.V.; Yong Lee, J.; Patil, P.S.; Ho Yun, J.; Hyeok Kim, J. Preparation and characterization of chemical bath deposited nanocrystalline ZnSe thin films using Na3-citrate and hydrazine hydrate: A comparative study. Mater. Lett. 2013, 106, 186–189. [Google Scholar] [CrossRef]
- Islam, R.; Rao, D.R. X-ray photoelectron spectroscopy of Zn1−xCdxSe thin films. J. Electron. Spectrosc. 1996, 81, 69–77. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Appendix B. In Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Eden Prairie, MN, USA, 1995; pp. 231–232, 242. ISBN 0-9648124-1-X. [Google Scholar]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Guimon, M.F.; Pfister-Guillouzo, G.; Bremont, M.; Brockmann, W.; Quet, C.; Chenard, J.Y. Application of X-ray photoelectron spectroscopy to the study of degradation mechanisms of epoxy-bonded joints of zinc coated steel. Appl. Surf. Sci. 1997, 108, 149–157. [Google Scholar] [CrossRef]
- Sadekar, H.K.; Ghule, A.V.; Sharma, R. Nanocrystalline ZnSe thin films prepared by solution growth technique for photosensor application. Compos. Part B-Eng. 2013, 44, 553–557. [Google Scholar] [CrossRef]
- Wei, A.; Zhao, X.; Liu, J.; Zhao, Y. Investigation on the structure and optical properties of chemically deposited ZnSe nanocrystalline thin films. Physica B 2013, 410, 120–125. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, D.; Zhai, G.; Zhang, J. Comparative study of ZnSe thin films deposited from modified chemical bath solutions with ammonia-containing and ammonia-free precursors. Mater. Chem. Phys. 2010, 120, 456–460. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Patil, P.S.; Tributsch, H.; Ennaoui, A. ZnSe thin films by chemical bath deposition method. Sol. Energy Mater. Sol. Cell 1998, 55, 379–393. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. Optical absorption edge of ZnO thin films: The effect of substrate. J. Appl. Phys. 1997, 81, 6357–6364. [Google Scholar] [CrossRef]
- Ernits, K.; Muska, K.; Danilson, M.; Raudoja, J.; Varema, T.; Volobujeva, O.; Altosaar, M. Anion effect of zinc source on chemically deposited ZnS(O,OH) films. Adv. Mater. Sci. Eng. 2009, 2009. [Google Scholar] [CrossRef]
- Doña, J.M.; Herrero, J. Chemical-bath deposition of ZnSe thin films process and material characterization. J. Electrochem. Soc. 1995, 142, 764–770. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Jan, Y.-L.; Chuang, W.-J.; Lu, P.-T. Investigation of Approaches to Control the Compositions of Zn(Se,OH) Buffers Prepared by Chemical Bath Deposition Process for Cu(In,Ga)Se2 (CIGS) Solar Cells. Crystals 2018, 8, 343. https://doi.org/10.3390/cryst8090343
Huang C-H, Jan Y-L, Chuang W-J, Lu P-T. Investigation of Approaches to Control the Compositions of Zn(Se,OH) Buffers Prepared by Chemical Bath Deposition Process for Cu(In,Ga)Se2 (CIGS) Solar Cells. Crystals. 2018; 8(9):343. https://doi.org/10.3390/cryst8090343
Chicago/Turabian StyleHuang, Chia-Hua, Yueh-Lin Jan, Wen-Jie Chuang, and Po-Tsung Lu. 2018. "Investigation of Approaches to Control the Compositions of Zn(Se,OH) Buffers Prepared by Chemical Bath Deposition Process for Cu(In,Ga)Se2 (CIGS) Solar Cells" Crystals 8, no. 9: 343. https://doi.org/10.3390/cryst8090343






