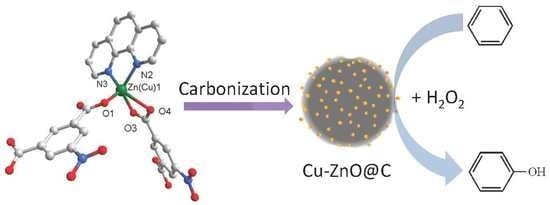
One-Step Synthesis of Cu–ZnO@C from a 1D Complex [Cu0.02Zn0.98(C8H3NO6)(C12H8N2)]n for Catalytic Hydroxylation of Benzene to Phenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Complex
2.3. Experimental Instruments
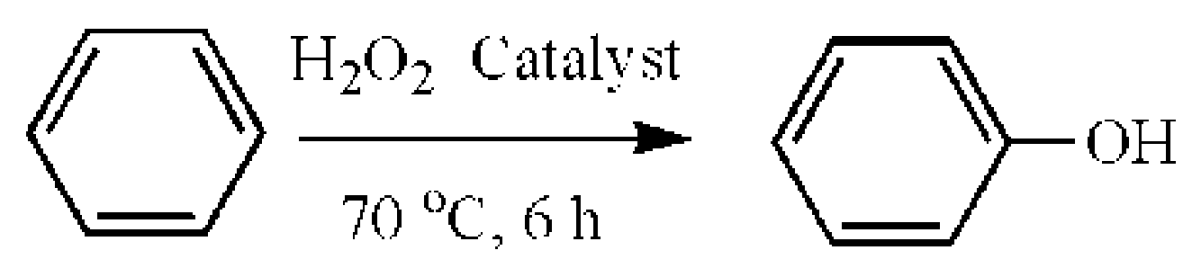
2.4. Evaluation of Catalytic Activity
3. Results and Discussion
3.1. Single Crystal X-ray Data Collection of Complex
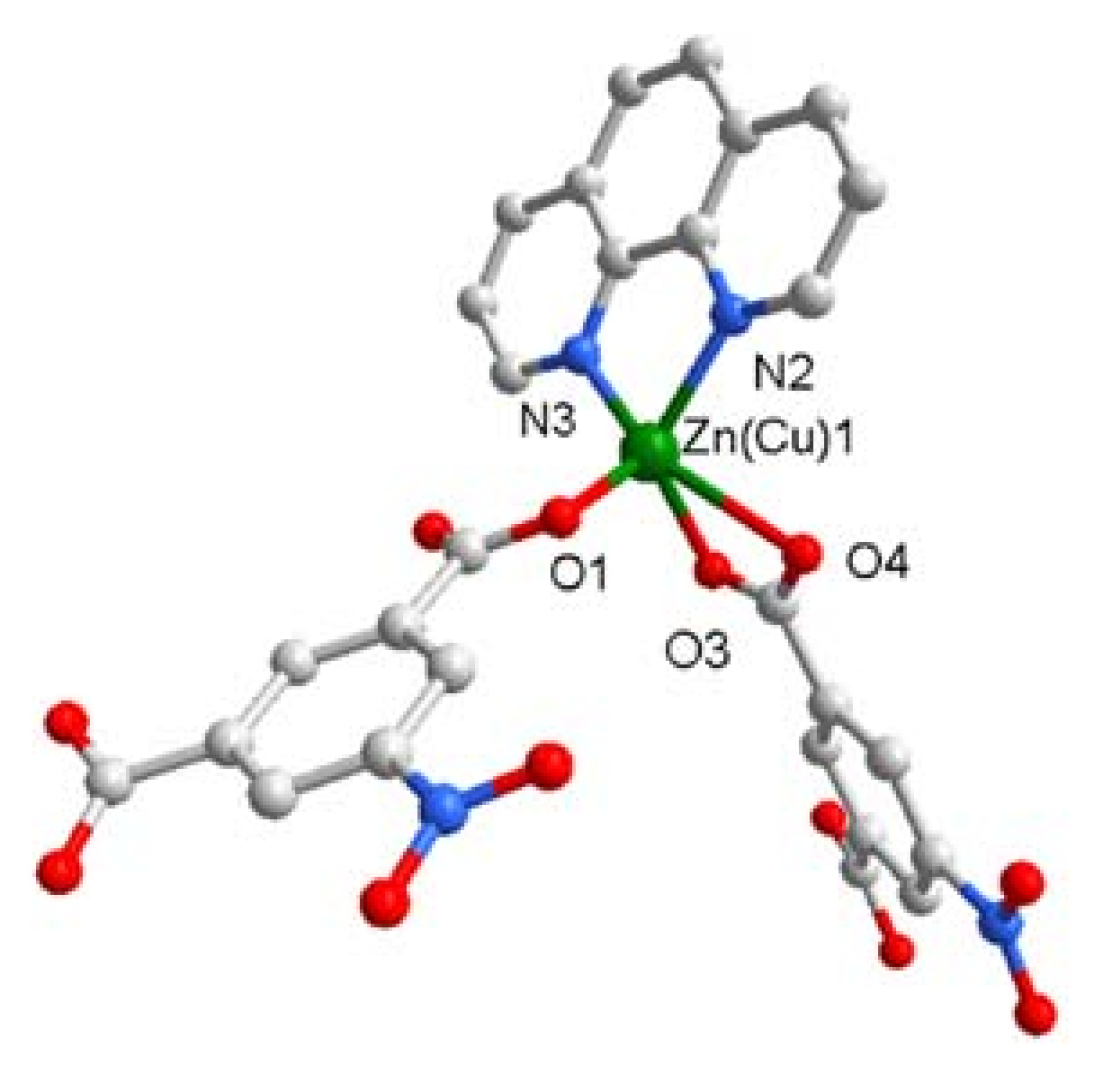
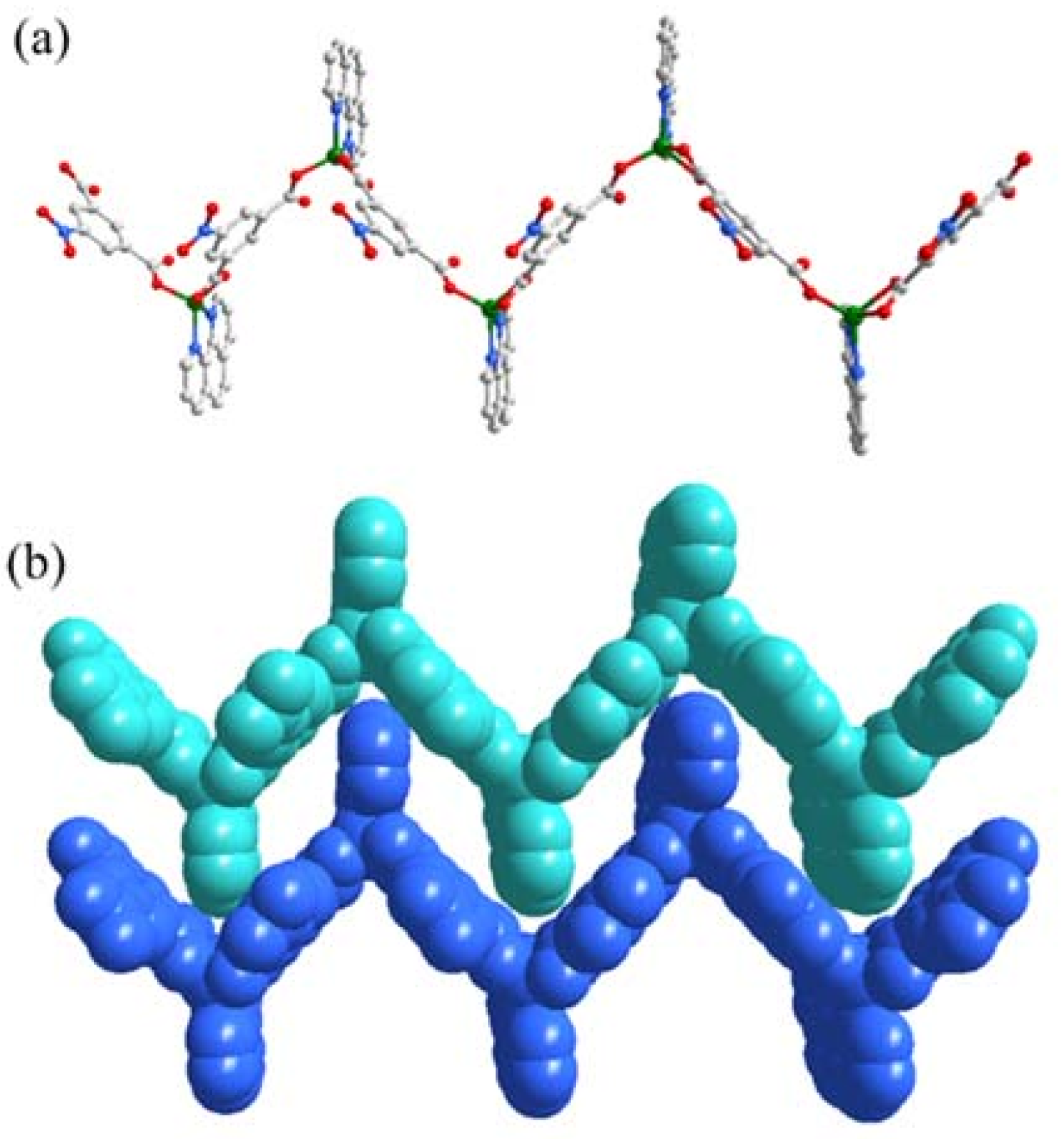
3.2. Description of Crystal Structure
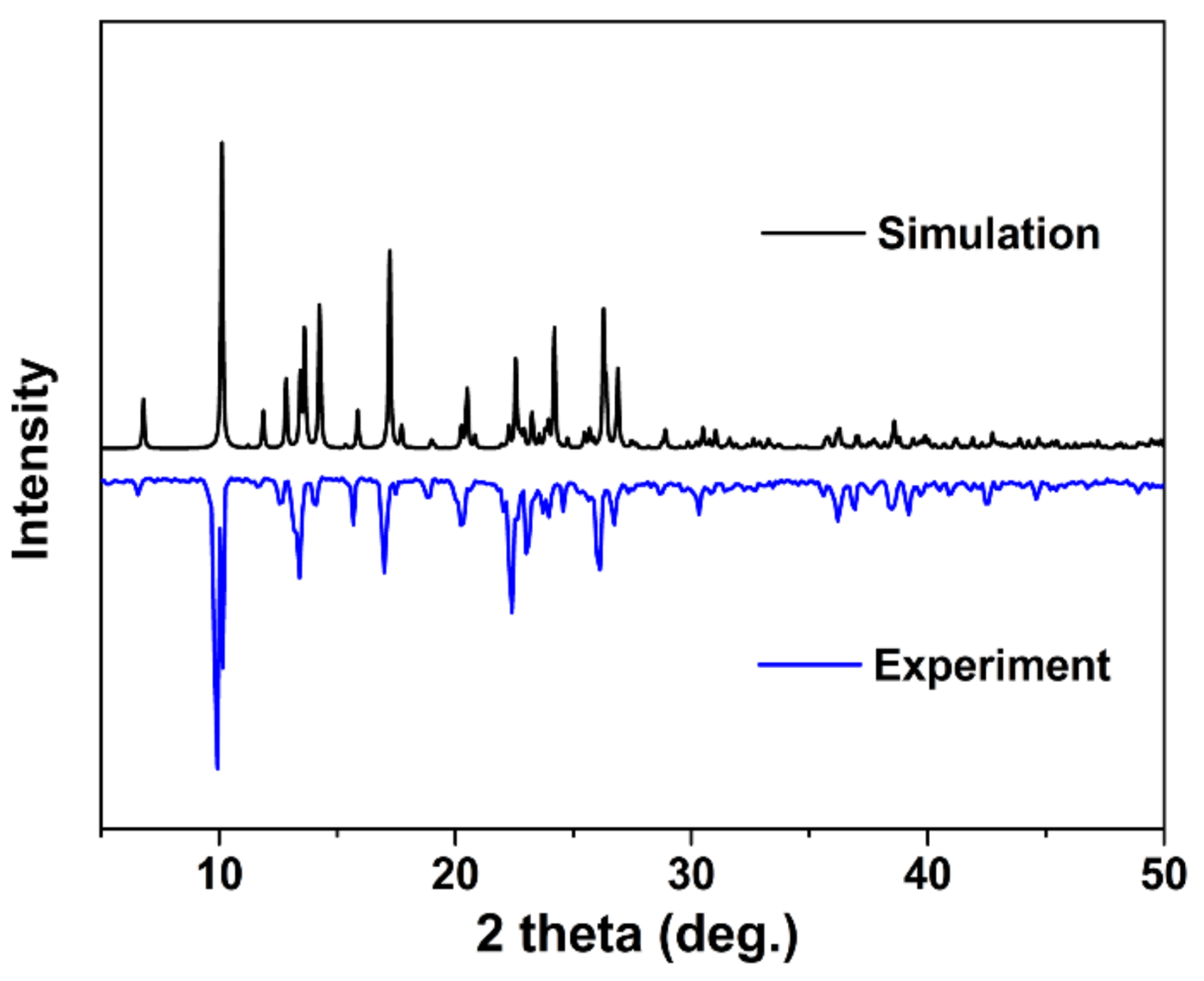
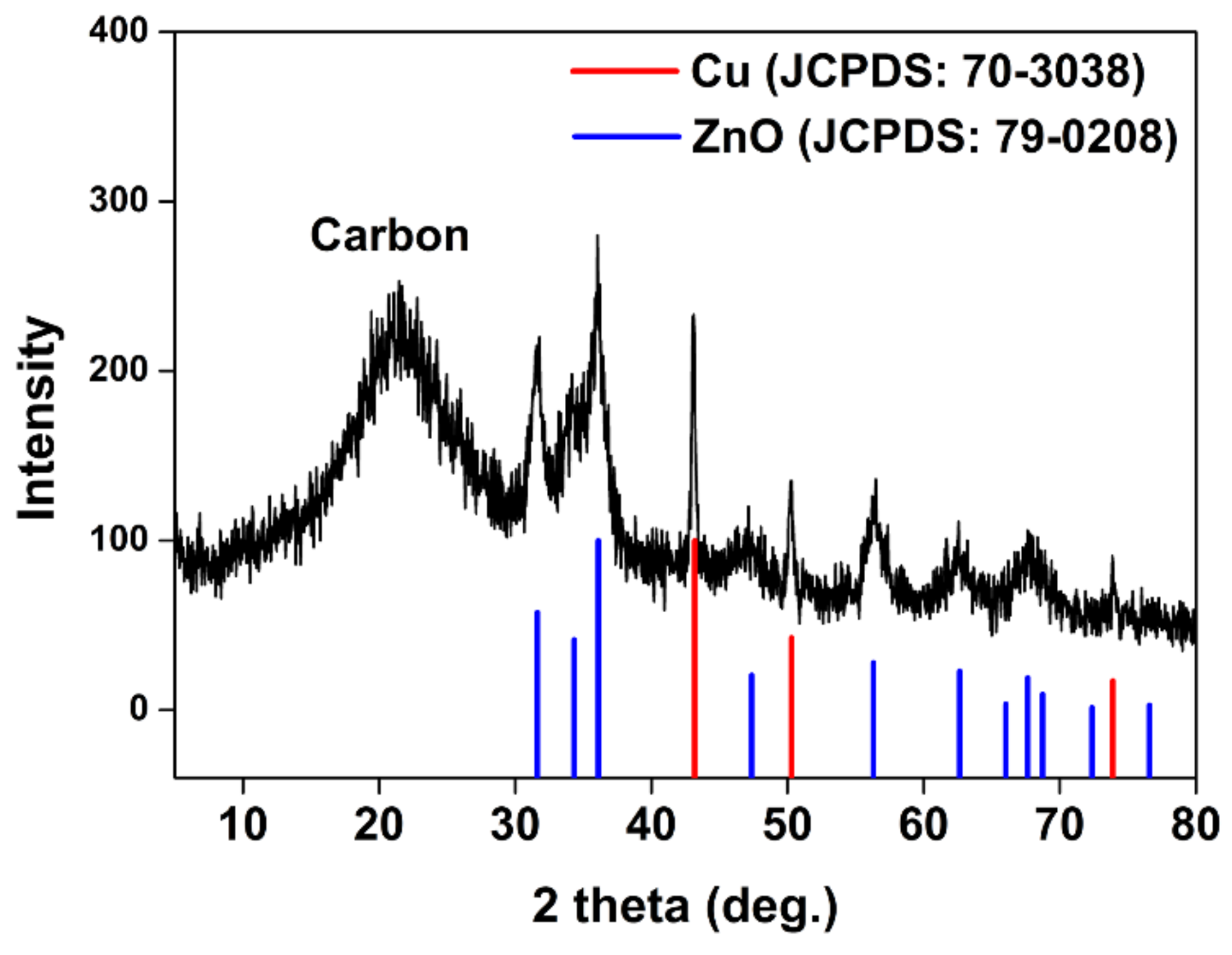
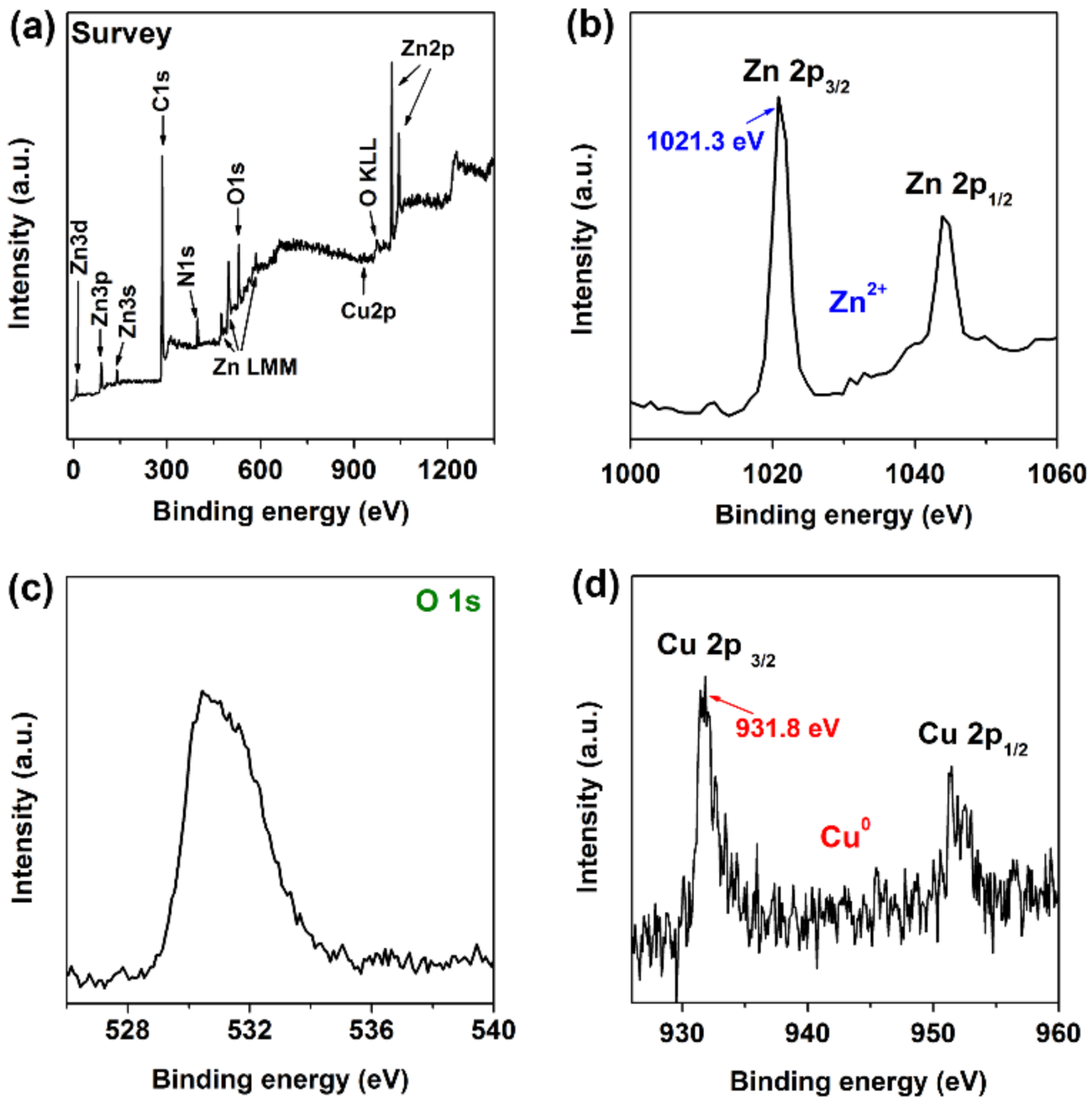
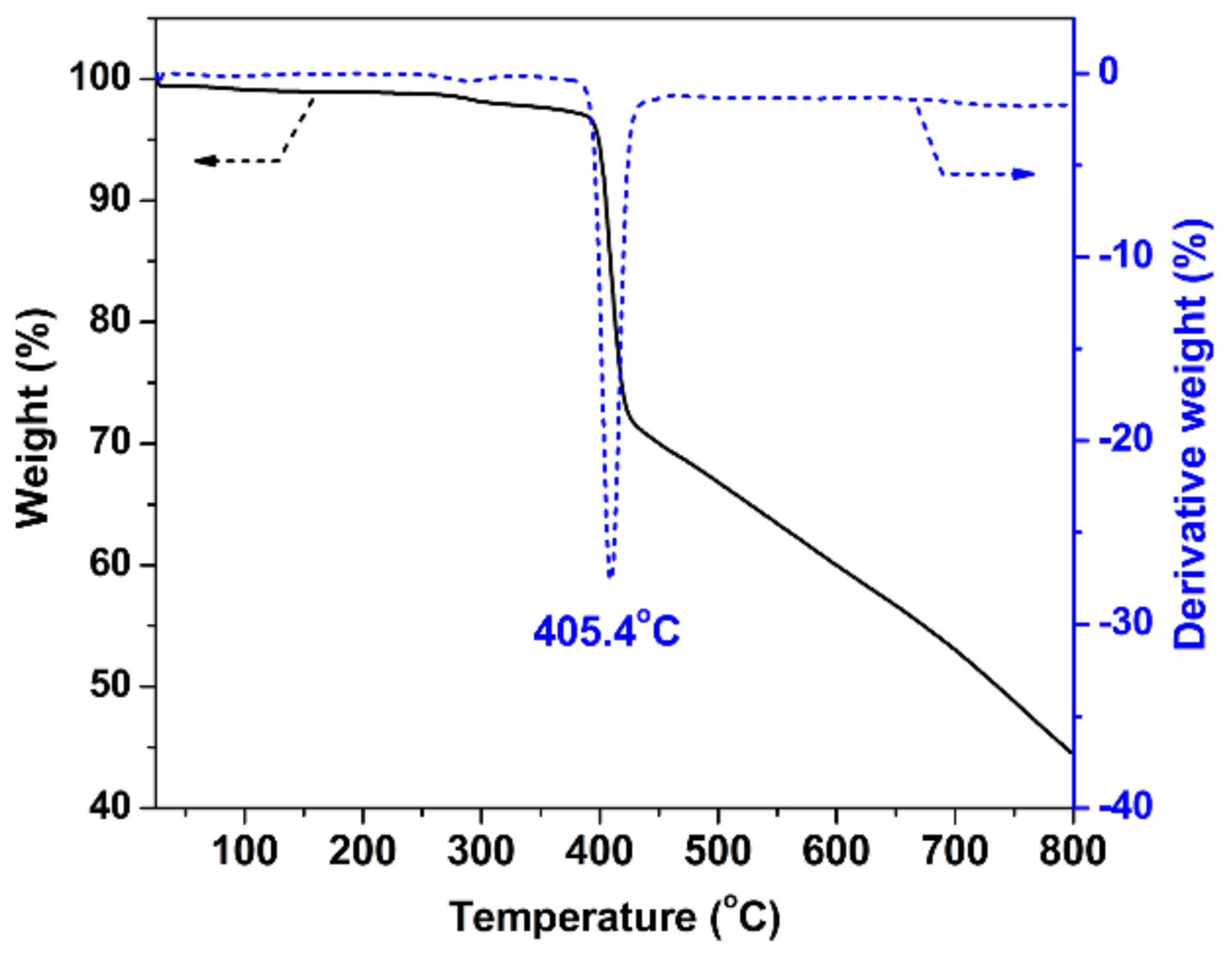
3.3. Characterization
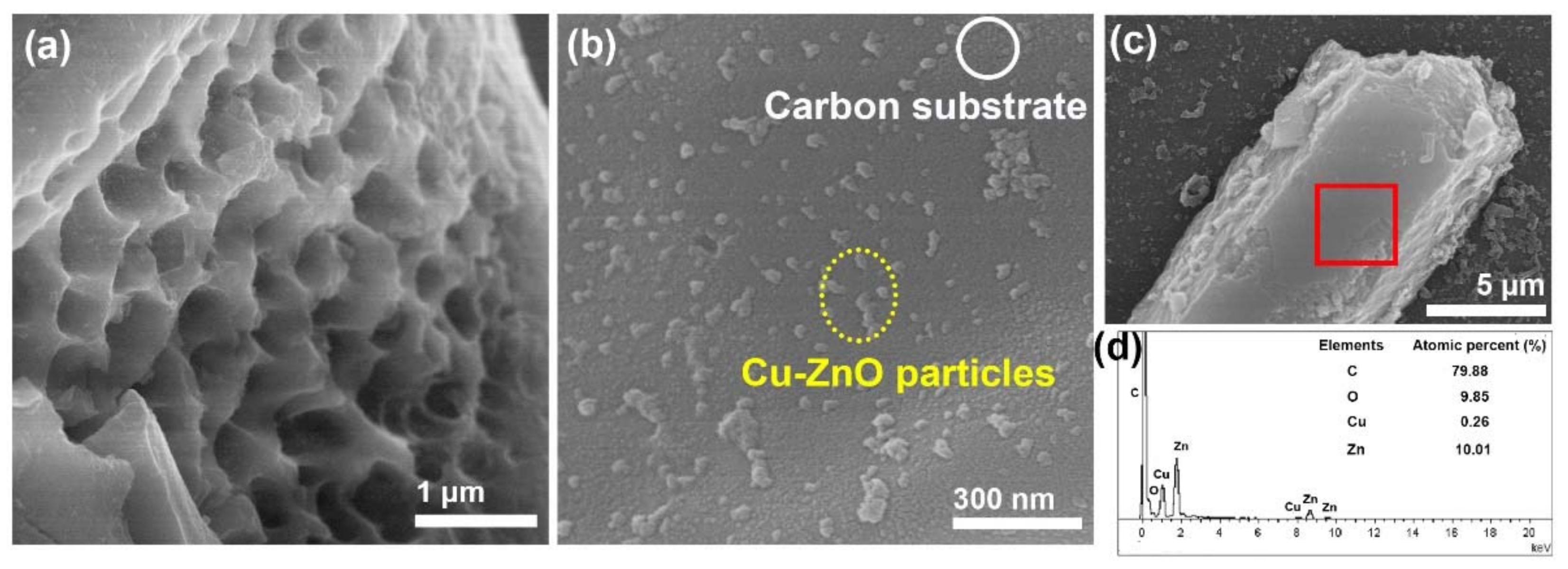
3.4. SEM Characterization
3.5. Measurement of Cu–ZnO@C Composite Catalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wei, C.; Li, X.; Xu, F.; Tan, H.; Li, Z.; Sun, L.; Song, Y. Metal organic frame work-derived anthill-like Cu@carbon nanocomposites for nonenzymatic glucose sensor. Anal. Methods 2014, 6, 1550–1557. [Google Scholar] [CrossRef]
- Yu, G.; Sun, J.; Muhammad, F.; Wang, P.; Zhu, G. Cobalt-based metal organic frame work as precursor to achieve superior catalytic activity for aerobic epoxidation of styrene. RSC Adv. 2014, 4, 38804–38811. [Google Scholar] [CrossRef]
- McDonald, T.M.; D’Alessandro, D.M.; Krishnac, R.; Long, J.R. Enhanced carbon dioxide capture upon incorporation of N,N′-dimethylethylenediamine in the metal-organic framework CuBTTri. Chem. Sci. 2011, 2, 2022–2028. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal-organic framework. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.-H.; Huang, Q.; Fang, X.-F.; Zhu, L.-W.; Yan, Z. Tuning the spin states of two apical iron(II) ions in a pentanuclear iron(II) cluster helicate through the choice of anions. Crystals 2018, 8, 119. [Google Scholar]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF derived nanoporous carbons and their promising applications. J. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A. Applications of metal-organic coordination polymers as precursors for preparation of nano-materials. Coord. Chem. Rev. 2012, 256, 2921–2943. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Q. Metal-organic frameworks as platforms for clean energy. Energy Environ. Sci. 2013, 6, 1656–1683. [Google Scholar] [CrossRef]
- Radhakrishnan, L.; Reboul, J.; Furukawa, S.; Srinivasu, P.; Kitagawa, S.; Yamauchi, Y. Preparation of microporous carbon fibers through carbonization of Al-based porous coordination polymer (Al-PCP) with furfuryl alcohol. Chem. Mater. 2011, 23, 1225–1231. [Google Scholar] [CrossRef]
- Song, J.; Zhu, C.; Xu, B.; Fu, S.; Engelhard, M.H.; Ye, R.; Du, D.; Beckman, S.P.; Lin, Y. Bimetallic cobalt-based phosphide zeolitic imidazolate framework: CoPx phase-dependent electrical conductivity and hydrogen atom adsorption energy for efficient overall water splitting. Adv. Energy Mater. 2017, 7, 1601555. [Google Scholar] [CrossRef]
- Zhen, W.; Jiao, W.; Wu, Y.; Jing, H.; Lu, G. The role of interlayer metallic copper for visible photocatalytic hydrogen generation over Cu/Cu2O/Cu/TiO2 catalyst. Catal. Sci. Technol. 2017, 7, 5028–5037. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Y.; Zheng, Q.; Zheng, Y.; Lin, X.; Li, D.; Zhu, J. Water-gas shift reaction over aluminum promoted Cu/CeO2 nanocatalysts characterized by XRD, BET, TPR and Cyclic Voltammetry (CV). Catal. Lett. 2007, 118, 91–97. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Chen, C.; Zhang, Y.; Zhan, Y.; Lin, X.; Zheng, Q.; Wang, H.; Ma, H.; Ding, L.; et al. Modified precipitation processes and optimized copper content of CuO-CeO2 catalysts for water-gas shift reaction. Int. J. Hydrogen Energy 2014, 39, 19570–19582. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Wang, H.; Chen, C.; She, Y.; Zhan, Y.; Lin, X.; Zheng, Q. Water-gas shift reaction over CuO/CeO2 catalysts: Effect of CeO2 supports previously prepared by precipitation with different precipitants. Int. J. Hydrogen Energy 2011, 36, 8839–8849. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Y.; Chen, C.; She, Y.; Lin, X.; Zheng, Q. Effect of CeO2 support prepared with different methods on the activity and stability of CuO/CeO2 catalysts for water-gas shift reaction. Acta Phys. Chim. Sin. 2009, 25, 1397–1404. [Google Scholar]
- Li, D.; Xu, S.; Cai, Y.; Chen, C.; Zhan, Y.; Jiang, L. Characterization and catalytic performance of Cu/ZnO/Al2O3 water-gas shift catalysts derived from Cu-Zn-Al layered double hydroxides. Ind. Eng. Chem. Res. 2017, 56, 3175–3210. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H. Selective hydrogenolysis of glycerol to propylene glycol on hydroxyl carbonate-derived Cu-ZnO-Al2O3 catalysts. Chin. J. Catal. 2014, 35, 631–643. [Google Scholar] [CrossRef]
- Shi, X.; Yang, X.; Gu, X.; Su, H. CuO-ZnO heterometallic hollow spheres: Morphology and defect structure. J. Solid State Chem. 2012, 186, 76–80. [Google Scholar] [CrossRef]
- Ding, L.; Li, L.; Chen, Y.; Hong, J.; Wang, L.; Hou, J.; Wang, H. Preparation of supported catalysts (CuO/TUD-1,CuO/MCM-41) and their application in one-step oxidation of benzene to phenol. Chin. J. Synth. Chem. 2015, 23, 300–304. [Google Scholar]
- Luo, Y.; Xiong, J.; Pang, C.; Li, G.; Hu, C. Direct hydroxylation of benzene to phenol over TS-1 catalysts. Catalysts 2018, 8, 49. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Song, T.; Xu, J.; He, X.; Wang, L.; Zhang, P.; Ye, J. Self-assembly of two zinc(II) supramolecular architectures with carboxylate and chelating aromatic amine ligands: [Zn(nba)2(phen)(H2O)] and [Zn(nip)(phen)]n (nba = 4-nitrobenzoic acid, nip = 5-nitroisoph thalic acid). Transit. Met. Chem. 2006, 31, 992–998. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kikuchi, R.; Takeguchi, T.; Eguchi, K. Steam reforming of dimethyl ether over composite catalysts of γ-Al2O3 and Cu-based spinel. Appl. Catal. B Environ. 2005, 57, 211–222. [Google Scholar] [CrossRef]
- Acharyya, S.; Ghosh, S.; Tiwari, R.; Pendem, C.; Sasaki, T.; Bal, R. Synergistic effect between ultrasmall Cu(II) oxide and CuCr2O4 spinel nanoparticles in selective hydroxylation of benzene to phenol with air as oxidant. ACS Catal. 2015, 5, 2850–2858. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, S.; Jiang, G.; Jiang, G.; Tang, R. A Cu-II-based metal-organic framework as an efficient photocatalyst for direct hydroxylation of benzene to phenol in aqueous solution. Asian J. Org. Chem. 2018, 7, 165–170. [Google Scholar] [CrossRef]
- Hu, L.; Yue, B.; Chen, X.; He, H. Direct hydroxylation of benzene to phenol on Cu-V bimetal modified HMS Catalysts. Catal. Commun. 2014, 43, 179–183. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Iqbal, A.; Rahman, N.R.A. Cu2+ coordinated graphitic carbon nitride (Cu-g-C3N4) nanosheets from melamine for the liquid phase hydroxylation of benzene and VOCs. Appl. Surf. Sci. 2016, 398, 43–55. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Kamizaki, Y.; Terai, N.; Taniya, K.; Tsuruya, S.; Nishiyama, S. One-Step oxidation of benzene to phenol over Cu/Ti/HZSM-5 Catalysts. Catal. Lett. 2010, 134, 324–329. [Google Scholar] [CrossRef]
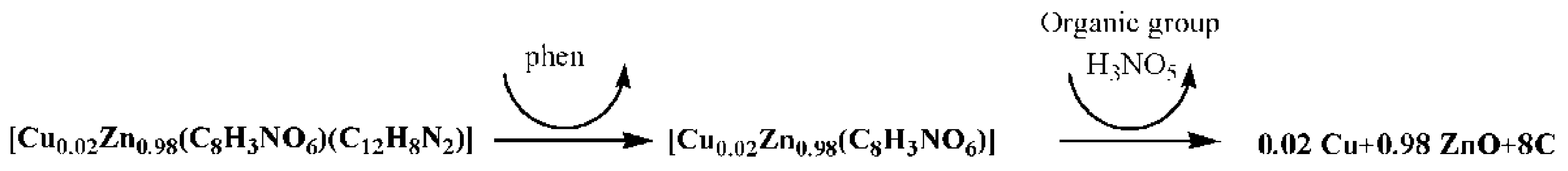
| Parameter | Complex |
|---|---|
| Empirical formula | Cu0.02Zn0.98C20H11N3O6 |
| Formula weight | 454.68 |
| Temperature (K) | 296 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a (Å) | 28.797 (6) |
| b (Å) | 9.2757 (19) |
| c (Å) | 14.965 (3) |
| α (°) | 90 |
| β (°) | 115.58 (3) |
| γ (°) | 90 |
| Volume (Å3) | 3605.4 (13) |
| Z | 8 |
| D (calc) (g cm−3) | 1.669 |
| Crystal size (mm) | 0.29 × 0.21 × 0.17 |
| Ref. collected | 3572 |
| Ref. unique | 2774 |
| Rint | 0.0307 |
| GOF | 1.031 |
| Completeness (%) | 100.00 |
| R1 a (I > 2σ(I)) | 0.0456 |
| wR2 b (all data) | 0.1413 |
| Complex | Bond Lengths (Angles) | Complex | Bond Lengths (Angles) |
|---|---|---|---|
| Zn(Cu)1–O1 | 1.929 (2) | Zn(Cu)1–O3 i | 2.015 (2) |
| Zn(Cu)1–O4 i | 2.433 (3) | Zn(Cu)1–N2 | 2.055 (3) |
| Zn(Cu)1–N3 | 2.072 (3) | - | - |
| O1–Zn(Cu)1–O3 i | 106.37 (11) | O1–Zn(Cu)1–O4 i | 87.21 (10) |
| O1–Zn(Cu)1–N2 | 115.98 (11) | O1–Zn(Cu)1–N3 | 118.44 (11) |
| O3 i–Zn(Cu)1–O4 i | 58.45 (9) | O3 i–Zn(Cu)1–N2 | 123.41 (10) |
| O3 i–Zn(Cu)1–N3 | 109.98 (10) | O4 i–Zn(Cu)1–N2 | 87.17 (9) |
| O4 i–Zn(Cu)1–N3 | 154.35 (10) | N2–Zn(Cu)1–N3 | 81.38 (11) |
| Entry | Post-Heat-Treatment Temperature (°C) | Benzene Conversion (%) | Phenol Selectivity (%) | Phenol Yield (%) |
|---|---|---|---|---|
| 1 | RT b | 17.3 | 26.2 | 4.5 |
| 2 | 200 | 18.7 | 27.8 | 5.2 |
| 3 | 400 | 30.9 | 43.3 | 13.4 |
| 4 | 500 | 45.6 | 54.2 | 24.7 |
| 5 | 600 | 55.7 | 57.5 | 32.0 |
| 6 | 800 | 49.8 | 40.8 | 20.3 |
| 7 | Commercial mixed sample c | 45.6 | 50.7 | 23.1 |
| 8 | Without any catalysts | - | - | - |
| Entry | Catalyst Name | Valence of Main Copper | TOF a (mmol mol−1 s−1) | Ref. |
|---|---|---|---|---|
| 1 | Cu–ZnO@C b | Cu0 | 117.9 | This Work |
| 2 | TS-1 c | - | 31.7 | [21] |
| 3 | CuO–CuCr2O4 | Cu2+ | 44.9 | [25] |
| 4 | CuII-Based complex | Cu2+ | 22.7 | [26] |
| 5 | Cu0.09-V-HMS | Cu2+ | 121.9 | [27] |
| 6 | Cu-g-C3N4 | Cu2+ | 1.5 | [28] |
| 7 | Cu/Ti/HZSM-5 | Cu2+/Cu+ | 2.0 | [29] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Yan, Z.; Song, L.; Li, L.; Zhu, J.; Wang, H. One-Step Synthesis of Cu–ZnO@C from a 1D Complex [Cu0.02Zn0.98(C8H3NO6)(C12H8N2)]n for Catalytic Hydroxylation of Benzene to Phenol. Crystals 2018, 8, 218. https://doi.org/10.3390/cryst8050218
Wang G, Yan Z, Song L, Li L, Zhu J, Wang H. One-Step Synthesis of Cu–ZnO@C from a 1D Complex [Cu0.02Zn0.98(C8H3NO6)(C12H8N2)]n for Catalytic Hydroxylation of Benzene to Phenol. Crystals. 2018; 8(5):218. https://doi.org/10.3390/cryst8050218
Chicago/Turabian StyleWang, Guanghui, Zheng Yan, Li Song, Lei Li, Jie Zhu, and Haidong Wang. 2018. "One-Step Synthesis of Cu–ZnO@C from a 1D Complex [Cu0.02Zn0.98(C8H3NO6)(C12H8N2)]n for Catalytic Hydroxylation of Benzene to Phenol" Crystals 8, no. 5: 218. https://doi.org/10.3390/cryst8050218