1. Introduction
Calcium carbonate (CaCO
3) biogenic precipitation is considered as an important process in nature with respect to its role in early diagenesis of marine sediments, hydrochemical evolution of karst streams, application of Geological and Civil Engineering and environmental treatments [
1]. From a geotechnical point of view, the potential of microbial carbonate precipitation (MCP) has been identified as a means of adapting soil properties to suit desired land-uses [
2]. The reaction is widely distributed in soil, freshwater, marine and subsurface environments. MCP can occur via a variety of processes whereby microbial activities results in the generation of carbonate in a calcium rich environment. The urea hydrolysis by the enzyme urease of microorganisms in a calcium-rich environment is the most commonly MCP studied. The CO
32− and NH
4+ from the hydrolysis of urea increase of the pH and carbonates concentration, which lead to the precipitation of calcium carbonate.
Through MCP, microbes play an important role in promoting calcite precipitation. Some mechanisms studied by researchers that can induce CaCO
3 deposition are: (1) the depositing particles are captured or adhered to by microbial mat or biofilm; (2) extracellular polymeric substances (EPS) can absorb Ca
2+ so that carbonate microcrystals form on the surface of the biofilm and result in CaCO
3 formation; (3) in the environment with higher content of dissolved inorganic carbon (DIC), EPS degradation will release the Ca
2+ ions that are chelated inside it, so that the supersaturation degree of Ca
2+ in the environment will be continuously enhanced, resulting in promoting the precipitation of CaCO
3; (4) Some bacteria can induce precipitation of CaCO
3 extracellularly through such processes as photosynthesis, ammonification, denitrification, sulfate reduction and anaerobic sulfide oxidation; (5) degradation of urea by urea-decomposing bacteria increases pH and alkalinity of the environment, leading to CaCO
3 precipitation [
3,
4,
5,
6,
7,
8,
9]. Mechanisms 1, 2 and 3 are defined as passive forms of precipitation in the presence of organic matter and mechanisms 4 and 5 involve indirect modifications of chemical conditions [
4,
6]
Considerable research on MCP has been performed using ureolytic bacteria, which influence the precipitation of CaCO
3, by the production of the enzyme urease. Urease catalyzes the hydrolysis of urea to CO
2 and ammonia. The resulting CaCO
3 precipitation is governed by (1) calcium concentration; (2) carbonate concentration; (3) pH and (4) the use of nucleation sites [
10]. The fact that hydrolysis of urea is a straightforward common microbial process and that a wide variety of microorganisms produce the urease enzyme makes it ideally suited for biotechnological applications. Urease activity is widespread among bacteria and this has been the approach most often applied for MCP for the production of calcite [
11,
12]. Urease-producing bacteria can be grouped into two categories according to their urease response to ammonium; those whose urease activity is not repressed (
S. pasteurii,
Helicobacter pyroli) and those whose urease activity is repressed (
Psuedomonas aeruginosa,
Klensiella aerogenes) [
13,
14]. Because high concentrations of urea are hydrolyzed during biocementation, only those microorganisms whose urease activity is not repressed by ammonium are useful. As well as meeting the needs for biocementation, the organism must also meet the needs for safe environmental application. In order to maintain the microbial ecological system in the environment, it will be suitable to apply and release those strains with non-pathogenic, non-genetically modified and non-transferable elements.
S. pasteurii has been widely used as a model organism for the MCP process because this strain is non-pathogenic with significantly high level of urease activity [
15]. Calcium carbonate precipitation, a widespread phenomenon among bacteria, has been investigated due to its wide range of scientific and technological implications. Calcite formation by
B. subtilis (which is a model laboratory bacterium that can produce calcite precipitates on suitable media supplemented with a calcium source) has also been studied [
16]. It is also well known that
B. subtilis was used to produce the varieties of exopolymeric substances, for example, biosurfactants of surfactin. At present, some studies have been done on the role of
B. subtilis in calcite precipitation. However, there are no adequate studies on the role of calcite precipitation by
B. subtilis in coculture environments for sand bioconsolidation. We selected
B. subtilis to study the influence of growth and metabolism of
S. pasteurii for biocementation processes. In order to clarify the contribution and influence of
B. subtilis, the monoculture
S. pasteurii and the coculture
S. pasteurii-
B. subtilis were studied in two different experimental systems. A main part of this research focuses on studying coculture environment for sand bioconsolidation through carbonate precipitation and geomicrobiology in the subsurface environment.
2. Results and Discussion
The effect of two types of microbial systems were evaluated to study the distribution of their activity and calcium carbonate precipitation in sand column through a number of steps elaborated in
Table 1.
Initially, immobilization of bacteria in the column was achieved by a two-phase injection. In order to fill the column volume, bacteria were injected. When bacteria were visualized in the outlet as seen by turbidity, 300 mL of 50 mM calcium chloride solution was injected into the column to immobilize the bacteria in a moving reaction front in the column. To start the cementation, 1.1 M equimolar urea and calcium chloride was injected. Samples were collected at regular intervals to measure OD, pH, urease activity, calcium ammonium concentration measurement [
2].
2.1. Optical Density and Urease Activity
Column 1 and column 2 with monoculture and coculture bacteria were studied to elucidate the distribution of urease activity and optical density over the column length. While flushing 300 mL of bacterial suspension, immediately followed by 300 mL fixation fluid of 50 mM CaCl
2 solution, measurement of both optical density and urease activity in the effluent of the sand column showed that a large proportion of the injected bacteria was retained in the column (
Figure 1a). Further, after injecting the cementation solution, no noticeable activity or bacteria were observed in the effluent (
Figure 1b). The effluent measurements showed that bacteria in the monoculture experiment resulted in more wash-out of cells and activity corresponding to that of coculture. This retainment of more urease activity and optical density in coculture may be attributed to the synergistic relationship between
S. pasteurii and
B. subtilis, where, in the environment with nutrient limiting conditions or microbes living in deep subsurface, majority of the populations can thrive without any input from the earth’s surface. This might be due to the symbiotic association for their survival where organic compounds or metabolites produced by one bacterial species may be utilized by the other to adapt to the harsh environment.
2.2. Solution Chemistry
The experiments conducted with monoculture and coculture ureolytic bacterial species were monitored for the measurements of pH and NH4+ after the column flush. Monoculture S. pasteurii and coculture S. pasteurii-B. subtilis show almost similar trend in pH over time. During the first batch flush of cementation fluid, the higher pH for column 1 (around 8.6 ± 1) and only a small pH increase (around 8.3 ± 1) in coculture experiments for column 2 were observed. Results obtained for the second and third batch flush also correspond to first batch flush with a slight increase of one-two unit pH, which indicates that S. pasteurii alone have a strong ureolytic capability to break down the urea while releasing NH4+ ions and increasing the solution pH. Similarly, an increase in solution pH for the coculture experiment also shows the strong urease activity in column 2. The control experiment evidenced no change in pH without any bacterial species.
The change in NH
4+ concentration over time caused by urea hydrolysis for
S. pasteurii is shown in
Figure 2. These values of urea hydrolysis are calculated from measured ammonium concentrations using the stoichiometric equation by Whiffin et al. [
2]. One mole of urea is assumed to hydrolyze into two moles of ammonium, and therefore, the amount of urea hydrolyzed at any given time is assumed to be equal to half the amount of ammonia produced. During the first batch flush of cementation, i.e., after 24 h, the maximum NH
4+ ions produced by
S. pasteurii in monoculture experiment was 398 mM which further increased to 1160 mM during the second batch flush after 48 h and 1921 mM during the third batch flush after 72 h respectively (
Figure 2a). However, the NH
4+ concentration increased linearly with flushed volume for the entire three-batch flush. To determine the urea hydrolysis under the influence of
B. subtilis in column 2, a detailed analysis of NH
4+ ion production was performed.
Figure 2b presents the corresponding NH
4+ concentration values determined during a period of 24, 48 and 72 h. The NH
4+ concentration does not increase above 157 mM until 24 h during the first batch flush. During the second batch flush with this experiment, NH
4+ concentration was found to increase to 953 mM, still lower than column 1, whereas, the third batch flush was shown to convert the urea to almost equimolar proportion with a further increase up to 1929 mM. This conclusively shows that urea hydrolysis is occurring for this system and that mono as well as coculture systems can convert the total urea with almost the same rate at the end of the experiment of around 72 h. The NH
4+ profiles shown in
Figure 2a demonstrate that bacterial growth was at its peak in the center of the column where the highest NH
4+ concentration was measured. In contrast to this, column 2 where no significant bacterial wash out was observed, showed higher NH
4+ concentrations at the top of the column and was lowest at the bottom.
The trend seen in the calcium precipitation rates mimicked those of NH
4+ production, i.e., the monoculture of
S. pasteurii exhibited a faster precipitation rate than coculture over first and second batch flush. Whereas, after a period of 72 h, soluble calcium was detected in the column 1 and column 2 were almost the same which shows that monoculture
S. pasteurii and coculture
S. pasteurii-
B. subtilis have almost the same ureolytic activity to precipitate CaCO
3. The dissolved calcium concentrations over time for monoculture and biculture experiments are shown in
Figure 3a,b. Whereas, residual calcium concentrations in the control experiments were higher and remain unreacted corresponding to that of other two systems. This decrease in calcium ion concentration for both the monoculture and biculture experiments could be due to passive precipitation of calcite, with
S. pasteurii cells acting as nucleation points, or due to sorption of calcium to the cells. Simultaneously, it can be seen from the data that the rate of calcite precipitation was directly proportional to the rate of urea hydrolysis or NH
4+ production. This results contrasts with that of Stocks-Fischer et al. [
17] who stated that calcite precipitation was directly linked to cell growth and indirectly linked to ammonia production. We assume that the results obtained in their study relates to pH where Stocks-Fischer et al. started their experiments at a pH of 8 which is related to the supersaturation degree of the solution for calcite; whereas in the present study, experiments were initiated at a pH of 7.
2.3. CaCO3 Profile along the Column
After completion of the experiment, all the three columns were flushed for 24 h with tap water and allowed to dry. Samples along the column length were scraped for evaluation of the CaCO
3 content. An average CaCO
3 content was determined from at least three samples from each column section and results were correlated with the NH
4+ production of each of the tested samples. For both the columns with monoculture of
S. pasteurii and coculture
S. pasteurii-
B. subtilis were almost shown to exhibit the same CaCO
3 precipitation at the bottom of the column as shown in
Figure 4. Whereas the highest strength of CaCO
3 formation measured were significantly highest in the middle of the column for monoculture and for biculture highest strength was obtained at the top of the column where maximum CaCO
3 precipitation occurred. Hence, by correlating both of these columns for CaCO
3 and NH
4+ concentration, it can be seen that the highest CaCO
3 precipitation was measured approximately at the same location where maximum NH
4+ production was observed with a CaCO
3 content of 1.5 kg/m
3 and 1.29 kg/m
3 for monoculture column 1 and coculture column 2, respectively.
2.4. Comparison of XRD Patterns among Monoculture and Biculture Experiments
Figure 5 indicates the results of XRD analyses of CaCO
3 precipitation in the sand columns. Sands were carefully removed from CaCO
3 precipitates with tweezers before XRD measurements. However, the peaks with strong intensity of 26.62° at the 2θ value that attributed to the Miller indices of (101) of quartz for all the samples were obtained. This is because the Ottawa sand with the size distribution of from 0.1 mm to 1 mm were used. The 2θ values at 20.85°, 26.62° and 40.28° were attributed to the tiny sand quartz particles that remained in CaCO
3 precipitates. The XRD peaks located at 2θ values of 29.39°, 36.0°, 39.41°, and 43.10°, which could be assigned to be due to those of calcite, were precipitated by monoculture and coculture bacteria in glass columns. This is coincides with the main characteristic peaks for quartz, and vaterite 26.63 and 26.99, respectively. Those peaks at 2θ of 24.92°, 26.99° and 32.78° might correspond to the vaterite. Aragonite was not detected with XRD and Raman analyses (
Supplementary Figures S1 and S2). The Raman active bands at 714 cm
−1 confirmed the main product of calcite from the MICP process (
Supplementary Figure S1). Vaterite is a metastable polymorph of calcium carbonate and is rare in natural environments. It is unstable and rapidly transforms into calcite at room temperature in an aqueous solution [
18]. Whereas, bacteria and its secretion (mainly organic matrix) may facilitate calcium carbonate precipitation. The results showed that both the monoculture
S. pasteurii and coculture
S. pasteurii-
B. subtilis could induce not only stable calcite crystals but also unstable vaterite crystals. The vaterite crystals have been lasting for more than two years without phase transformation.
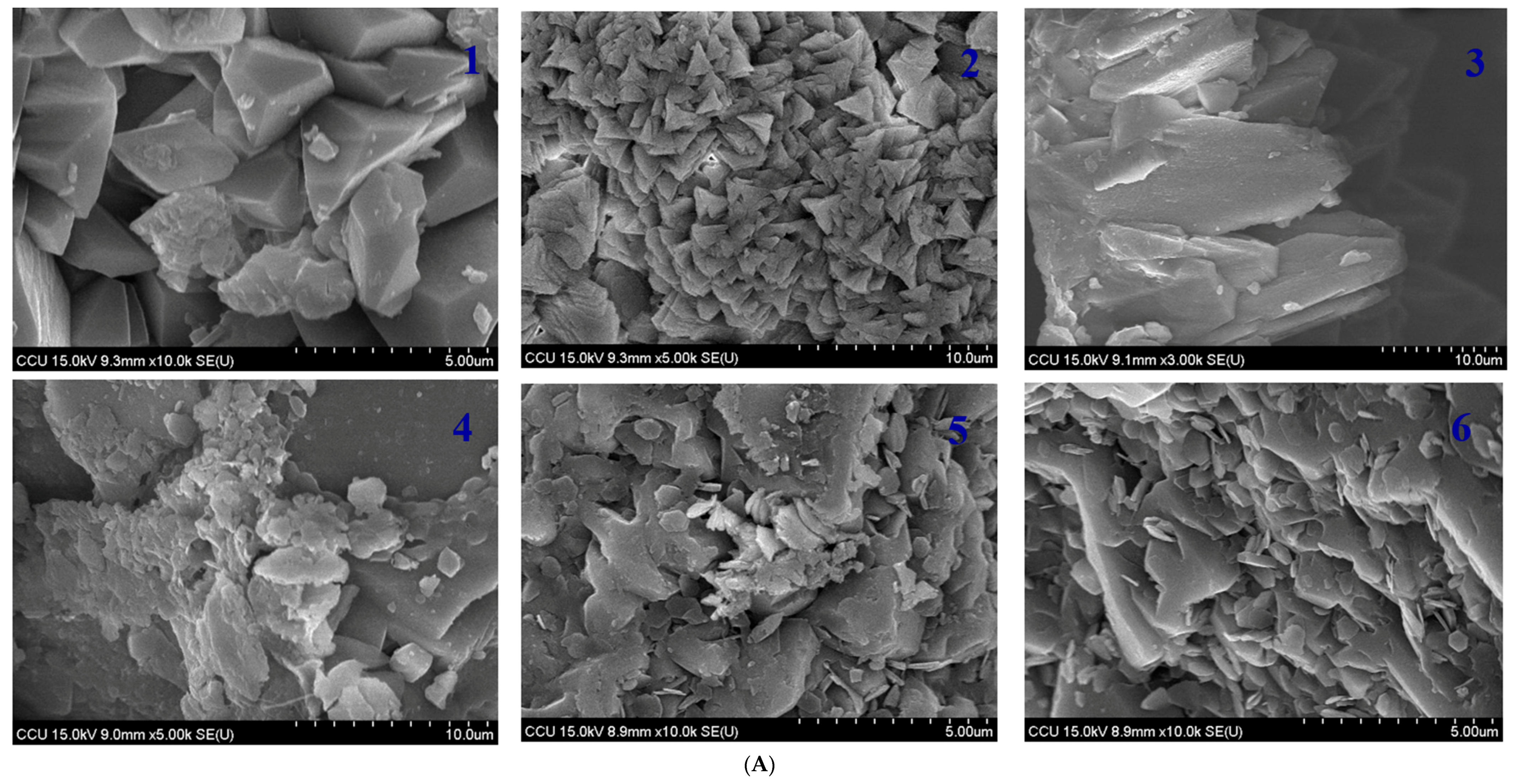
2.5. Comparison of CaCO3 Crystal Morphology Induced by Biological Factors
The CaCO
3 crystal morphologies induced by monoculture and biculture experiments are shown in
Figure 6. There were obvious differences in the size and morphology of CaCO
3 crystals induced in both the experiments as seen in SEM. The column with monoculture
S. pasteurii exhibited that large amount of CaCO
3 particles formed on the top of the column with trigonal prism CaCO
3 (about 3 μm) and rod-shaped calcite (25 μm) in
Figure 6A, which were accumulated on the sand surface. Furthermore, it was observed that CaCO
3 particles were accumulated by compact calcite with irregular flakes of CaCO
3 crystals at the bottom of the column (
Figure 6A). Those CaCO
3 crystals were confirmed by the XRD spectrum in
Figure 5. This SEM result evidenced that the action of
S. pasteurii on specific CaCO
3 morphology increased with the increasing depth of the column. However, specific morphology of crystals disappeared at the bottom of the column and irregular compact plate like flakes of CaCO
3 appeared probably because the control action of
S. pasteurii weakened with the gradual bigger and denser crystal size at the top (
Figure 6B). Despite the same reaction conditions, coculture bacterial sand columns induced the CaCO
3 crystals formation, which were of regular shape; the granular calcite as well as rod-shaped calcite on the top of the column whereas at the bottom of the column compact and lamellar shaped crystals and spherulitic vaterite
. crystals were induced (
Figure 6B). The length/thickness ratio of the rod-shaped crystals in biculture is completely different from monoculture. The main characteristic peaks of calcite can be found from the XRD peaks located at 2θ values of 29.40 in
Figure 5. Vaterite was confirmed from the peaks at 2θ of 24.92°, 26.99° and 32.78° (
Figure 5). The appearance of vaterite is probably related to the local higher supersaturation of a metastable phase.
The observed carbonate formation under the influence of two bacteria indicates that both the microbes directly participate in crystallization process. In addition, SEM investigation also shows that CaCO
3 crystals precipitate in this experimental condition and are approximately of same size of 2–30 μm diameter. However, compared with monoculture, biculture experiments exhibited the three different crystal morphologies, i.e., lamellar, spherulitic and rod. It has been demonstrated that specific biofilm-forming bacteria which could produce exopolysaccharides (EPS) and amino acids play an essential role in the morphology and mineralogy of bacterially induced carbonate precipitation. In a review article, Marvasi et al. [
19] described the production of sorptive EPS, poly-γ-glutamate that is anionic, nontoxic and biodegradable viscous polymer produced mainly by wild type
B. subtilis. In addition, the glutamic acid solution was also shown to induce trigonal prism crystal morphology of CaCO
3 precipitate studied by Li et al. [
1]. We hypothesize that this is why this crystal morphology was selected in the biculture microbial environment, where the EPS might have been produced by
B. subtilis which acts as glue to specifically binds Ca
2+ ions. However, due to its viscous nature, EPS binds more Ca
2+ ions on the top of the column and it flows slowly down towards the bottom of the column where the CaCO
3 precipitation was less. Research conducted by Buczynski and Chafetz [
20] showed that EPS plays an important role in the formation of carbonate crystals by providing nucleation sites and by attaching small crystals to each other to increase the size of the bioliths. In accordance with Le Me’tayer-Levrel et al. [
21],
B. subtilis can be applied as biocalcifiers producing carbonates, whereas this activity did not allow the establishment of other bacterial species to function.
Although the present hypothesis that B. subtilis EPS/EPS like chemical might release into the environment and influence the CaCO3 formation is from the laboratory experiment, there are wide implications for natural carbonate precipitation, since bacteria are ubiquitous in nature. Thus, this needs to be examined in detail to study the role of S. pasteurii and B. subtilis monoculture and coculture environment for CaCO3 precipitation and its morphology. Further studies are needed to determine whether the mineralogical biosignatures found in nutrient-rich media can also be found in the subsurface environments.
2.6. Engineering Assessment of the Effect of Consolidation
In addition, to verify the effectiveness in consolidation of sand with the two ureolytic stains, S. pasteurii and B. subtilis, an engineering experiment of mixing standard sand, Portland cement, and bacterial solution into porous cement binder, casting 5 cm cube mortar specimen for testing of compressive strength was carried out.
Figure 7 shows the results of the mortar’s compressive strength with different bacterial solutions. The average compressive strength of control specimen without bacteria is 18.2 MPa at 14 days. The mortars incorporated monoculture
S. pasteurii (SP group) and coculture
S. pasteurii-
B subtilis (SP + BS group) and present the compressive strength of 27.7 MPa and 26.9 MPa, respectively. This indicates that both the monoculture
S. pasteurii and coculture
S. pasteurii-
B subtilis can greatly enhance the strength of the mortars. It is significant for geotechnical engineering to improve the mechanical property of soil by the invented consolidation method.