Effects of Alloying Atoms on Antiphase Boundary Energy and Yield Stress Anomaly of L12 Intermetallics: First-Principles Study †
Abstract
:1. Introduction
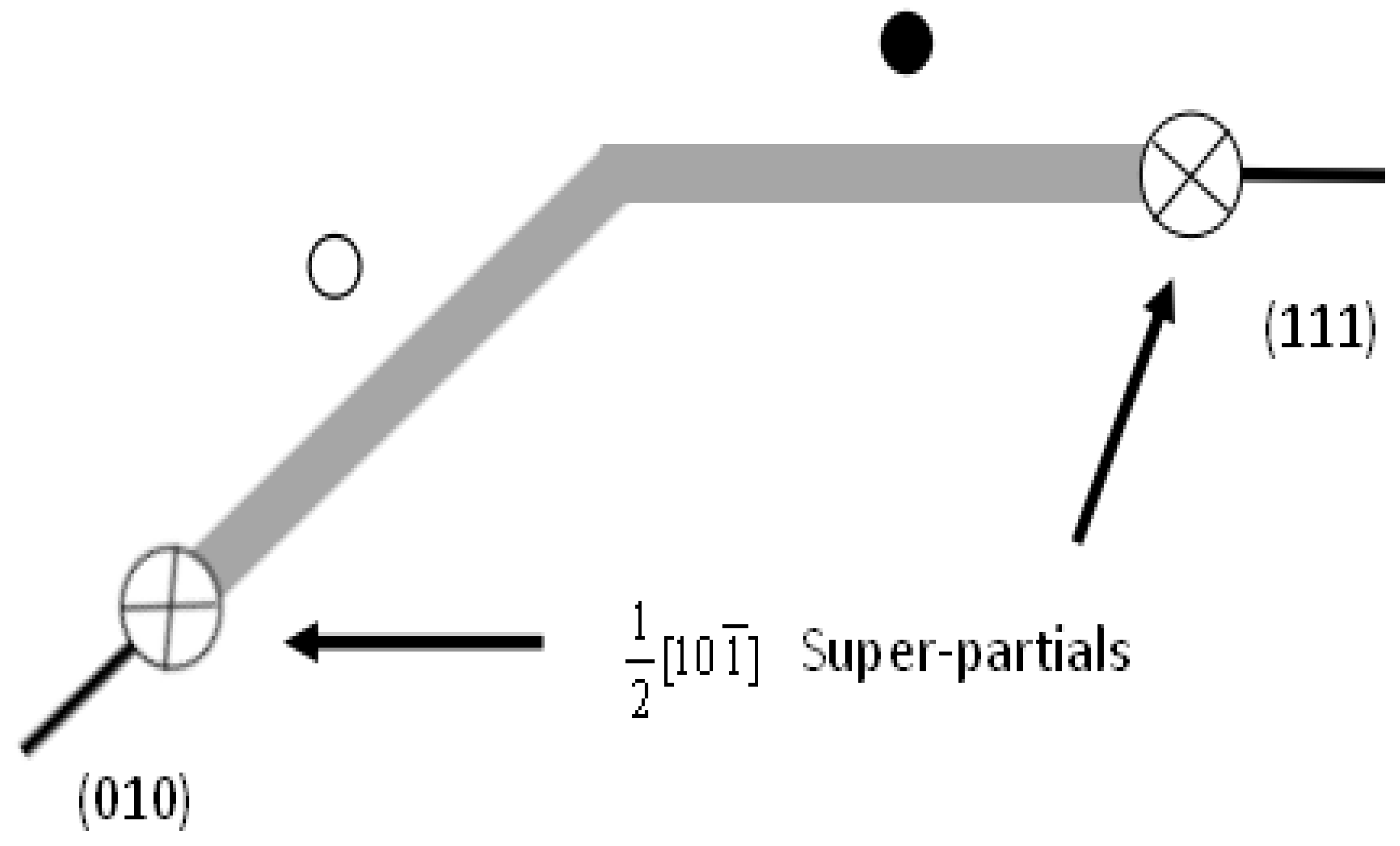
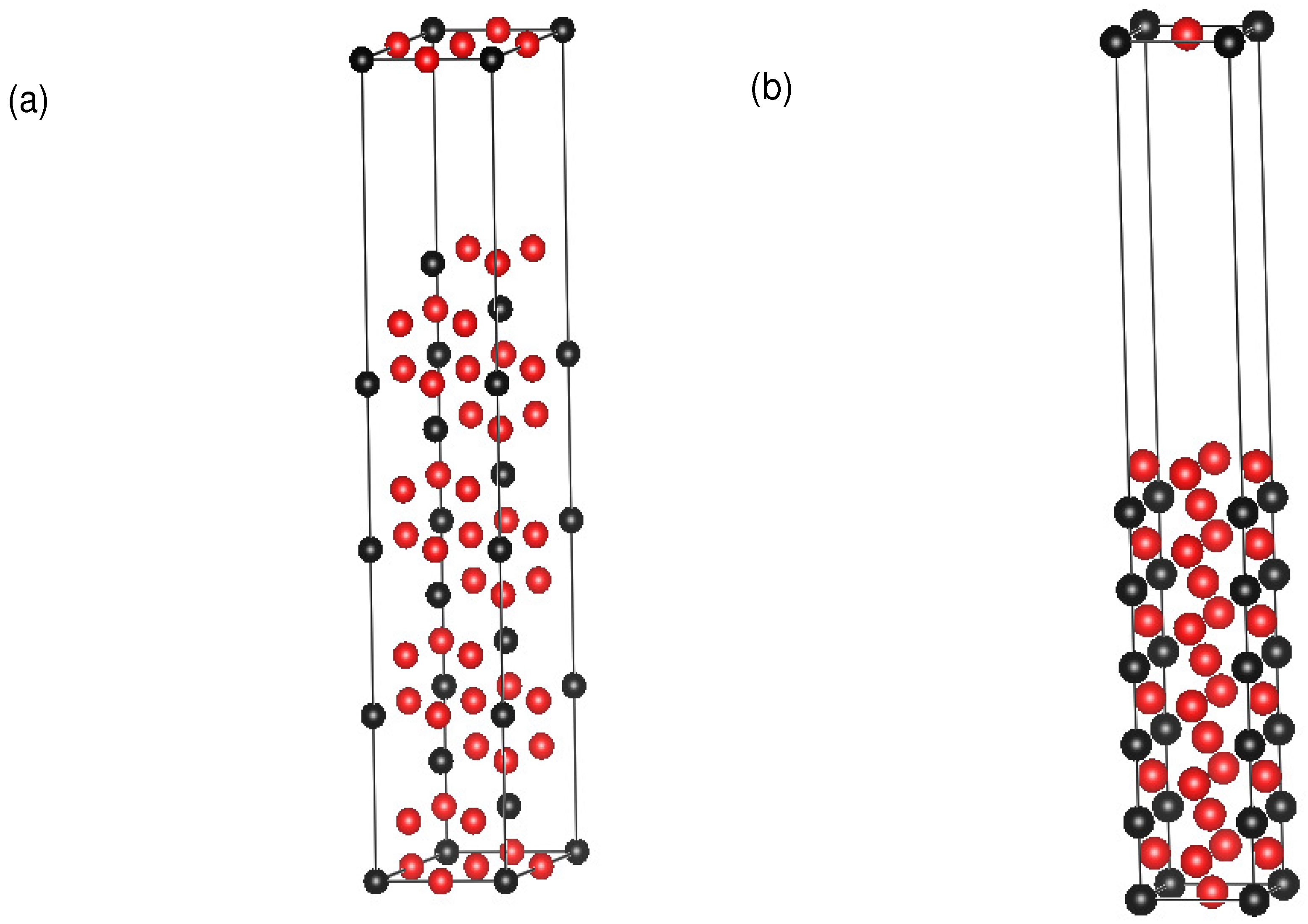
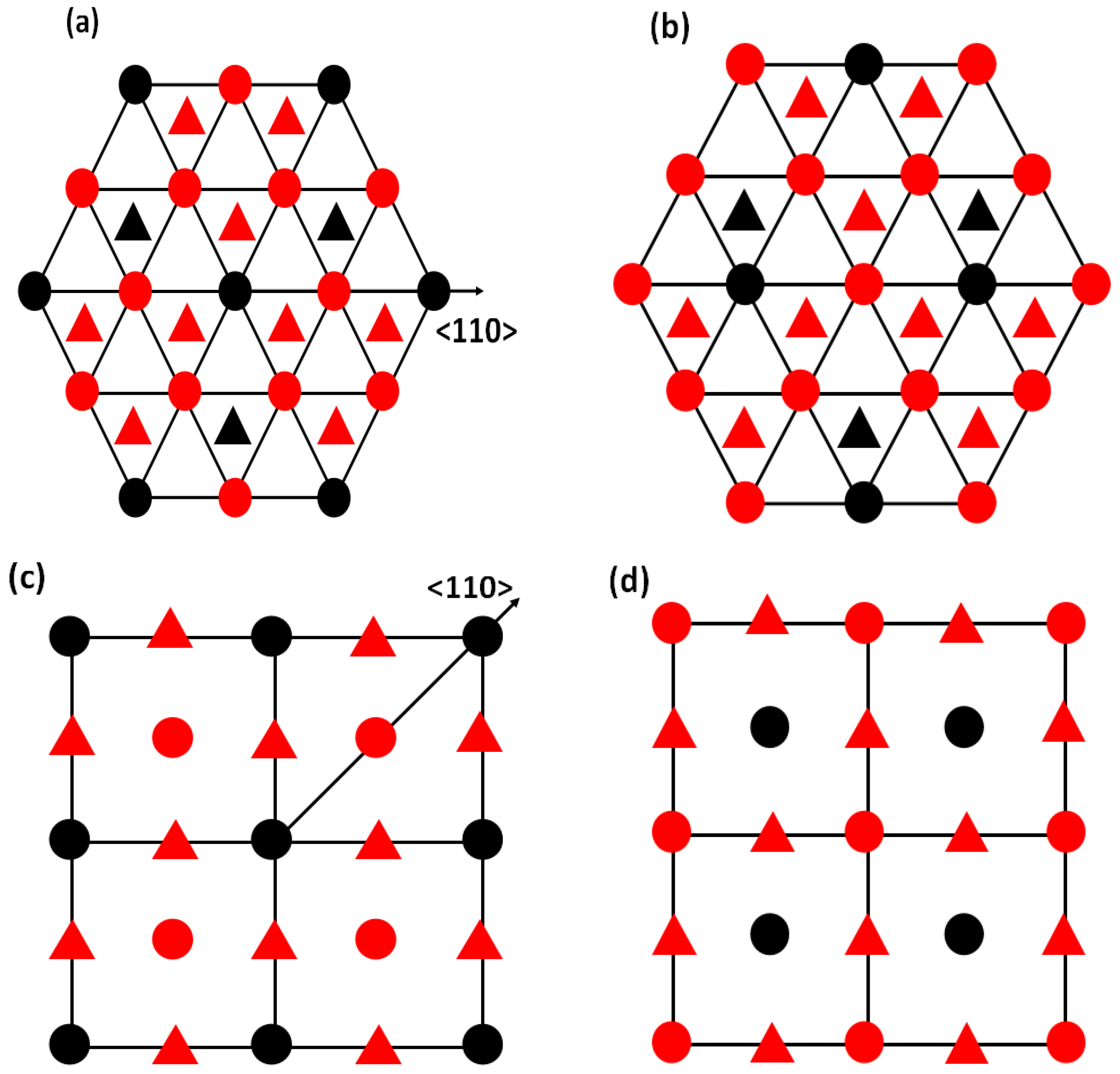
2. Computational Methodology and Models
3. Effects of Pressure on Stacking Fault Energy and p-Factor
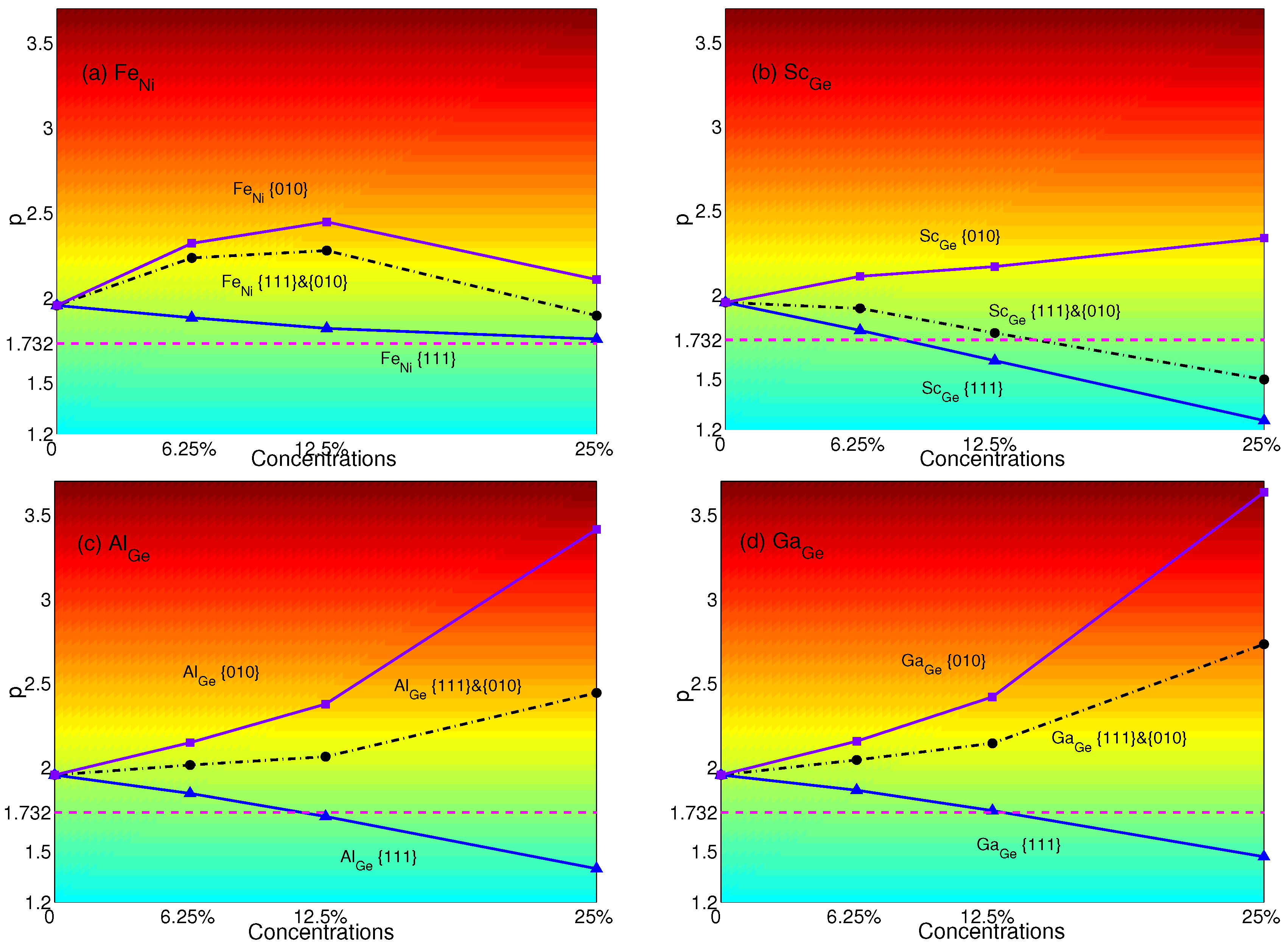
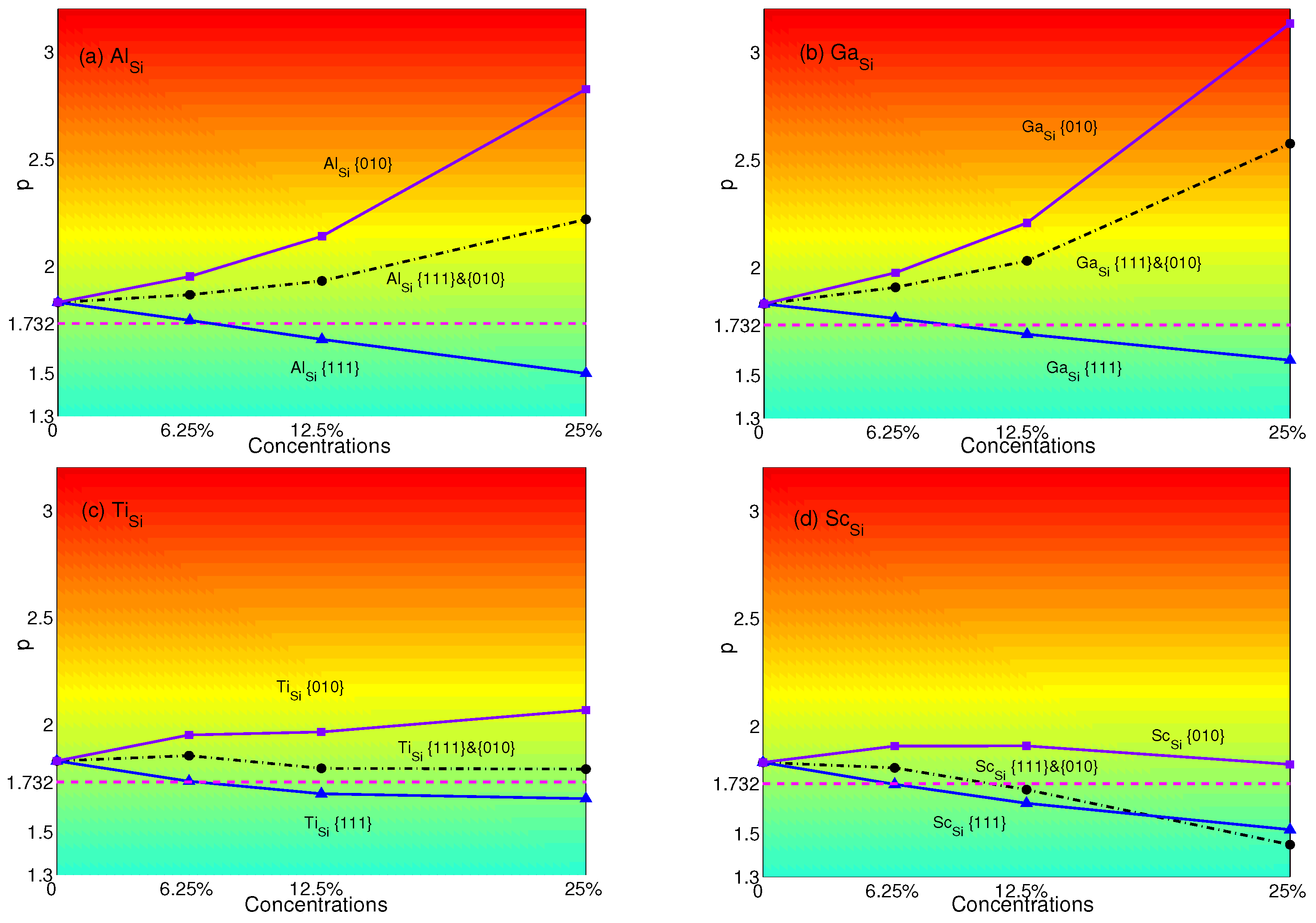
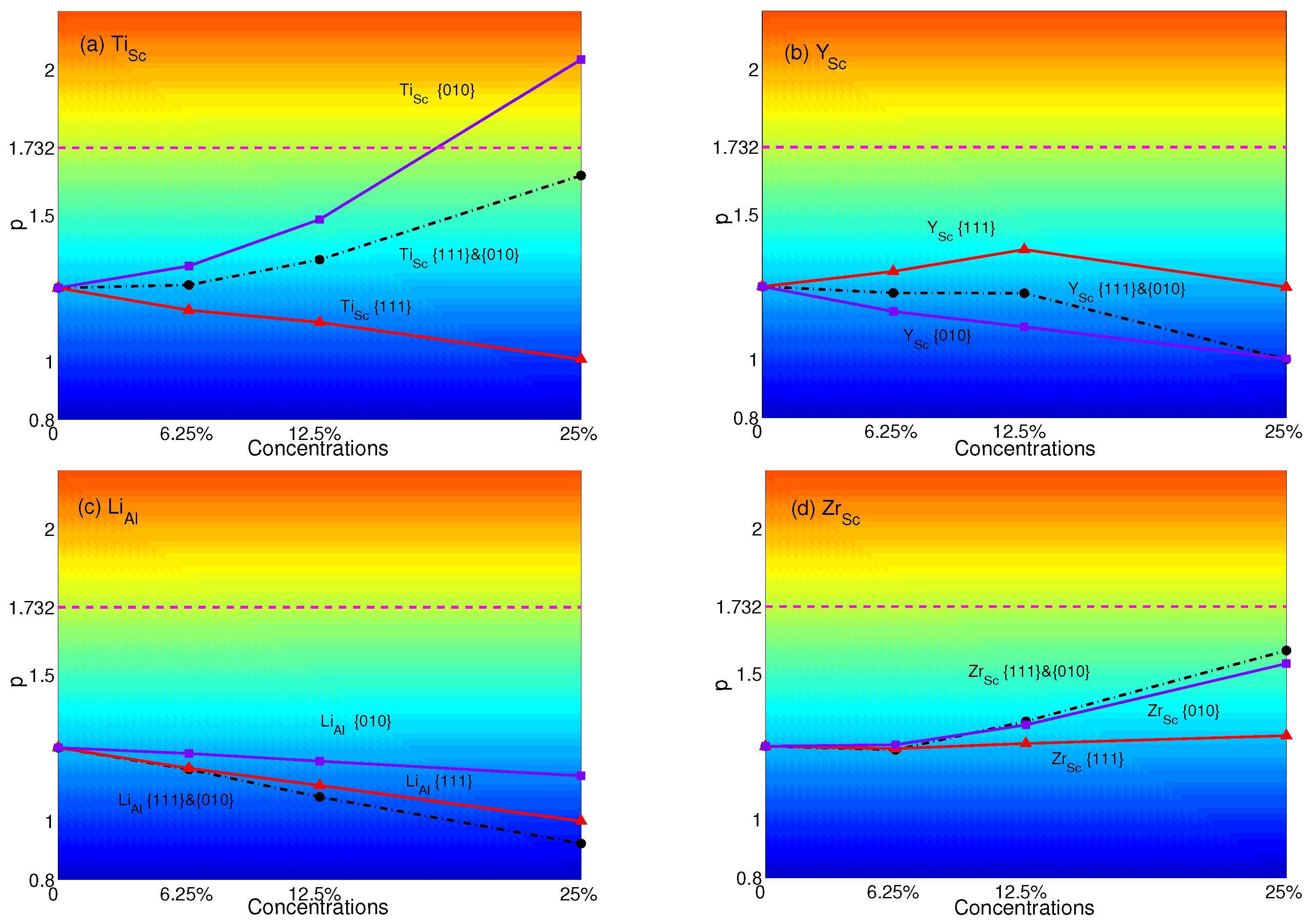
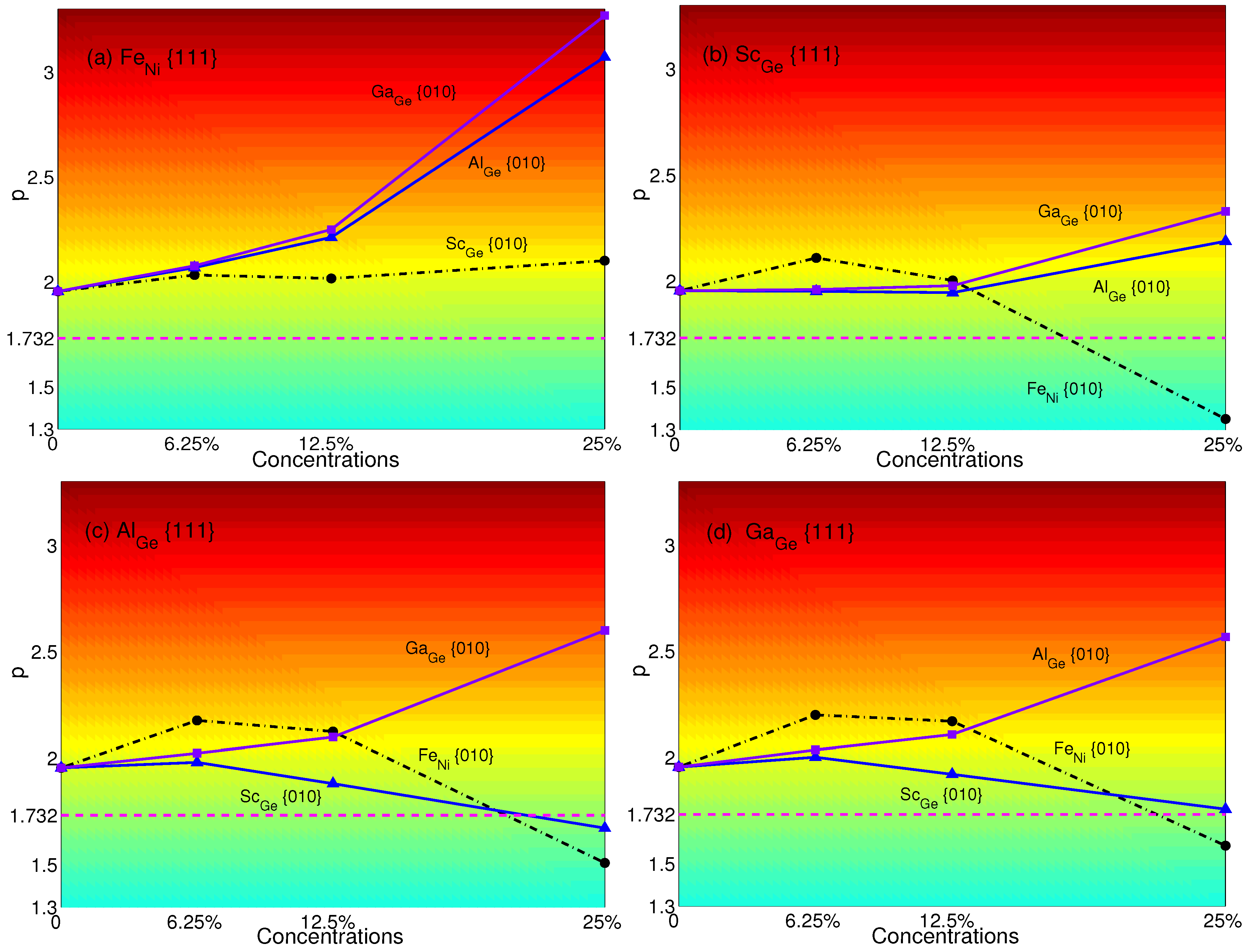
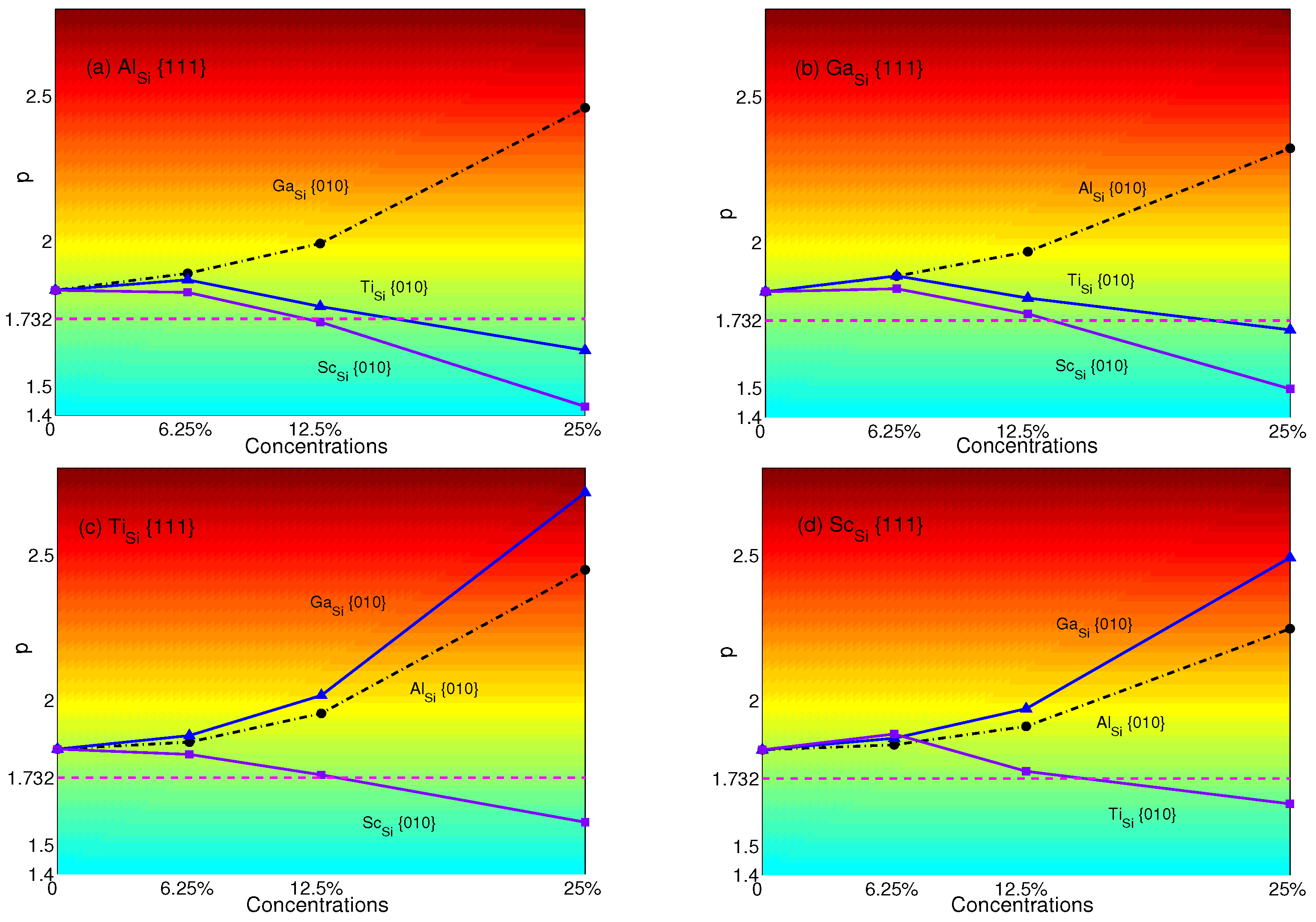
4. Effects of Substituting Atoms on Stacking Fault Energy and p-Factor
4.1. Substituted by a Single Atom
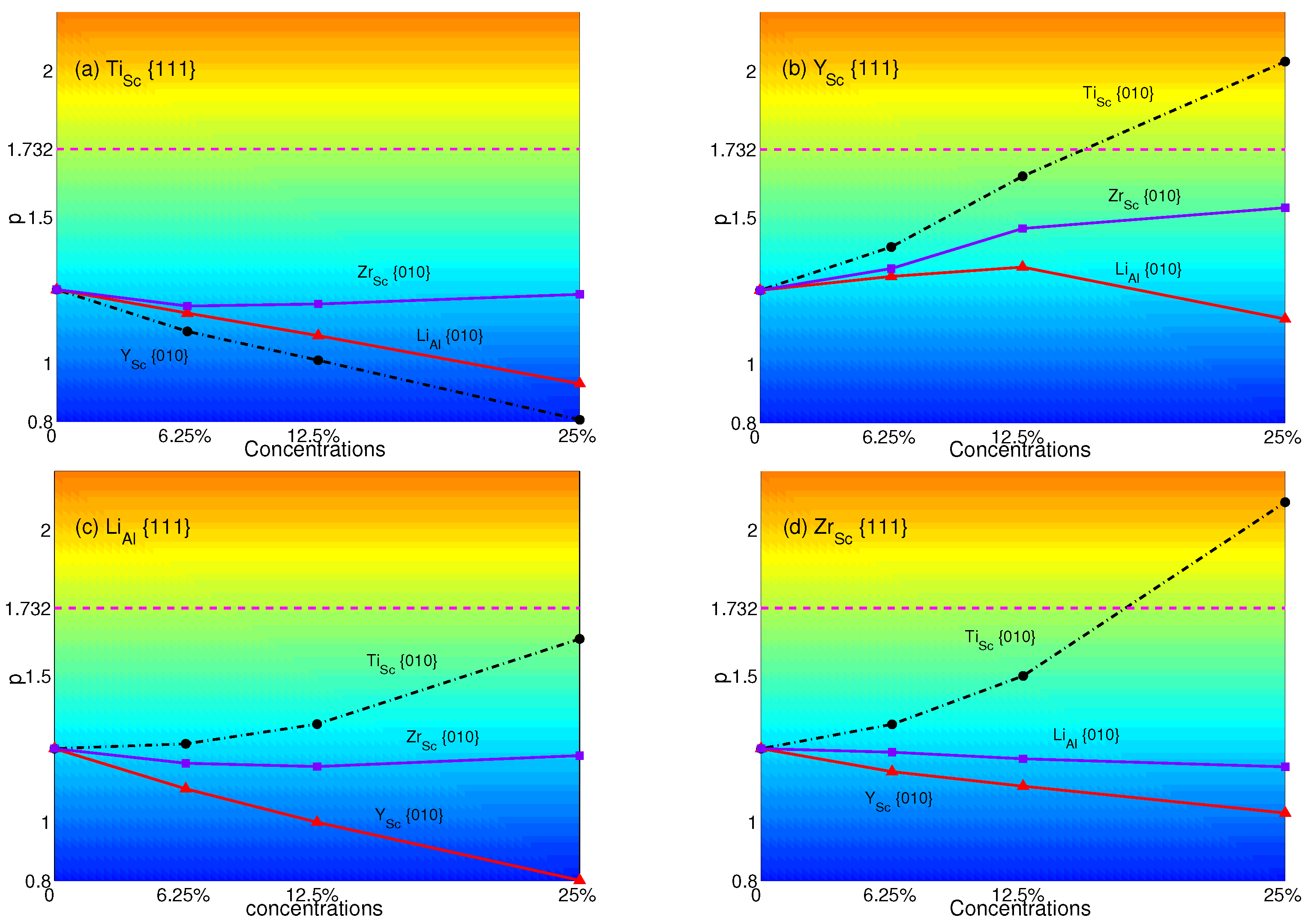
4.2. Substituted by Two Different Atoms
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Westbrook, J.H. Defect structure and temperature dependence of hardness of an intermetallic compound. Trans. TMS-AIME 1957, 209, 898. [Google Scholar] [CrossRef]
- Flinn, P. Theory of deformation and superlattices. Trans. TMS-AIME 1960, 218, 145–154. [Google Scholar]
- Davies, R.G.; Stoloff, N.S. Yield stress of aged Ni-Al alloys. Trans. TMS-AIME 1965, 233, 714. [Google Scholar]
- Copley, S.M.; Kear, B.H. Working-hardening in off-stoichiometric. Trans. TMS-AIME 1967, 239, 977. [Google Scholar]
- He, L.Z.; Zheng, Q.; Sun, X.F.; Hou, G.C.; Guan, H.R.; Hu, Z.Q. Low ductility at intermediate temperature of NiCbase superalloy M963. Mater. Sci. Eng. A 2004, 380, 340–348. [Google Scholar] [CrossRef]
- Sheng, L.Y.; Fang, Y.; Guo, J.T.; Xi, T.F. Anomalous yield and intermediate temperature brittleness behaviors of directionally solidified nickel-based superalloy. Trans. Nonferrous Met. Soc. 2014, 24, 673–681. [Google Scholar] [CrossRef]
- Chu, Z.K.; Yu, J.J.; Sun, X.F.; Guan, H.R.; Hu, Z.Q. Tensile property and deformation behavior of a directionally solidified Ni-base superalloy. Mater. Sci. Eng. A 2010, 527, 3010–3014. [Google Scholar] [CrossRef]
- Pope, D.P. Physical Metallurgy, 4th ed.; Elsevier Press: Amsterdam, The Netherlands, 1996; Volume 3, p. 2075. [Google Scholar]
- Vitek, V.; Pope, D.P.; Bassani, J.L. Disloactions in Solids; Elsevier Press: Amsterdam, The Netherlands, 1996; Volume 10, p. 135. [Google Scholar]
- Veyssière, P.; Saada, G.; Duesbery, M.S. Disloactions in Solids; Elsevier Press: Amsterdam, The Netherlands, 1996; Volume 10, p. 253. [Google Scholar]
- Veyssière, P. Yield stress anomalies in ordered alloys: A review of microstructural findings and related hypotheses. Mater. Sci. Eng. A 2001, 309, 44–48. [Google Scholar] [CrossRef]
- Caillard, D.; Molénat, G.; Paidar, V. On the role of incomplete Kear-Wilsdorf locks in the yield stress anomaly of Ni3Al. Mater. Sci. Eng. A 1997, 234, 695–698. [Google Scholar] [CrossRef]
- Bonneville, J.; Coupeau, C. Quantitative atomic force microscopy analysis of slip traces in Ni3Al yield stress anomaly. Mater. Sci. Eng. A 2008, 483, 87–90. [Google Scholar] [CrossRef]
- Michel, J.; Coupeau, C.; Nahas, Y.; Drouet, M.; Beonneville, J. What can be learnt on the yield stress anomaly of Ni3Al using AFM observations. Intermetallics 2014, 50, 86–93. [Google Scholar] [CrossRef]
- Caillard, D.; Couret, A. Disloactions in Solids; Elsevier Press: Amsterdam, The Netherlands, 1996; Volume 10, p. 69. [Google Scholar]
- Rao, S.I.; Dimiduk, D.M.; Parthasarathy, T.A.; Unchic, M.D.; Woodward, C. Atomistic simulations of intersection cross-slip nucleation in L12 Ni3Al. Scr. Mater. 2012, 66, 410–413. [Google Scholar] [CrossRef]
- Paidar, V.; Pope, D.P.; Vitek, V. A theory of the anomalous yield behavior in L12 ordered alloys. Acta Metall. 1984, 32, 435–448. [Google Scholar] [CrossRef]
- Umakoshi, Y.; Pope, D.P.; Vitek, V. The asymmetry of the flow stress in Ni3(Al,Ta) single crystals. Acta Metall. 1984, 32, 449–456. [Google Scholar] [CrossRef]
- Kear, B.H.; Wilsdolf, H.G. Dislocation configurations in plastically deformed polycrystalline Cu3Au alloys. Trans. Metall. Soc. AIME 1962, 224, 382. [Google Scholar]
- Gorbatov, O.I.; Lomaev, I.L.; Gornostyrev, Y.N. Effect of composition on antiphase boundary energy in Ni3Al based alloys: Ab initio calculations. Phys. Rev. B 2016, 93, 224106. [Google Scholar] [CrossRef]
- Sun, R.; Van de Walle, A. Automating impurity-enhanced antiphase boundary energy calculations from ab initio Monte Carlo. CALPHAD 2016, 53, 20–24. [Google Scholar] [CrossRef]
- Koizumi, Y.; Mizuno, M.; Sugihara, A. Effects of substitutional impurity Au and Si atoms on antiphase boundary energies in Ti3Al: A first principles study. Philos. Mag. 2010, 90, 3919–3934. [Google Scholar] [CrossRef]
- Vamsi, K.V.; Karthikeyan, S. MATEC Web of Conferences. 2014; 14, 11005. [Google Scholar]
- Shoeck, G.; Kohlhammer, S.; Fähnle, M. Planar dissociations and recombination energy of [110] superdislocations in Ni3Al: Generalized Peierls model in combination with ab initioelectron theory. Philos. Mag. Lett. 1999, 79, 849–857. [Google Scholar] [CrossRef]
- Yoo, M.H. On the theory of anomalous yield behavior of Ni3Al-effect of elastic anisotropy. Scr. Metall. 1986, 20, 915. [Google Scholar] [CrossRef]
- Paxton, A.T.; Sun, Y.Q. The role of planar fault energy in the yield anomaly in L12 intermetallics. Philos. Mag. A 1998, 78, 85–104. [Google Scholar]
- Kumar, K.; Sankarasubramanian, R.; Waghmare, U.V. The effect of γ-γ’ interface on the tensile and shear strengths of nickel-based superalloys: A first-principles study. Comput. Mater. Sci. 2015, 97, 26–31. [Google Scholar] [CrossRef]
- Manga, V.R.; Shang, S.L.; Wang, W.Y.; Wang, Y.; Liang, J.; Crespi, V.H.; Liu, Z.K. Anomalous phonon stiffening associated with the (111) antiphase boundary in L12 Ni3Al Original research article. Acta Mater. 2015, 82, 287–294. [Google Scholar] [CrossRef]
- Demura, M.; Golberg, D.; Hirano, T. An athermal deformation model of the yield stress anomaly in Ni3Al. Intermetallics 2007, 15, 1322–1331. [Google Scholar] [CrossRef]
- Abzaev, Y.A.; Starenchenko, V.A.; Solo’eva, Y.V.; Kozlov, E.V. Effect of orientation on the peak temperature of the yield-stress anomaly in single crystals of the Ni3Ge alloy. Phys. Met. Metall. 2006, 101, 591–595. [Google Scholar] [CrossRef]
- Suzuki, T.; Oya, Y.; Wee, D.M. Transition from positive to negative temperature dependence of the strength in Ni3Ge-Fe3Ge solid solution. Acta Metall. 1980, 28, 301–310. [Google Scholar] [CrossRef]
- Pak, H.R.; Saubri, T.; Nenno, S. Temperature and Orientation Dependence of the Yield Stress in Ni3Ge Single Crystals. Trans. Jpn. Inst. Met. 1977, 18, 617–626. [Google Scholar] [CrossRef]
- Thornton, P.H.; Davies, R.G. The temperature dependence of the flow stress of gamma prime phases having the Ll2 structure. Metall. Trans. 1970, 1, 549–550. [Google Scholar] [CrossRef]
- Dyck, S.V.; Delaey, L.; Froyen, L.; Buekenhout, L. Microstructural evolution and its influence on the mechanical properties of a nickel silicide based intermetallic alloy. Intermetallics 1997, 5, 137–145. [Google Scholar] [CrossRef]
- Takasugi, T.; Yoshida, M. Strength anomaly and dislocation structure at 4.2 k in ni3(si, ti) single crystals. Philos. Mag. A 1992, 65, 613–624. [Google Scholar] [CrossRef]
- Lunt, M.J.; Sun, Y.Q. Creep and the anomalous yield stress of Ni3Ga. Mater. Sci. Eng. A 1997, 239, 445–449. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kuramoto, E. Anomalous Temperature Dependence of the Yield Stress in Ni3Ga. J. Phys. Soc. Jpn. 1971, 31, 1282. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kuramoto, E. Temperature and orientation dependence of the yield stress in Niin3Ga single crystals. Acta Metall. 1973, 21, 415–425. [Google Scholar] [CrossRef]
- Wu, Z.L.; Pope, D.P.; Vitek, V. Deformation and fracture of L12 (Al,Fe)3Ti. Scr. Metall. 1990, 24, 2187. [Google Scholar] [CrossRef]
- Geng, P.J.; Li, W.G.; Zhang, X.H.; Deng, Y.; Kou, H.B.; Ma, J.Z.; Shao, J.X.; Chen, L.M.; Wu, X.Z. A theoretical model for yield strength anomaly of Ni-base superalloys at elevated temperature. J. Alloy. Compd. 2017, 706, 340–343. [Google Scholar] [CrossRef]
- Liu, J.B.; Johnson, D.D.; Smirnov, A.V. Predicting yield-stress anomalies in L12 alloys: Ni3Ge-Fe3Ge pseudo-binaries. Acta Mater. 2005, 53, 3601–3612. [Google Scholar] [CrossRef]
- Hagihara, K.; Tanaka, T.; Nakano, T.; Veyssière, P.; Umakoshi, Y. Effects of the anisotropy of the anti-phase boundary energy on the yield-stress anomaly in Ni3X compounds with close-packed crystal structures. Philos. Mag. Lett. 2007, 87, 705–712. [Google Scholar] [CrossRef]
- Hagihara, K.; Tanaka, T.; Izumo, H.; Umakoshi, Y.; Nakano, T. Non-basal slip in Ni3(Ti, Nb) and Ni3(Ti, Al) single crystals with various long-period stacking ordered structures. Acta Mater. 2013, 61, 4365. [Google Scholar] [CrossRef]
- Nishino, Y.; Tanahashi, T. Effect of molybdenum substitution on the yield stress anomaly in Fe3Al-based alloys. Mater. Sci. Eng. A 2004, 387, 973–976. [Google Scholar] [CrossRef]
- George, E.P.; Baker, I. Thermal vacancies and the yield anomaly of FeAl. Intermetallics 1998, 6, 759–763. [Google Scholar] [CrossRef]
- Mitchell, T.E.; Baskes, M.I.; Hoagland, R.G.; Misra, A. Dislocation core structures and yield stress anomalies in molybdenum disilicide. Intermetallics 2001, 9, 849–856. [Google Scholar] [CrossRef]
- Nakano, T.; Hagihara, K. Yield stress anomaly controlled by the phase stability in NbSi2 single crystals. Scr. Mater. 2013, 68, 313–316. [Google Scholar] [CrossRef]
- Liu, L.L.; Wu, X.Z.; Wang, R.; Li, W.G.; Liu, Q. Stacking fault energy, yield stress anomaly, and twinnability of Ni3Al: A first principle study. Chin. Phys. B 2015, 24, 077102. [Google Scholar] [CrossRef]
- Lü, B.L.; Chen, G.Q.; Qu, S.; Su, H.; Zhou, W.L. First-principle calculation of yield stress anomaly of Ni3Al-based alloys. Mater. Sci. Eng. A 2013, 565, 317–320. [Google Scholar] [CrossRef]
- Golovin, I.S.; Jäger, S.; Mennerich, C.; Siemers, C.; Neuhäuser, H. Structure and anelasticity of Fe3Ge alloy. Intermetallics 2007, 15, 1548–1557. [Google Scholar] [CrossRef]
- Balk, T.J.; Kumar, M.; Hemker, J. Influence of Fe substitutions on the deformation behavior and fault energies of Ni3Ge-Fe3Ge L12 intermetallic alloys. Acta Mater. 2001, 49, 1725–1736. [Google Scholar] [CrossRef]
- Hu, W.C.; Liu, Y.; Li, D.J.; Zeng, X.Q.; Xu, C.S. Mechanical and thermodynamic properties of Al3Sc and Al3Li precipitates in Al-Li-Sc alloys from first-principles calculations. Phys. B 2013, 427, 85–90. [Google Scholar] [CrossRef]
- Saal, J.E.; Wolverton, C. Energetics of antiphase boundaries in γ’ Co3(Al,W)-based superalloys. Acta Mater. 2016, 103, 57–62. [Google Scholar] [CrossRef]
- Wu, X.Z.; Wang, R.; Wang, S.F.; Wei, Q.Y. Ab initio calculations of generalized-stacking-fault energy surfaces and surface energies for FCC metals. Appl. Surf. Sci. 2010, 256, 6345–6349. [Google Scholar] [CrossRef]
- Frankel, J.; Vassiliou, J.; Jamieson, J.C.; Dandekar, D.P.; Scholz, W. The elastic constants of Ni3Al to 1.4 GPa. Physica B+C 1986, 139, 198–201. [Google Scholar] [CrossRef]
- Duan, Y.H.; Sun, Y.; Peng, M.J.; Zhou, S.G. Ab-initio investigations on elastic properties in L12 structure Al3Sc and Al3Y under high pressure. J. Alloy. Compd. 2014, 585, 587–593. [Google Scholar] [CrossRef]
- Boucetta, S.; Zegrar, F. First-Principles Study of the Structural, Elastic, and Mechanical Properties of Ni3Ga Compound under Pressure. Acta Phys. Pol. A 2014, 125, 54. [Google Scholar] [CrossRef]
- Pearson, W.B. A Handbook of Lattice Spacings and Structures of Metals and Alloys; Pergamon Press: Pergamon, Turkey; New York, NY, USA, 1958. [Google Scholar]
- Li, J.; Zhang, M.; Luo, X. Theoretical investigations on phase stability, elastic constants and electronic structures of D022- and L12-Al3Ti under high pressure. J. Alloy. Compd. 2013, 556, 214–220. [Google Scholar] [CrossRef]
- Tian, T.; Wang, X.F.; Li, W. Ab initio calculations on elastic properties in L12 structure Al3X and X3Al-type (X = transition or main group metal) intermetallic compounds. Solid State Commun. 2013, 156, 69–75. [Google Scholar] [CrossRef]
- Fu, C.L.; Ye, Y.Y.; Yoo, M.H. Theoretical investigation of the elastic constants and shear fault energies of Ni3Si. Philos. Mag. Lett. 1993, 67, 179–185. [Google Scholar] [CrossRef]

| P | a | A | ||||
|---|---|---|---|---|---|---|
| NiAl | 0 | 3.569 | 228.42 | 151.76 | 116.89 | 3.05 |
| 1.4 [55] | - | 223.50 | 149.00 | 122.90 | 3.30 | |
| 20 | 3.463 | 330.25 | 231.54 | 161.43 | 3.27 | |
| 40 | 3.389 | 415.88 | 302.55 | 197.93 | 3.49 | |
| NiGe | 0 | 3.585 | 253.81 | 149.28 | 98.83 | 1.89 |
| 0 [41] | 3.500 | 263.00 | 143.00 | 103.00 | 1.72 | |
| 20 | 3.484 | 367.87 | 231.14 | 141.99 | 2.08 | |
| 40 | 3.413 | 474.76 | 310.69 | 183.49 | 2.24 | |
| NiSi | 0 | 3.511 | 298.15 | 166.93 | 129.24 | 1.97 |
| 20 | 3.420 | 410.44 | 247.02 | 174.86 | 2.14 | |
| 40 | 3.354 | 514.69 | 322.28 | 216.06 | 2.25 | |
| NiGa | 0 | 3.588 | 226.66 | 154.68 | 105.40 | 2.93 |
| 0 [57] | 3.521 | 288.86 | 192.53 | 127.74 | 2.65 | |
| 0 [57] | 3.570 | 264.14 | 169.99 | 116.39 | 2.47 | |
| 0 [58] | 3.580 | 191.00 | 123.00 | 108.00 | 3.17 | |
| 20 | 3.482 | 329.99 | 240.69 | 147.18 | 3.30 | |
| 40 | 3.408 | 424.04 | 320.88 | 184.82 | 3.58 | |
| AlTi | 0 | 3.980 | 190.74 | 64.27 | 75.41 | 1.19 |
| 0 [59] | 3.985 | 184.40 | 64.21 | 74.61 | 1.24 | |
| 0 [59] | 3.984 | 184.32 | 62.41 | 72.89 | 1.20 | |
| 0 [59] | 3.900 | 207.54 | 69.05 | 87.29 | 1.26 | |
| 20 | 3.797 | 299.66 | 122.13 | 127.42 | 1.44 | |
| 20 [59] | 3.799 | 292.10 | 120.50 | 126.80 | 1.48 | |
| AlSc | 0 | 4.106 | 182.21 | 39.39 | 71.37 | 1.00 |
| 0 [60] | - | 180.67 | 40.62 | 72.00 | 1.03 | |
| 0 [56] | 4.101 | 187.84 | 35.14 | 73.32 | 0.96 | |
| 20 | 3.887 | 293.69 | 94.29 | 124.15 | 1.25 | |
| 20 [56] | - | 312.83 | 88.19 | 128.13 | 1.14 |
| P | 0 | 20 | 40 | |
|---|---|---|---|---|
| NiAl | {111} | 0.344 | 0.406 | 0.459 |
| {010} | 0.117 | 0.173 | 0.205 | |
| 2.940 | 2.346 | 2.239 | ||
| p | 5.352 | 4.626 | 4.282 | |
| NiGe | {111} | 0.509 | 0.587 | 0.651 |
| {010} | 0.381 | 0.415 | 0.442 | |
| 1.336 | 1.414 | 1.473 | ||
| p | 1.950 | 2.163 | 2.331 | |
| NiSi | {111} | 0.455 | 0.515 | 0.565 |
| {010} | 0.395 | 0.453 | 0.506 | |
| 1.152 | 1.137 | 1.117 | ||
| p | 1.718 | 1.766 | 1.777 | |
| NiGa | {111} | 0.272 | 0.308 | 0.337 |
| {010} | 0.016 | 0.024 | 0.029 | |
| 17.000 | 12.833 | 11.621 | ||
| p | 31.396 | 24.485 | 22.320 | |
| AlTi | {111} | 0.258 | 0.643 | - |
| {010} | 0.094 | 0.173 | - | |
| 2.745 | 3.717 | - | ||
| p | 5.615 | 4.748 | - | |
| AlSc | {111} | 0.741 | 1.025 | - |
| {010} | 0.575 | 0.851 | - | |
| 1.289 | 1.204 | - | ||
| p | 1.280 | 1.394 | - |
| NiGe | Fe | Sc | Al | Ga |
| Ni | −36.21 | −32.22 | −32.89 | −32.25 |
| Ge | −34.69 | −33.02 | −33.52 | −33.01 |
| NiSi | Al | Ga | Ti | Sc |
| Ni | −53.90 | −53.23 | −53.58 | −52.64 |
| Si | −54.02 | −53.43 | −53.87 | −52.98 |
| AlSc | Ti | Y | Li | Zr |
| Al | −50.86 | −49.45 | −50.86 | −50.41 |
| Sc | −51.15 | −51.15 | −49.46 | −51.63 |
| APB | APB | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 6.25% | 12.5% | 25% | 0% | 6.25% | 12.5% | 25% | 0% | 6.25% | 12.5% | 25% | ||
| Fe | 0.691 | 0.665 | 0.643 | 0.621 | 0.515 | 0.434 | 0.412 | 0.478 | 1.955 | 2.235 | 2.278 | 1.895 | |
| Sc | 0.691 | 0.628 | 0.566 | 0.442 | 0.515 | 0.477 | 0.465 | 0.431 | 1.955 | 1.919 | 1.774 | 1.497 | |
| Al | 0.691 | 0.648 | 0.600 | 0.494 | 0.515 | 0.469 | 0.424 | 0.295 | 1.955 | 2.014 | 2.064 | 2.443 | |
| Ga | 0.691 | 0.655 | 0.613 | 0.519 | 0.515 | 0.467 | 0.417 | 0.277 | 1.955 | 2.044 | 2.143 | 2.732 | |
| NiSi | Al | 0.666 | 0.635 | 0.602 | 0.523 | 0.520 | 0.507 | 0.464 | 0.351 | 1.831 | 1.866 | 1.931 | 2.219 |
| Ga | 0.666 | 0.641 | 0.614 | 0.548 | 0.520 | 0.500 | 0.450 | 0.317 | 1.831 | 1.907 | 2.031 | 2.575 | |
| Ti | 0.666 | 0.631 | 0.609 | 0.577 | 0.520 | 0.506 | 0.505 | 0.480 | 1.831 | 1.855 | 1.796 | 1.792 | |
| Sc | 0.666 | 0.629 | 0.596 | 0.530 | 0.520 | 0.518 | 0.521 | 0.545 | 1.831 | 1.806 | 1.704 | 1.449 | |
| AlSc | Ti | 0.698 | 0.666 | 0.637 | 0.562 | 0.558 | 0.527 | 0.472 | 0.343 | 1.251 | 1.262 | 1.349 | 1.637 |
| Y | 0.698 | 0.738 | 0.775 | 0.697 | 0.558 | 0.600 | 0.630 | 0.696 | 1.251 | 1.229 | 1.228 | 1.001 | |
| Li | 0.698 | 0.669 | 0.630 | 0.558 | 0.558 | 0.568 | 0.582 | 0.604 | 1.251 | 1.177 | 1.083 | 0.924 | |
| Zr | 0.698 | 0.704 | 0.708 | 0.718 | 0.558 | 0.557 | 0.529 | 0.454 | 1.251 | 1.239 | 1.337 | 1.581 | |
| Materials | Substitutions | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 6.25% | 12.5% | 25% | 6.25% | 12.5% | 25% | ||
| NiGe | 1.955 | 1.883 | 1.820 | 1.758 | 2.320 | 2.447 | 2.108 | |
| Sc | 1.955 | 1.789 | 1.609 | 1.253 | 2.110 | 2.168 | 2.337 | |
| Al | 1.955 | 1.845 | 1.708 | 1.399 | 2.147 | 2.376 | 3.415 | |
| Ga | 1.955 | 1.865 | 1.743 | 1.470 | 2.155 | 2.419 | 3.635 | |
| NiSi | Al | 1.831 | 1.746 | 1.657 | 1.499 | 1.951 | 2.139 | 2.826 |
| Ga | 1.831 | 1.763 | 1.670 | 1.569 | 1.975 | 2.207 | 3.133 | |
| Ti | 1.831 | 1.736 | 1.677 | 1.654 | 1.952 | 1.966 | 2.068 | |
| Sc | 1.831 | 1.729 | 1.641 | 1.518 | 1.907 | 1.907 | 1.822 | |
| AlSc | Ti | 1.251 | 1.175 | 1.134 | 1.007 | 1.327 | 1.487 | 2.035 |
| Y | 1.251 | 1.304 | 1.379 | 1.250 | 1.166 | 1.113 | 1.002 | |
| Li | 1.251 | 1.182 | 1.122 | 1.001 | 1.232 | 1.206 | 1.155 | |
| Zr | 1.251 | 1.244 | 1.261 | 1.288 | 1.257 | 1.325 | 1.536 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Wang, J.; Wu, X.; Wang, R.; Jia, Z. Effects of Alloying Atoms on Antiphase Boundary Energy and Yield Stress Anomaly of L12 Intermetallics: First-Principles Study. Crystals 2018, 8, 96. https://doi.org/10.3390/cryst8020096
Gao X, Wang J, Wu X, Wang R, Jia Z. Effects of Alloying Atoms on Antiphase Boundary Energy and Yield Stress Anomaly of L12 Intermetallics: First-Principles Study. Crystals. 2018; 8(2):96. https://doi.org/10.3390/cryst8020096
Chicago/Turabian StyleGao, Xiaojun, Jianwei Wang, Xiaozhi Wu, Rui Wang, and Zhihong Jia. 2018. "Effects of Alloying Atoms on Antiphase Boundary Energy and Yield Stress Anomaly of L12 Intermetallics: First-Principles Study" Crystals 8, no. 2: 96. https://doi.org/10.3390/cryst8020096




