Effect of Alginate from Chilean Lessonia nigrescens and MWCNTs on CaCO3 Crystallization by Classical and Non-Classical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactants
2.2. In Vitro Gas Diffusion (GD) Crystallization of CaCO3.
2.3. Electrodeposition of Alginate/MWCNTs.
2.4. Electrocrystallization (EC) Experiments of CaCO3.
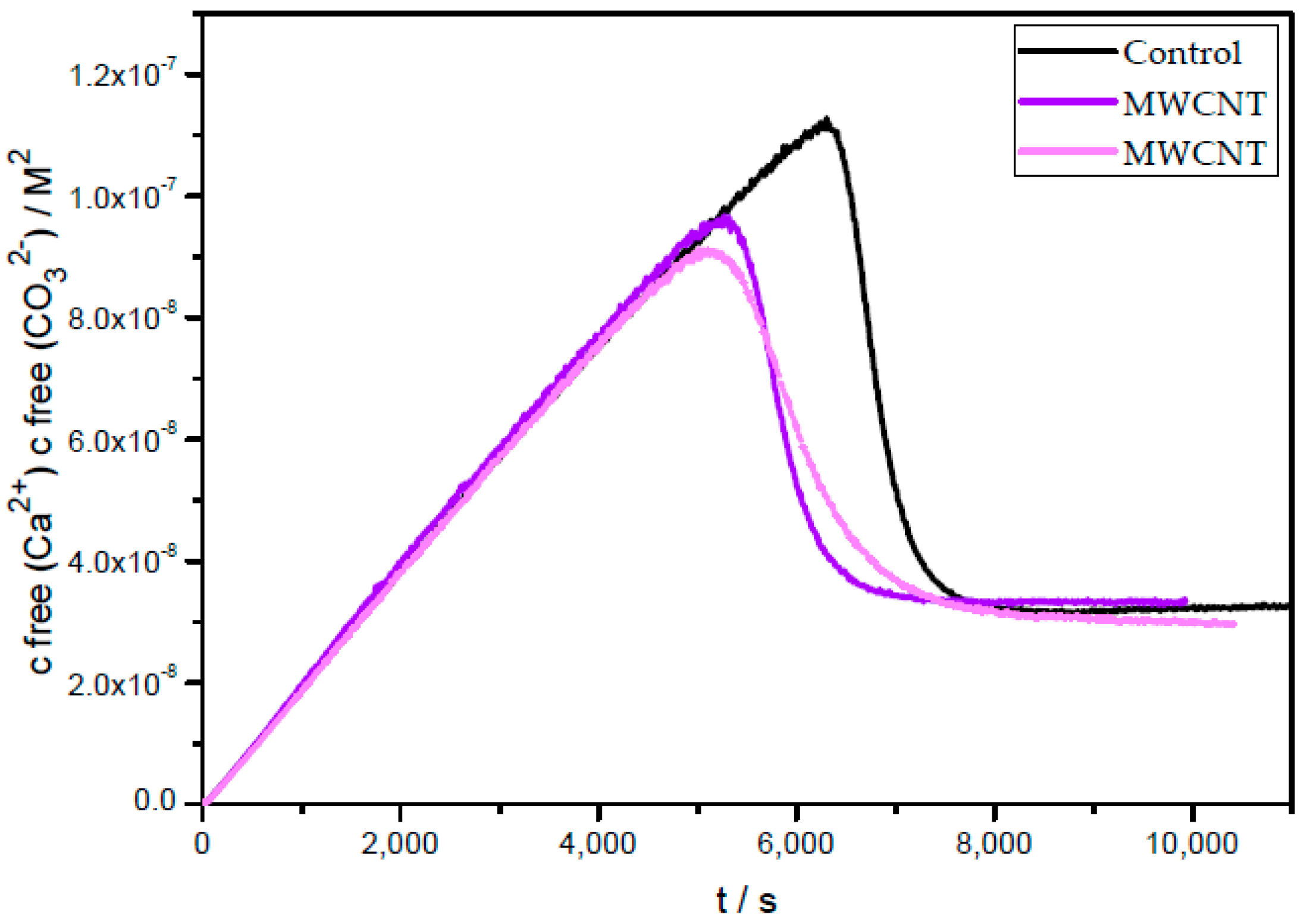
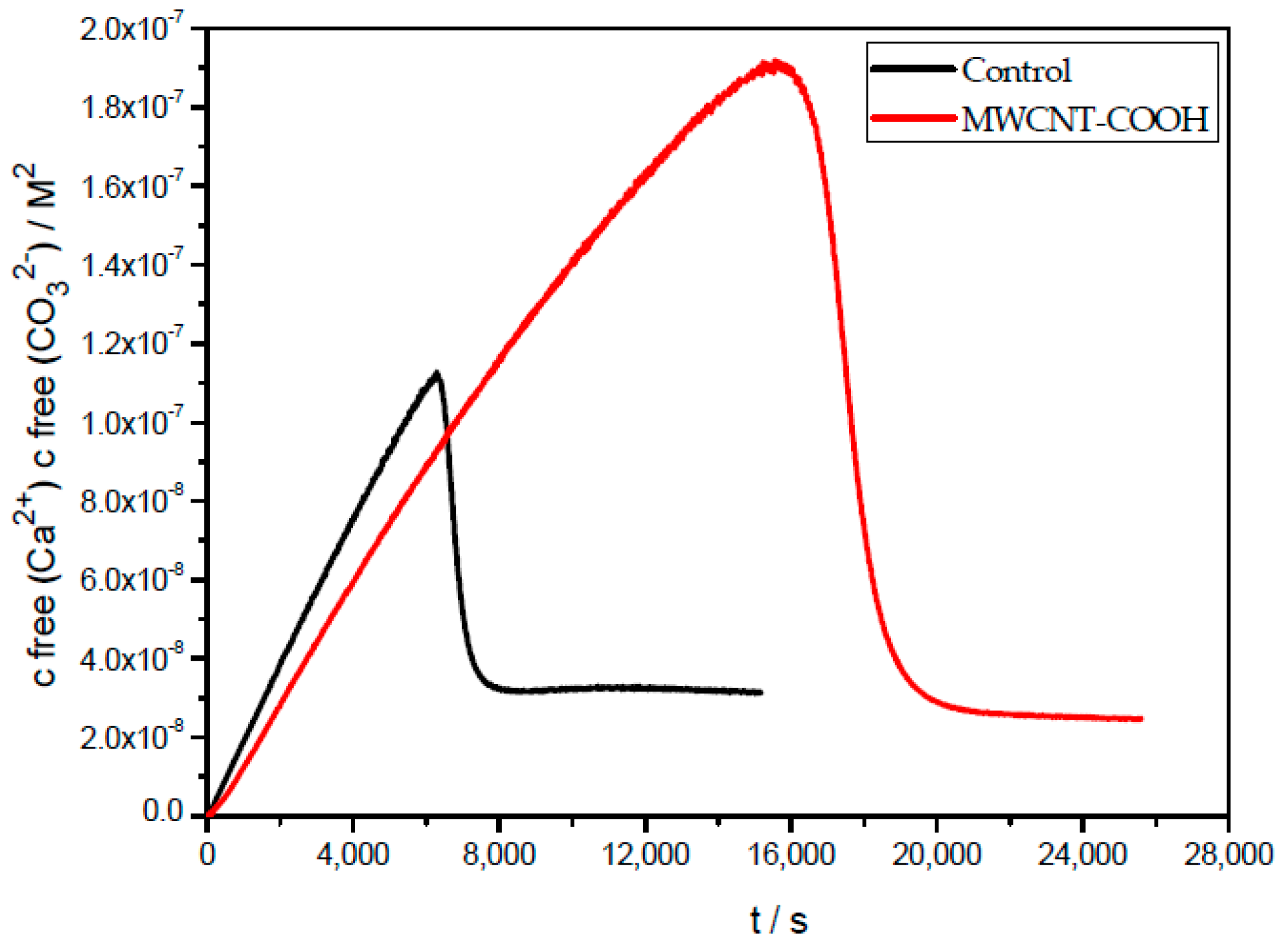
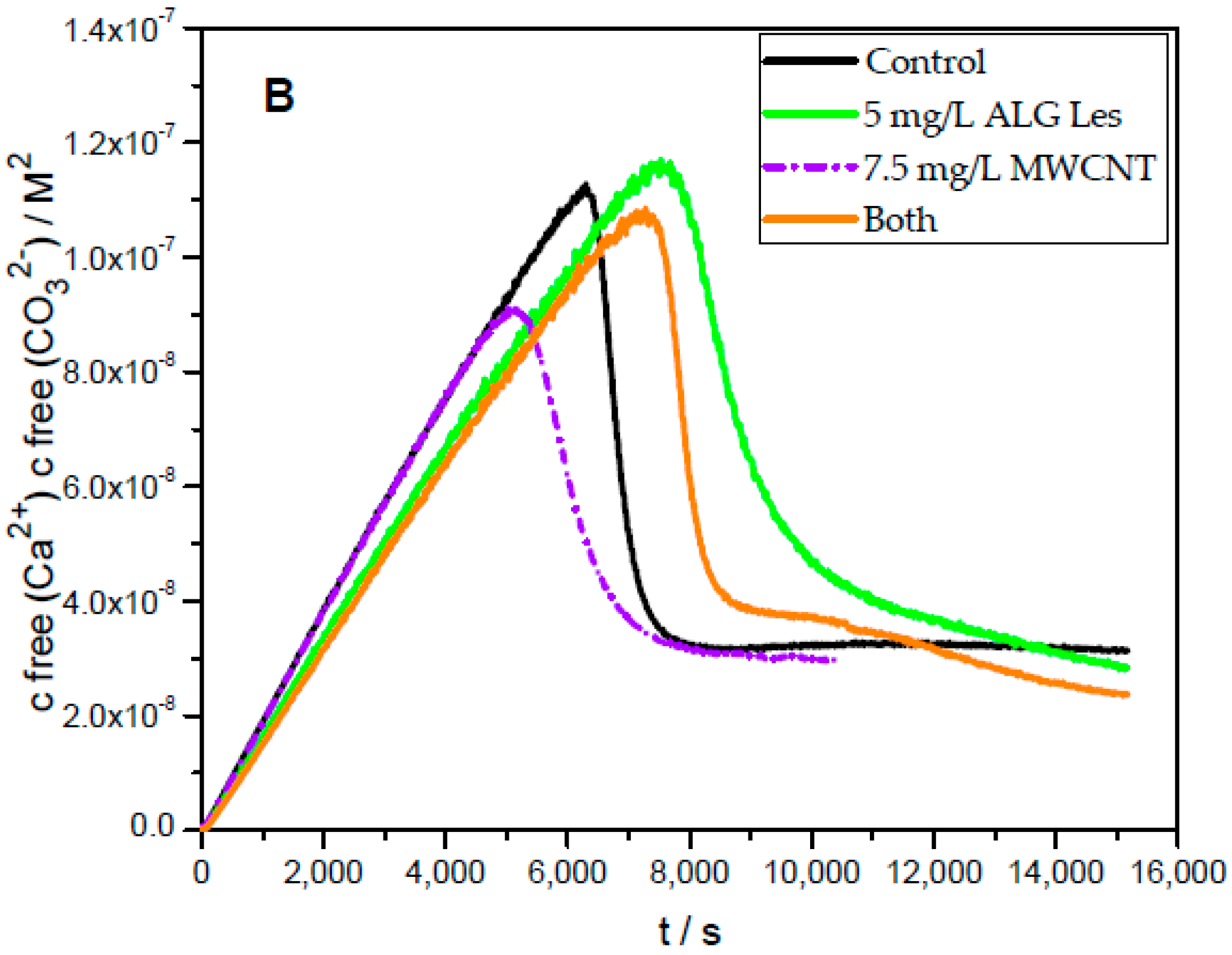
2.5. Prenucleation Clusters (PNC) Assay of CaCO3
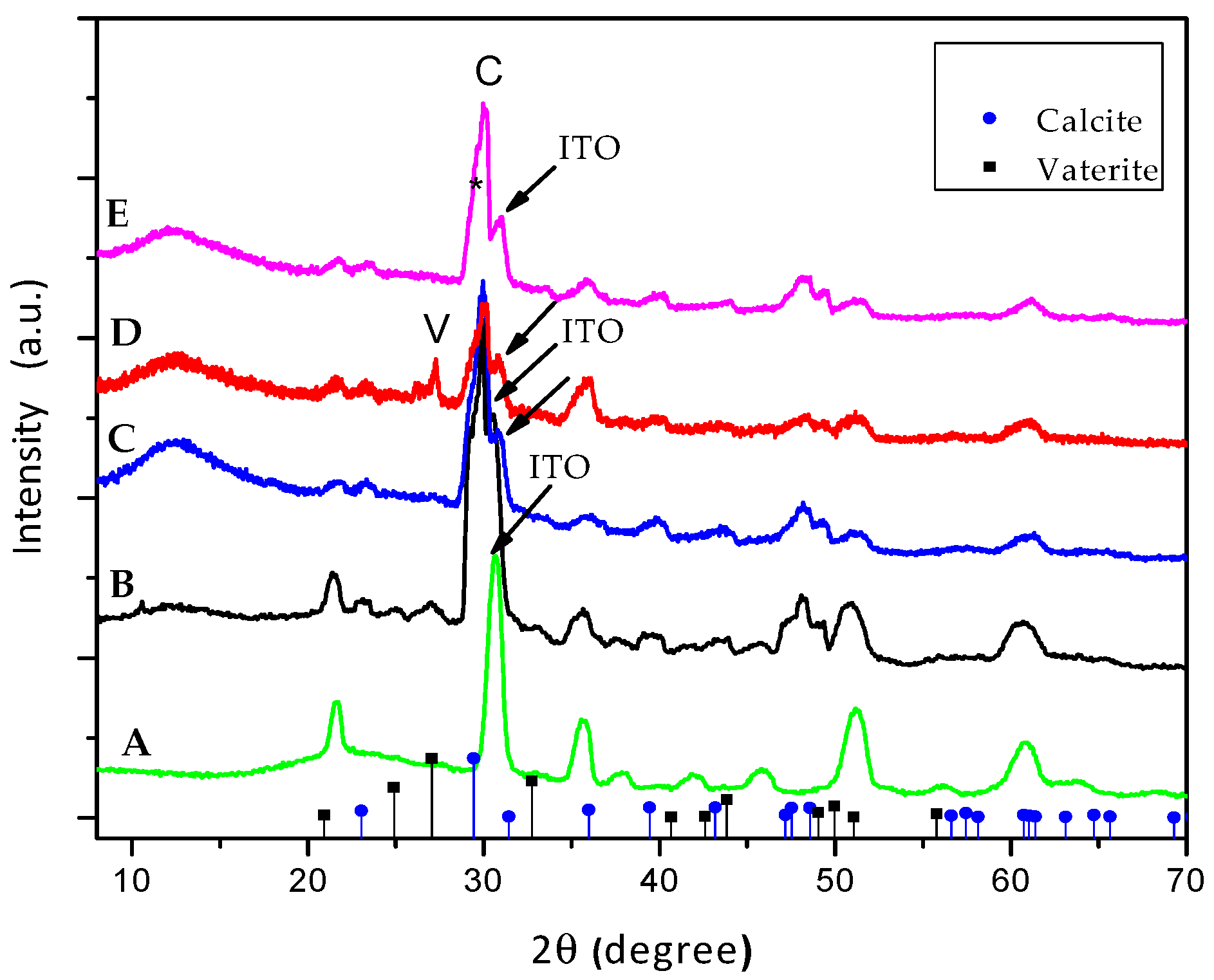
2.6. Characterization
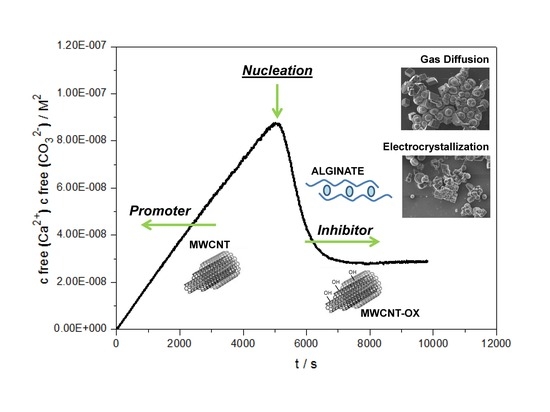
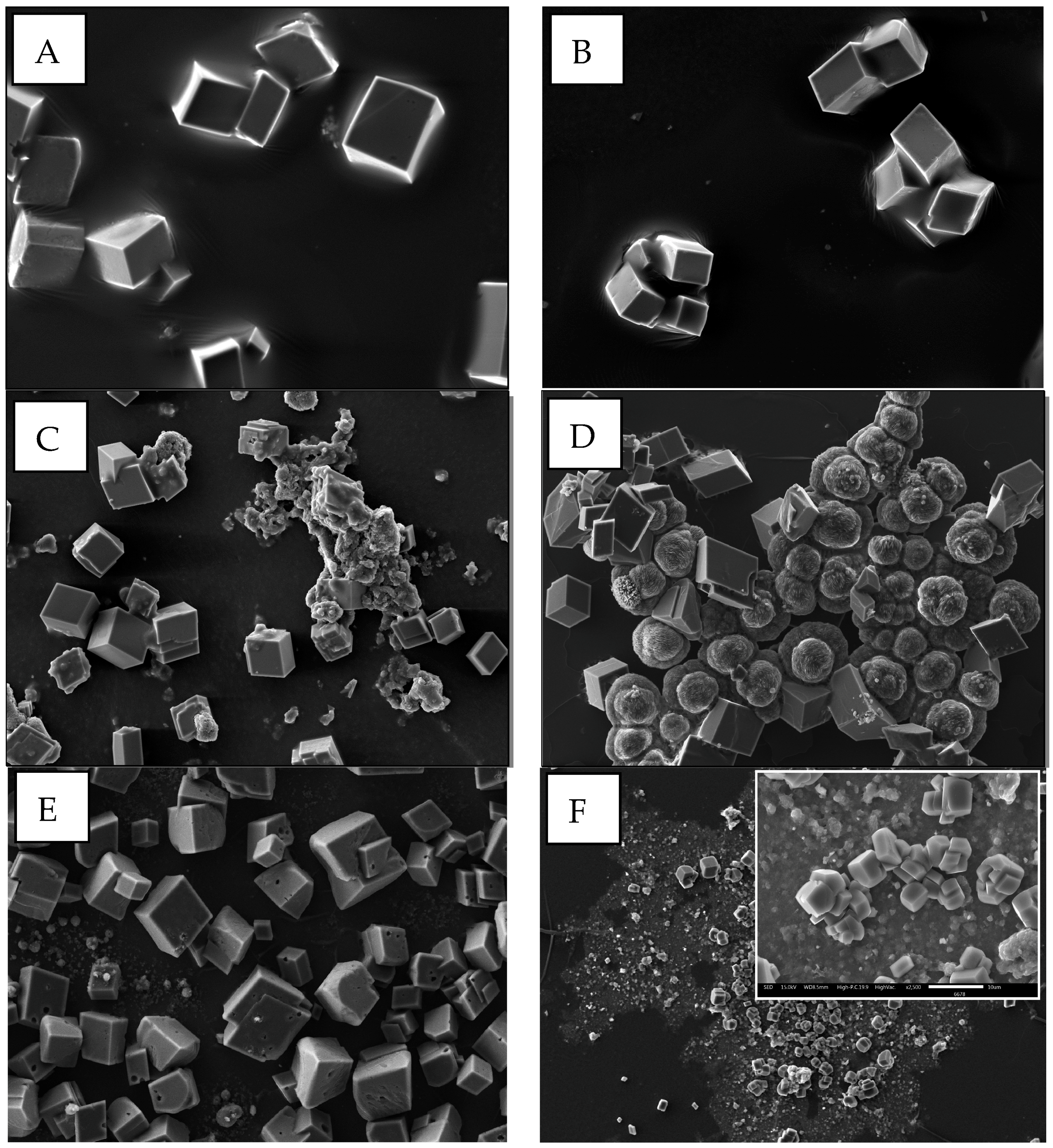
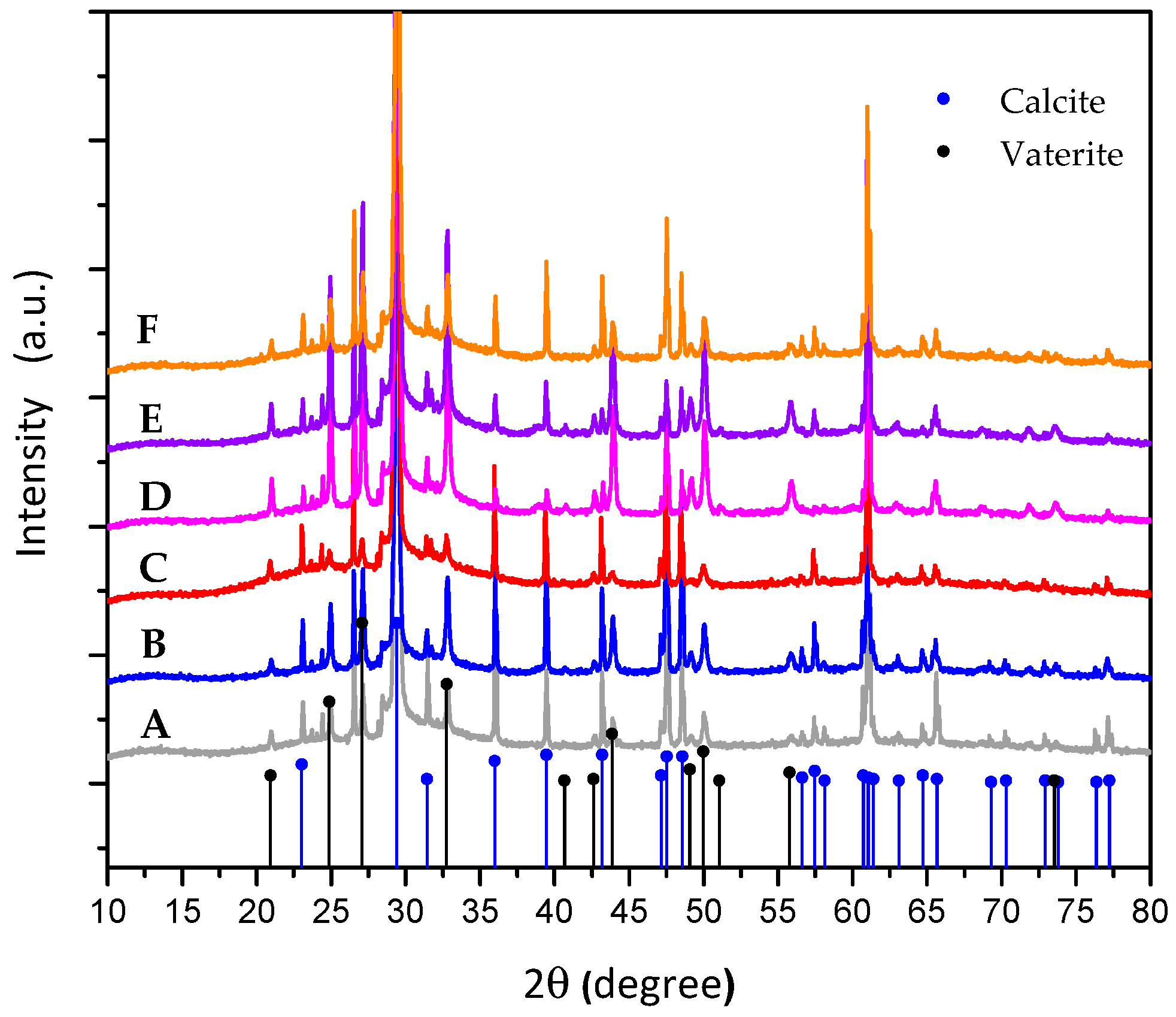
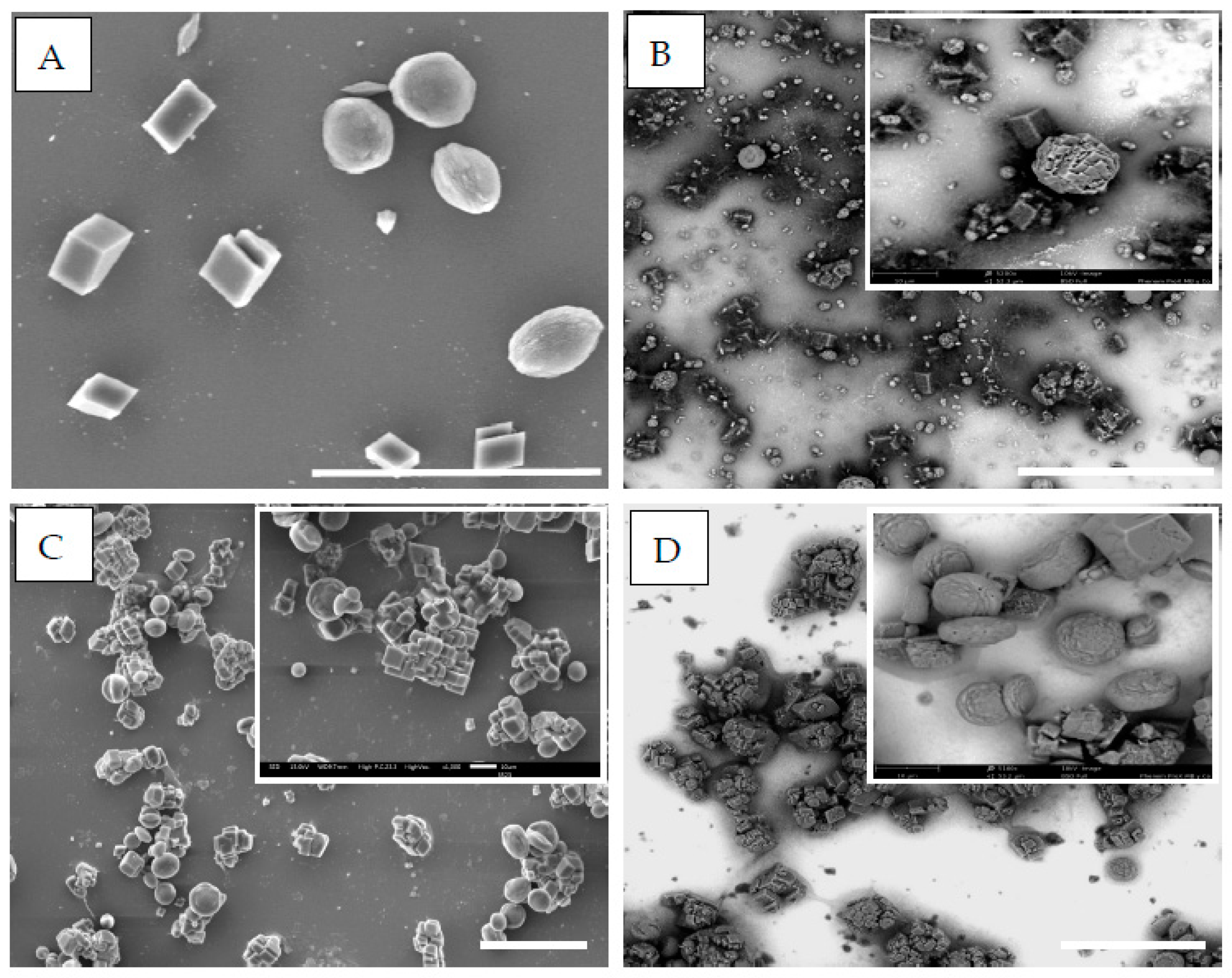
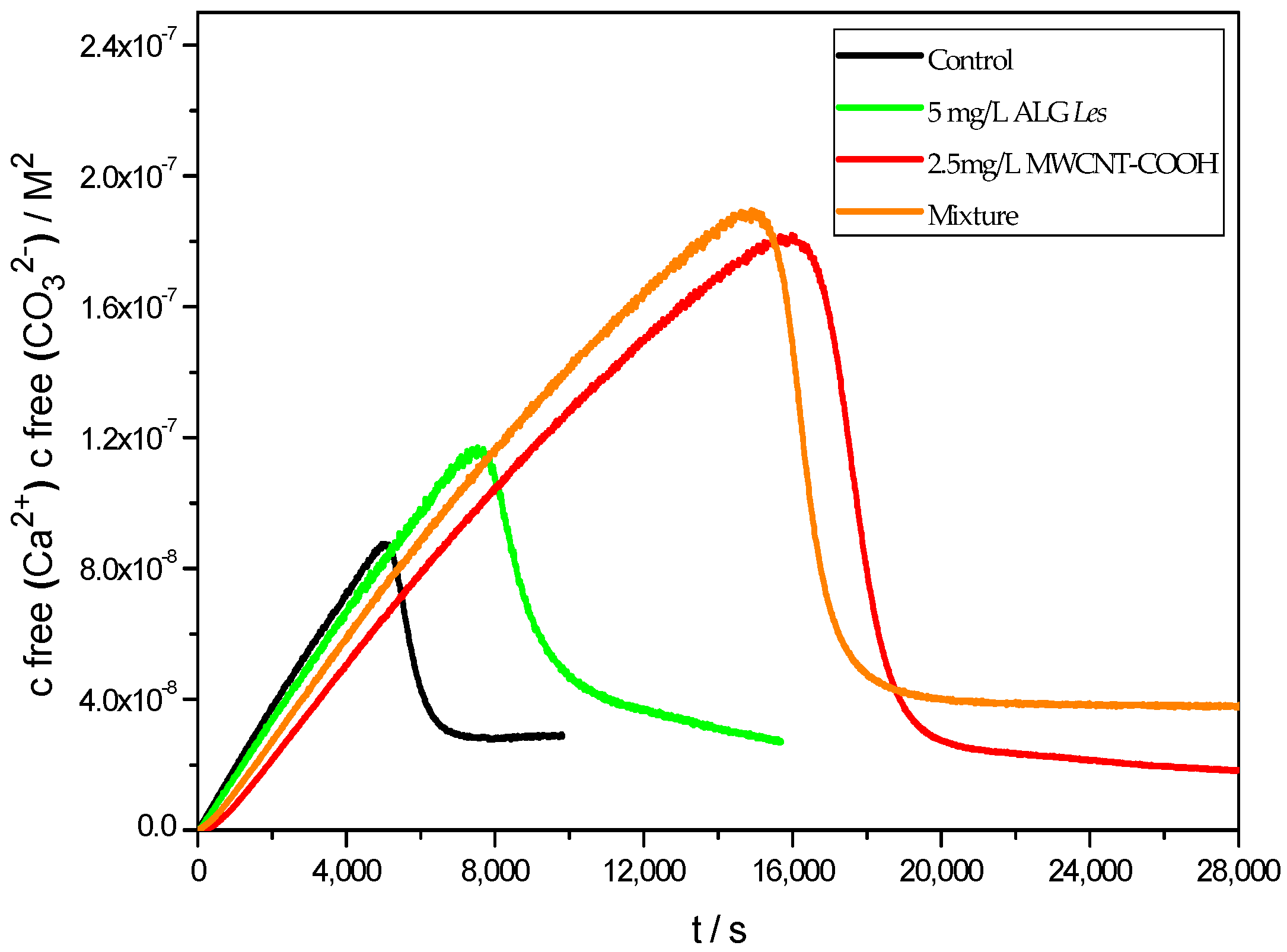
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix A1. ALG Extraction from L. nigrescens
Appendix A2. Purifying of ALG Extracted from L. nigrescens
References
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, 1st ed.; Oxford University Press: New York, NY, USA, 2001; pp. 1–30. ISBN 0198508824. [Google Scholar]
- Arias, J.L.; Neira-Carrillo, A.; Arias, J.I.; Escobar, C.; Bodero, M.; David, M.; Fernández, M.S. Sulfated polymers in biological mineralization: A plausible source for bio-inspired engineering. J. Mater. Chem. 2004, 14, 2154–2160. [Google Scholar] [CrossRef]
- Arias, J.L.; Fernández, M.S. Polysaccharides and proteoglycans in calcium carbonate-based biomineralization. Chem. Rev. 2008, 108, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Young, J.R.; Henriksen, K. Biomineralization within vesicles: The calcite of coccoliths. Rev. Mineral. Geochem. 2003, 54, 189–215. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Cölfen, H. Prenucleation clusters and non-classical nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Kellermeier, M.; Cölfen, H.; Gebauer, D. Investigating the early stages of mineral precipitation by potentiometric titration and analytical ultracentrifugation. Methods Enzymol. 2013, 532, 45–69. [Google Scholar] [PubMed]
- Cartwright, J.H.; Checa, A.G.; Gale, J.D.; Gebauer, D.; Sainz-Díaz, C.I. Calcium carbonate polyamorphism and its role in biomineralization: How many amorphous calcium carbonates are there? Angew. Chem. Int. Ed. 2012, 51, 11960–11970. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Cölfen, H.; Antonietti, M.; Yu, S.-H. Ethanol assisted synthesis of pure and stable amorphous calcium carbonate nanoparticles. ChemComm 2013, 49, 9564–9566. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Berg, J.K.; Kellermeier, M.; Gebauer, D. Sweet on biomineralization: Effects of carbohydrates on the early stages of calcium carbonate crystallization. Eur. J. Miner. 2014, 26, 537–552. [Google Scholar] [CrossRef]
- Rao, A.; Vásquez-Quitral, P.; Fernández, M.S.; Berg, J.K.; Sánchez, M.; Drechsler, M.; Neira-Carrillo, A.; Arias J., L.; Gebauer, D.; Cölfen, H. pH-dependent schemes of calcium carbonate formation in the presence of alginates. Cryst. Growth Des. 2016, 16, 1349–1359. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Krishna Pai, R.; Fernández, M.S.; Carreño, E.; Vásquez-Quitral, P.; Arias, J.L. Synthesis and characterization of sulfonated polymethylsiloxane polymer as template for crystal growth of CaCO3. Colloid Polym. Sci. 2009, 287, 385–393. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Vásquez-Quitral, P.; Díaz, M.P.; Fernández, M.S.; Arias, J.L.; Yazdani-Pedram, M. Control of calcium carbonate crystallization by using anionic polymethylsiloxanes as templates. J. Solid State Chem. 2012, 194, 400–408. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Vásquez Quitral, P.; Yazdani-Pedram, M.; Arias, J.L. Crystal growth of CaCO3 induced by new carboxylated monomethylitaconate grafted polymethylsiloxane. Eur. Polym. J. 2010, 46, 1184–1193. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Asenath-Smith, E.; Li, H.; Keene, E.C.; Seh, Z.W.; Estroff, L.A. Crystal growth of calcium carbonate in hydrogels as a model of biomineralization. Adv. Funct. Mater. 2012, 22, 2891–2914. [Google Scholar] [CrossRef]
- Salgado, L.T.; Amado Filho, G.M.; Fernández, M.S.; Arias, J.L.; Farina, M. The effect of alginates, fucans and phenolic substances from the brown seaweed Padina gymnospora in calcium carbonate mineralization in vitro. J. Cryst. Growth 2011, 321, 65–71. [Google Scholar] [CrossRef]
- Manoli, F.; Dalas, E. The effect of sodium alginate on the crystal growth of calcium carbonate. J. Mater. Sci. Mater. Med. 2002, 13, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Olderøy, M.Ø.; Xie, M.; Strand, B.L.; Flaten, E.M.; Sikorski, P.; Andreassen, J.P. Growth and nucleation of calcium carbonate vaterite crystals in presence of alginate. Cryst. Growth Des. 2009, 9, 5176–5183. [Google Scholar] [CrossRef]
- Ma, Y.; Feng, Q. Alginate hydrogel-mediated crystallization of calcium carbonate. J. Solid State Chem. 2011, 184, 1008–1015. [Google Scholar] [CrossRef]
- Egaña Palma, R. Preparación de Esferas de Alginato Purificado de Origen Comercial y Desde Algas Chilenas Lessonia nigrescens Como Implante Para Terapia Celular. MVD Thesis, Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile, Santiago, Chile, 2013. [Google Scholar]
- Gebauer, D.; Cölfen, H.; Verch, A.; Antonietti, M. The multiple roles of additives in CaCO3 crystallization: A quantitative case study. Adv. Mater. 2009, 21, 435–439. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhong, G.J.; Hsiao, B.S.; Fu, Q.; Li, Z.M. Low-dimensional carbonaceous nanofiller induced polymer crystallization. Prog. Polym. Sci. 2014, 39, 555–593. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Makar, J.M.; Chan, G.W. Growth of cement hydration products on single-walled carbon nanotubes. J. Am. Ceram. Soc. 2009, 92, 1303–1310. [Google Scholar] [CrossRef]
- Balázsi, C.; Wéber, F.; Kövér, Z.; Shen, Z.; Konya, Z.; Kasztovszky, Z.; Vértesy, Z.; Biró, L.P.; Kiricsi, I.; Arato, P. Application of carbon nanotubes to silicon nitride matrix reinforcements. Curr. Appl. Phys. 2006, 6, 124–130. [Google Scholar] [CrossRef]
- Lupo, F.; Kamalakaran, R.; Scheu, C.; Grobert, N.; Rühle, M. Microstructural investigations on zirconium oxide–carbon nanotube composites synthesized by hydrothermal crystallization. Carbon 2004, 42, 1995–1999. [Google Scholar] [CrossRef]
- Tasis, D.; Pispas, S.; Galiotis, C.; Bouropoulos, N. Growth of calcium carbonate on non-covalently modified carbon nanotubes. Mater. Lett. 2007, 61, 5044–5046. [Google Scholar] [CrossRef]
- Li, W.; Gao, C. Efficiently stabilized spherical vaterite CaCO3 crystals by carbon nanotubes in biomimetic mineralization. Langmuir 2007, 23, 4575–4582. [Google Scholar] [CrossRef] [PubMed]
- Pavez, J.; Silva, J.F.; Melo, F. Effects of alginic acid from marine algae on calcium carbonate electrodeposited coating. J. Cryst. Growth 2005, 282, 438–447. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Acevedo, D.F.; Miras, M.C.; Barbero, C.A.; Gebauer, D.; Cölfen, H.; Arias, J.L. Influence of Conducting Polymers Based on Carboxylated Polyaniline on in vitro CaCO3 Crystallization. Langmuir 2008, 24, 12496–12507. [Google Scholar] [CrossRef] [PubMed]
- Neira-Carrillo, A.; Yazdani-Pedram, M.; Retuert, J.; Díaz-Dosque, M.; Gallois, S.; Arias, J.L. Selective crystallization of calcium salts by poly(acrylate)-grafted chitosan. J. Colloid Interface Sci 2005, 286, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Neira-Carrillo, A.; Retuert, J.; Martinez, F.; Arias, J.L. Effect of crosslinked chitosan as a constrained volume on the in vitro calcium carbonate crystallization. J. Chil. Chem. Soc. 2008, 53, 1367–1372. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Pai, R.; Fuenzalida, V.; Fernandez, M.S.; Retuert, J.; Arias, J.L. Calcium carbonate growth modification by constituents releases from porous cellulose filter membranes. J. Chil. Chem. Soc. 2008, 53, 1469–1473. [Google Scholar] [CrossRef]
- Yang, X.; Kim, E.; Liu, Y.; Shi, X.W.; Rubloff, G.W.; Ghodssi, R.; Payne, G.F. In-film bioprocessing and immunoanalysis with electroaddressable stimuli-responsive polysaccharides. Adv. Funct. Mater. 2010, 20, 1645–1652. [Google Scholar] [CrossRef]
- Lédion, J.; Leroy, P.; Labbé, J.-P. Détermination du caractère incrustant d’une eau par un essai d'entartrage accéléré. Tech. Sci. Munic. (1971) 1985, 7–8, 323–328. [Google Scholar]
- Butler, M.F.; Glaser, N.; Weaver, A.C.; Kirkland, M.; Heppenstall-Butler, M. Calcium carbonate crystallization in the presence of biopolymers. Cryst. Growth Des. 2006, 6, 781–794. [Google Scholar] [CrossRef]
- Díaz-Dosque, M.; Aranda, P.; Darder, M.; Retuert, J.; Yazdani-Pedram, M.; Arias, J.L.; Ruiz-Hitzky, E. Use of biopolymers as oriented supports for the stabilization of different polymorphs of biomineralized calcium carbonate with complex shape. J. Cryst. Growth 2008, 310, 5331–5340. [Google Scholar] [CrossRef]
- Ketrane, R.; Saidani, B.; Gil, O.; Leleyter, L.; Baraud, F. Efficiency of five scale inhibitors on calcium carbonate precipitation from hard water: Effect of temperature and concentration. Desalination 2009, 249, 1397–1404. [Google Scholar] [CrossRef]
- Butler, M.F.; Frith, W.J.; Rawlins, C.; Weaver, A.C.; Heppenstall-Butler, M. Hollow calcium carbonate microsphere formation in the presence of biopolymers and additives. Cryst. Growth Des. 2008, 9, 534–545. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, M.; Cui, Y.L.; Zhao, L.; Zhou, X. Synthesis of quercetin loaded nanoparticles based on alginate for Pb (II) adsorption in aqueous solution. Nanoscale Res. Lett. 2015, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Lakouraj, M.M.; Mojerlou, F.; Zare, E.N. Nanogel and superparamagnetic nanocomposite based on sodium alginate for sorption of heavy metal ions. Carbohydr. Polym. 2014, 106, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Jiang, F.; Lu, K.; Ming, W.; Shao, Z. Growth of calcium carbonate mediated by slowly released alginate. CrystEngComm 2010, 12, 730–736. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Vásquez-Quitral, P.; Sánchez, M.; Farhadi-Khouzani, M.; Aguilar-Bolados, H.; Yazdani-Pedram, M.; Cölfen, H. Evaluation of functionalized multi-walled CNTs as template for classical and non-classical CaCO3 crystallization. Unpublished work. 2018. [Google Scholar]
- Zhao, B.; Hu, H.; Mandal, S.K.; Haddon, R.C. A Bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. Chem. Mater. 2005, 17, 3235–3241. [Google Scholar] [CrossRef]
- Gomez, C.G.; Lambrecht, M.V.P.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction–purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.; Vásquez-Quitral, P.; Butto, N.; Díaz-Soler, F.; Yazdani-Pedram, M.; Silva, J.F.; Neira-Carrillo, A. Effect of Alginate from Chilean Lessonia nigrescens and MWCNTs on CaCO3 Crystallization by Classical and Non-Classical Methods. Crystals 2018, 8, 69. https://doi.org/10.3390/cryst8020069
Sánchez M, Vásquez-Quitral P, Butto N, Díaz-Soler F, Yazdani-Pedram M, Silva JF, Neira-Carrillo A. Effect of Alginate from Chilean Lessonia nigrescens and MWCNTs on CaCO3 Crystallization by Classical and Non-Classical Methods. Crystals. 2018; 8(2):69. https://doi.org/10.3390/cryst8020069
Chicago/Turabian StyleSánchez, Marianela, Patricio Vásquez-Quitral, Nicole Butto, Felipe Díaz-Soler, Mehrdad Yazdani-Pedram, Juan Francisco Silva, and Andrónico Neira-Carrillo. 2018. "Effect of Alginate from Chilean Lessonia nigrescens and MWCNTs on CaCO3 Crystallization by Classical and Non-Classical Methods" Crystals 8, no. 2: 69. https://doi.org/10.3390/cryst8020069