2.1. Theoretical Predictions Using USPEX
We constructed the convex hull, which connects the phases that are stable against decomposition into elements or binaries at a given pressure. The convex hulls were obtained from the enthalpies of the most stable structures of compounds at a given pressure. The enthalpy of formation of the lowest enthalpy Ga-Ti-oxynitrides at 0, 5, 10 and 20 GPa are given in
Table 1, which simply shows a tendency for structures with 1:1 and 1:2 ratios of GaN:TiO
2 to move to lower formation enthalpy with increasing pressure. This trend suggests their potential stability with increasing pressure.
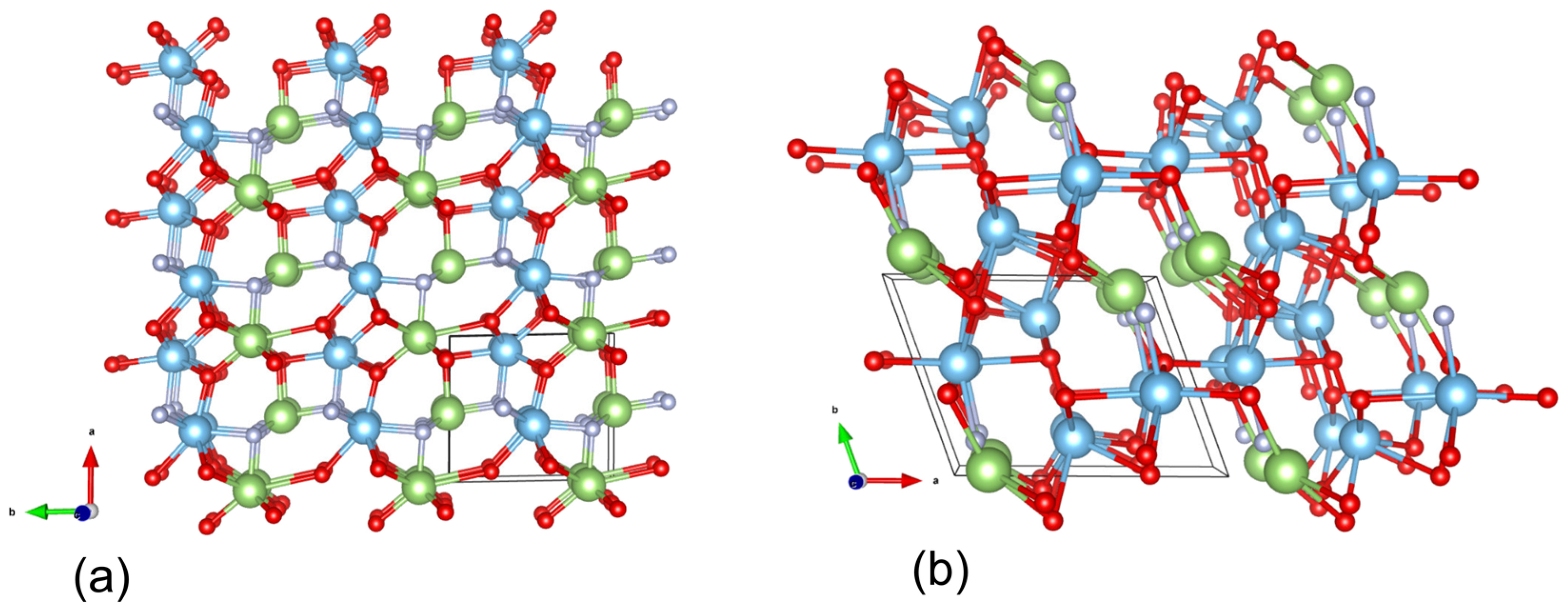
The structures of the predicted ordered GaTiO
2N and GaTi
2O
4N phases are illustrated in
Figure 1 and the Crystallographic Information Files (CIF) are described in the
appendix (
Table A2). Although not all structural aspects of these ordered phases did match with the disordered experimental phase described in
Section 2.2, ab initio calculations at 0 K provided a useful guide to target compositions that are within thermodynamically synthesizable range.
Analysis of X-ray and neutron scattering data suggests that the experimentally synthesized new phase has the composition of Ga10Ti8O28N2. After obtaining this result, we performed phase stability calculations for Ga10Ti8O28N2 against the decomposition to oxynitrides and oxide endmembers i.e., Ti3O3N2 and Ga2TiO5, however, the decomposition reaction energies indicate that Ga10Ti8O28N2 is an entropy-stabilized oxynitride. The decomposition reaction energies are obtained as follow: 15 meV/atom at 10 GPa and 4 meV/atom at 20 GPa, which indicates its stability with increasing pressure.
Entropy can have a significant impact on free energy calculations of disordered structures; however, incorporating entropy is computationally expensive. Entropy is a sum of the electronic entropy (
Sel), configurational entropy (
Sconf) and vibrational entropy [
21]. The electronic entropy
Sel is easy to calculate, but does not have a significant effect on the free energy. On the other hand, vibrational
Svib and configurational
Sconf entropies are very expensive and can have significant effect on free energy. The configurational entropies are calculated based on Boltzmann’s formula which relates entropy values to the number of the accessible arrangement of atoms in the lattice [
22].
where
is the Boltzmann constant and
is the number of all possible configurations. The general formula for the configurational entropy can be obtained from:
where
Pj is the probability of a certain configuration
j. Configurational entropies are calculated based on the above formula and the stabilizing temperatures are calculated from:
where
is the difference between enthalpies of ordered and disordered structures and
. Configurational entropies and required temperatures for stabilizing GaTiO
2N, GaTi
2O
4N and Ga
10Ti
8O
28N
2 are listed in
Table 2. The experimentally-observed mixed tetrahedral and octahedral coordination of Ga is accurately predicted with USPEX; however, Ti is predicted to exist in a mixture of octahedral and heptahedral coordination while experiments only observe octahedral Ti. In addition, bond valences are calculated according to
, where B is 0.37 and
is obtained from published tables [
23]. Bond valences for Ga and Ti of the predicted structures are consistent with the experimental phase.
Theoretical and experimental structural studies revealed that the synthesized phase has positional disorder among both the cations and the anions sites. Since oxygen and nitrogen have similar ionic size, electronegativities and coordination number, these anions are usually disordered across anion sites [
9], and hence makes it a real challenge for crystal structure prediction methods. Partial ordering have been observed in some oxynitrides [
24,
25,
26], however, ideally ordered oxynitrides are scarce between known oxynitrides e.g., TaON and Si
2N
2O [
27,
28]. While incorporating disorder is not practical with crystal structure prediction methods at the current stage of development, the predicted structures give insights into the chemistry of the system, may guide experimental efforts, and contain structural features, such as the separation of Ti and Ga into different structural blocks, which is confirmed in the experimental analysis. In addition, the average bond lengths of the predicted phases, (
Figure 1 &
Table A3) are in a good agreement with the experimental data.
2.2. Crystallographic Characterization
In anticipation of the inability of X-rays to distinguish between O and N in the anion sites, and because of the favorable scattering contrast between Ga and Ti, b
N = 7.29 fm and −3.44 fm, respectively, initially only neutron diffraction data, collected on the POWGEN instrument at the Spallation Neutron Source at Oak Ridge National Laboratory, TN, were utilized. Unit cell indexing and structure solution identified the new phase designated presumptively as “Ga-Ti-O-N”, is isomorphic with monoclinic V
2GaO
5,
C2/m,
Z = 6,
a = 17.758(5) Å,
b = 2.990(1) Å,
c = 9.323(3) Å and
β = 98.44(2)° [
30]. From the crystal structure of V
2GaO
5 we can construct a solid solution that accounts for the coupled substitution of Ti/N into the full unit cell contents (
Z = 6) of V
12Ga
6O
30. The oxide and oxynitride endmembers of this solid solution would then be Ga
12Ti
6O
30 and Ti
18O
18N
12, respectively. More generally, the solid solution is formulated as Ti
6+xGa
12−xO
30−xN
x; empirical formula Ti
1+xGa
2−xO
5−xN
x. This formulation provided chemical constraints utilized in the latter stages of structure refinement.
An X-ray diffraction pattern was collected on the high resolution powder [
31,
32] instrument at beamline 11-BM at the Advanced Photon Source, IL, by taking advantage of the mail-in service at this facility [
33]. The X-ray data provides an independent means of determining the Ga/Ti content; this is particularly important since the presumed unknown phase designated isomorphous to V
2GaO
5 phase is nonstoichiometric [
30]. Any sample related variables, such as residual strain, can also be best determined from the high angular resolution X-ray data. Further, the small size of the sample, its multiphase nature, and the relative complexity of the Ga-Ti-O-N phase, is well matched to the high angular resolution at the 11-BM instrument. The initial X-ray refinements were constrained by assuming a pure oxide composition, Ga and Ti distributed randomly over all cation sites consistent with the presumed chemistry derived from V
2GaO
5, that is Ga
2TiO
5, and with displacement parameters for all atoms fixed to a common value (U
iso = 0.01Å
2). The peak width parameters were fixed to values determined from a LaB
6 standard, which also provided the X-ray wavelength, 0.412447(5) Å. The sample on which X-ray data were collected was identical to the one used for neutron-data collection. Because of the superior counting statistics (
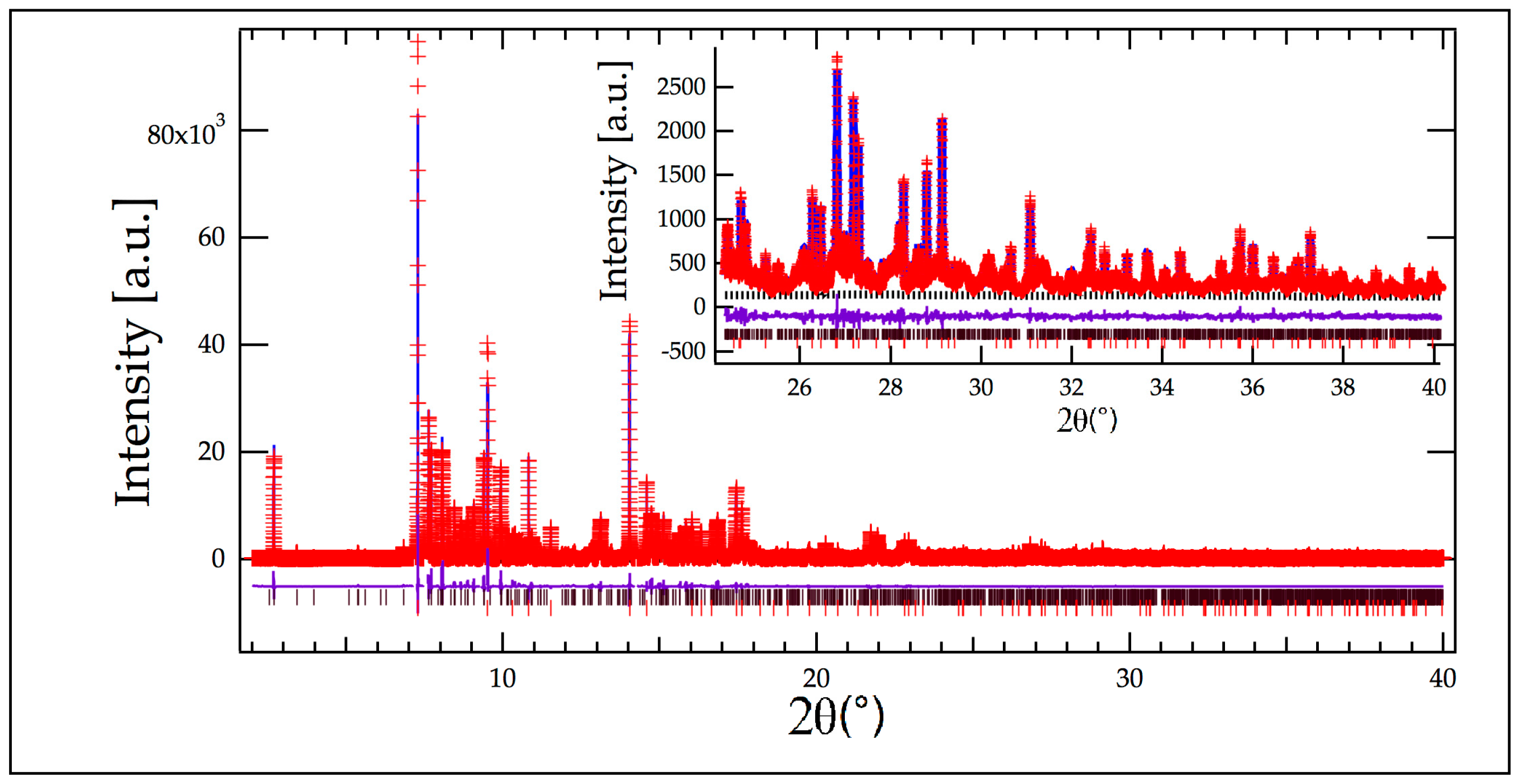
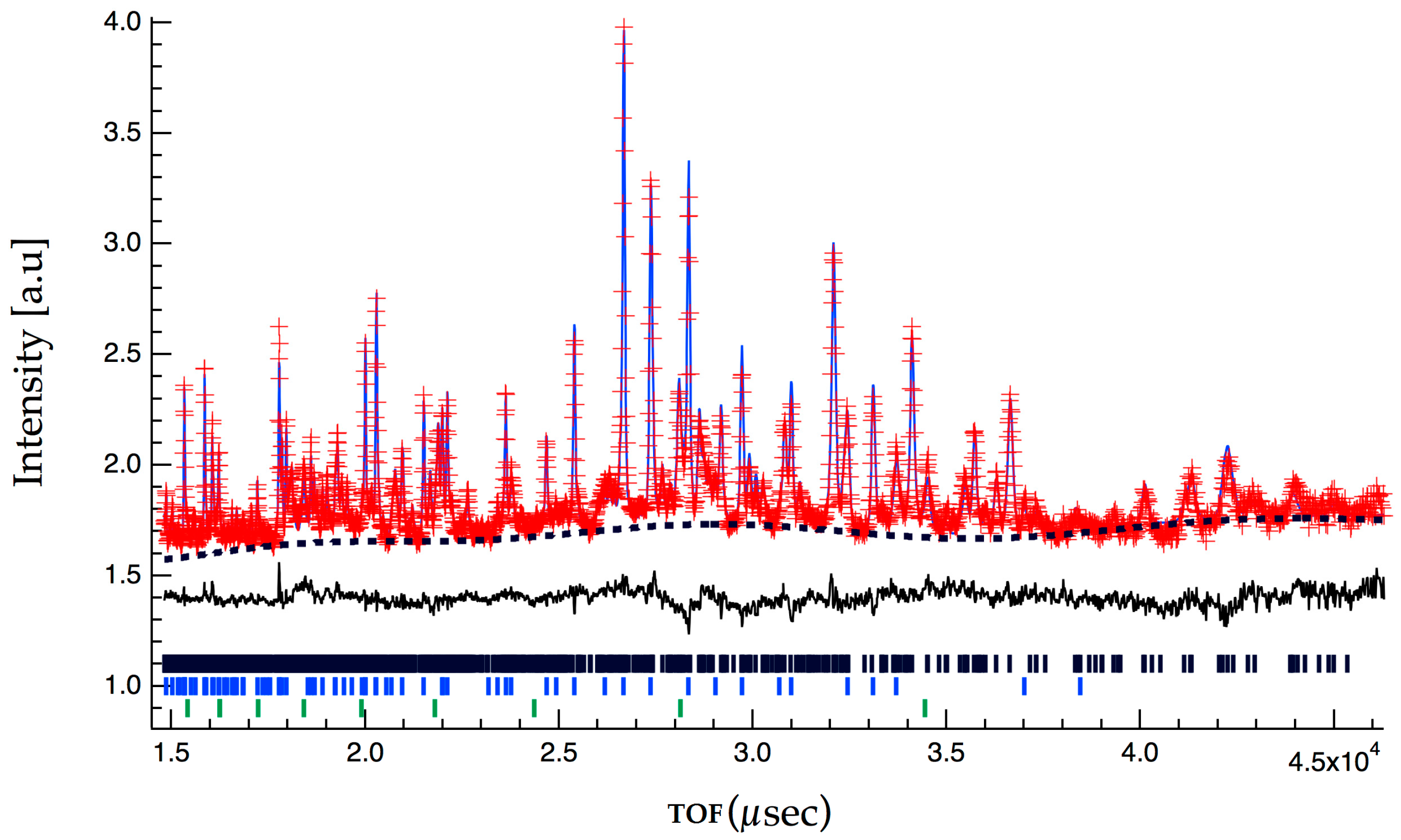
Figure 2 and
Figure 3) a small amount of an unidentified phase was obvious in the XRD pattern. The largest peaks from this phase were around 7.2, 7.5, 9.3, and 14.2° in 2θ and the most intense peak of the unindexed phase match that of rutile and Ga-Ti-O-N phases; the amounts were estimated to be 1% and 2% of those phases, respectively. Those regions containing peaks from the unidentified phase were excluded from refinements utilizing the X-ray data. These peaks were not observed in the neutron data, probably because of insufficient peak-to-background discrimination.
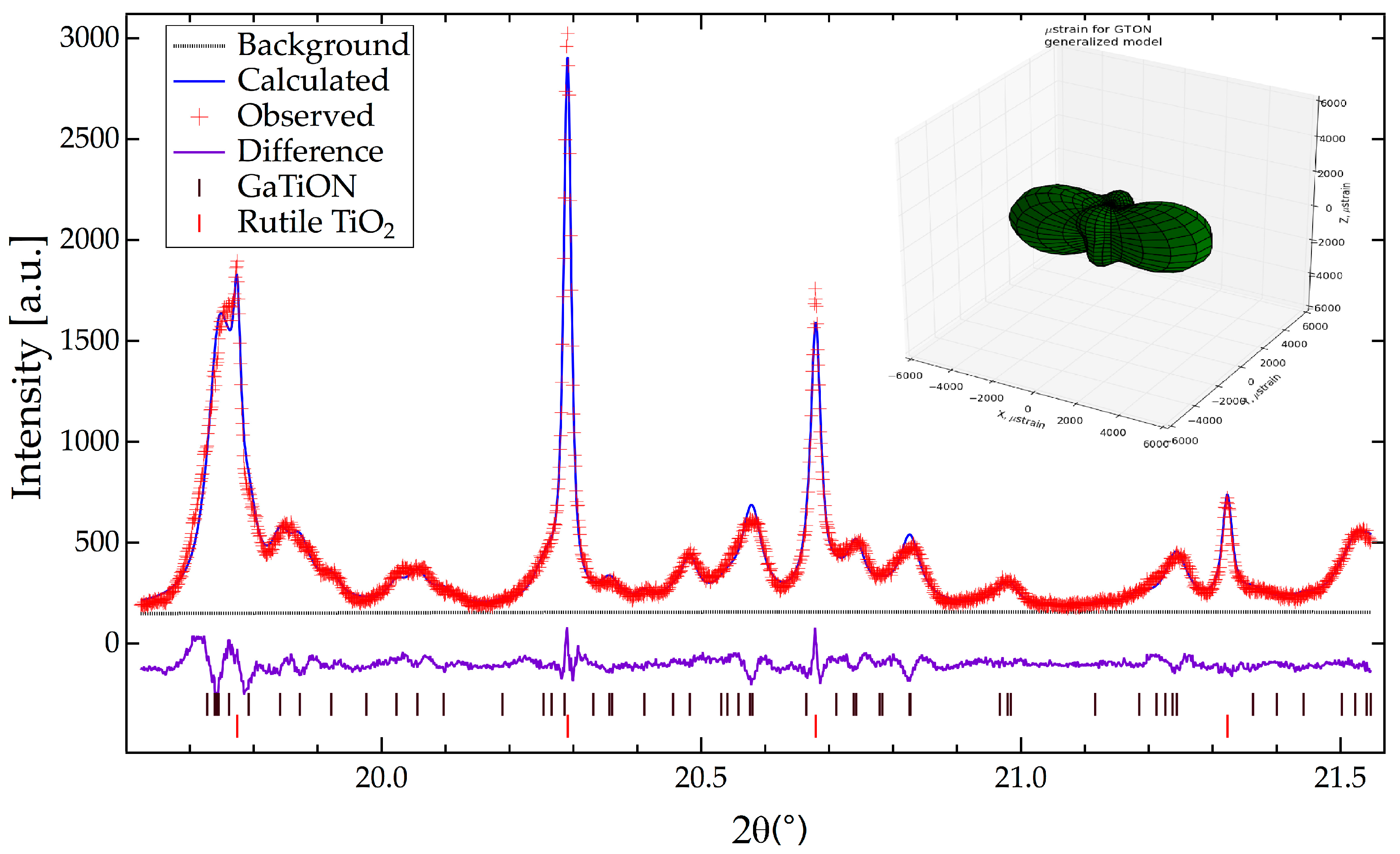
Refinement began with background parameters (cosine series, 12 parameters) and scale factor. Following convergence, cell parameters for both rutile and Ga-Ti-O-N phases were added to the refinement. The differences between observed and calculated patterns was dominated by anisotropic broadening of the Ga-Ti-O-N phase. Refinement of a generalized anisotropic broadening model [
34,
35] for Ga-Ti-O-N, improved the fit considerably as shown in
Figure 2, which is a smaller region of the fit.
The question of stoichiometry was addressed by refining positional parameters, followed by refinement of all metal site occupancies with fixed displacement parameters for all atoms (U
iso = 0.005 Å
2), the total occupancy of anion sites fixed to one oxygen, and metal sites constrained to be fully occupied. These assumptions were tested further in joint X-ray-neutron refinements discussed below. At this stage, refinement using just the X-ray data suggested some cation sites were partially disordered but that all were preferred by either Ga or Ti, or indeed were fully occupied by one or other of these metals. For example, the tetrahedral site (
Figure 3) is occupied exclusively by Ga, while the block of sites at
x = 0 and
x = ½ are essentially pure Ti, with about 20% Ga-substitution in the remaining TiGa sites (
Figure 3). The refined Ga/Ti ratio was approximately 1.25, which would correspond to a unit cell composition of Ga
10Ti
8O
28N
2.
A joint refinement utilizing both X-ray and neutron data was then initiated. The strain parameters for rutile and Ga-Ti-O-N were refined independently (
Figure 4) but constrained to be equal for each phase between X-ray and neutron datasets. The neutron data was weighted at 1.5, compared to the X-ray data weighted at 1.0, to emphasize the favorable scattering differences between O/N and Ga/Ti pairs occupying anion and cation sites, respectively. Starting with the unit cell composition consistent with refinement of cation site occupancies from the X-ray data, Ga
10Ti
8O
28N
2, atoms were distributed randomly over the cation and anion sites. The neutron refinement was initiated by sequentially refining overall parameters, background and scale, for individual data sets, and then sample parameters common to both datasets, strain and unit cell dimensions. To account for slight differences arising due to the temperatures at which neutron data were collected, elastic strain components were refined for both phases for the X-ray data set. The neutron dataset was contaminated by peaks arising from the vanadium sample holder, and this phase was added to account for this. Following convergence of a refinement of the above parameters and the site positional parameters for the Ga-Ti-O-N phase, the anion and cation site occupancies were determined.
To set a baseline for comparison of the overall fit, all anion sites were filled with oxygen while the Ga-Ti occupancies of cation sites were refined along with overall displacement parameters (U); the value of U for all cation sites were constrained to be equal, as were those of the anion sites. The results of this refinement suggested the following approximate compositions for the five cation sites (see
Table A1, which summarizes the final results for Ga-Ti-O-N): site 1, Ga
¾Ti
¼; site2, Ti; site3, Ga
0.8Ti
0.2; site 4, Ti; site5, Ga. The fit to the neutron data, summarized by the weighted profile R-value (R
wp) and goodness of fit (GOF) were 5.81% and 3.70, respectively. The fits to the Ga-Ti-O-N phase Bragg intensities, R
B(F
2), comparable to the usual single crystal discrepancy factor, were 5.27% and 12.86%, for the X-ray and neutron data, respectively. The site occupancies of all metal and anion sites were then varied, with U
iso constrained as above. The results of this refinement were: neutron overall R
wp = 5.34%, GOF = 3.64, and R
B(F
2) = 5.25% and 10.14%, for the X-ray and neutron data, respectively. Sites that were within the margin of error of full occupancy by Ga, Ti and O were fixed at those compositions; no anion site was fully occupied by nitrogen. A new set of refinements was performed, maintaining restraints on strain and displacement parameters as described above. Trial refinements with displacement parameters refined without constraint did not significantly improve the overall fit. Finally, the chemical composition was constrained to be consistent with the formula derived for the solid solution Ti
6+xGa
12−xO
30−xN
x; the amount of nitrogen per unit cell distributed over sites occupied by both oxygen and nitrogen, designated as “ON” sites ON1, 2, 4 and 8, was set equal to the amount of titanium in sites GaTi1 and 3 (
Table A1). After convergence, the results summarized in
Table A1 were obtained. The overall fits to the X-ray and neutron data are given in
Figure 2 and
Figure 3, respectively. The CIF file for the experimental phase is deposited into Crystallography Open Database (COD entry 3000168) as prepublication data.