Effect of Sonication Output Power on the Crystal Structure and Magnetism of SrFe12O19 Nanoparticles
Abstract
:1. Introduction
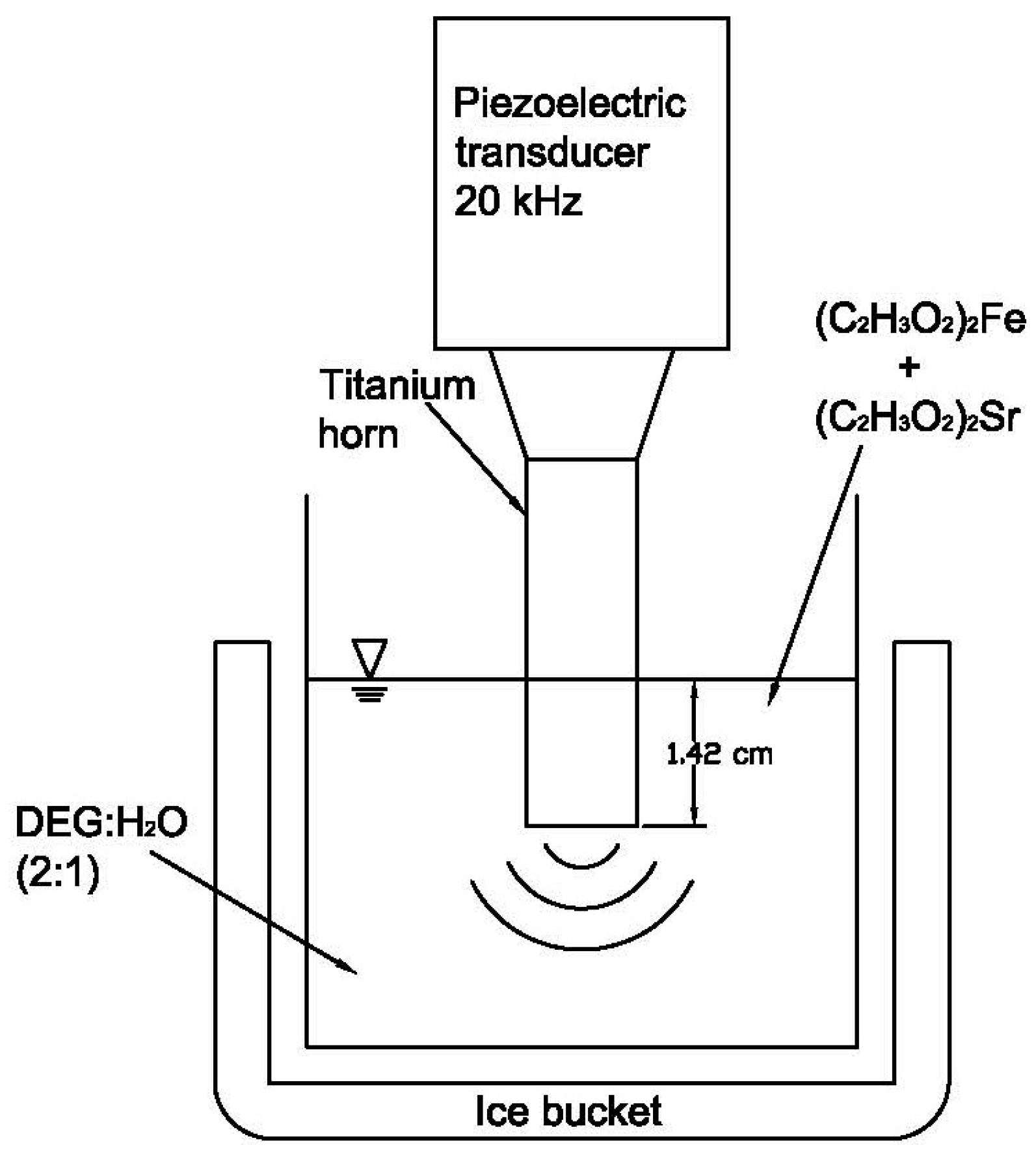
2. Materials and Methods
3. Results
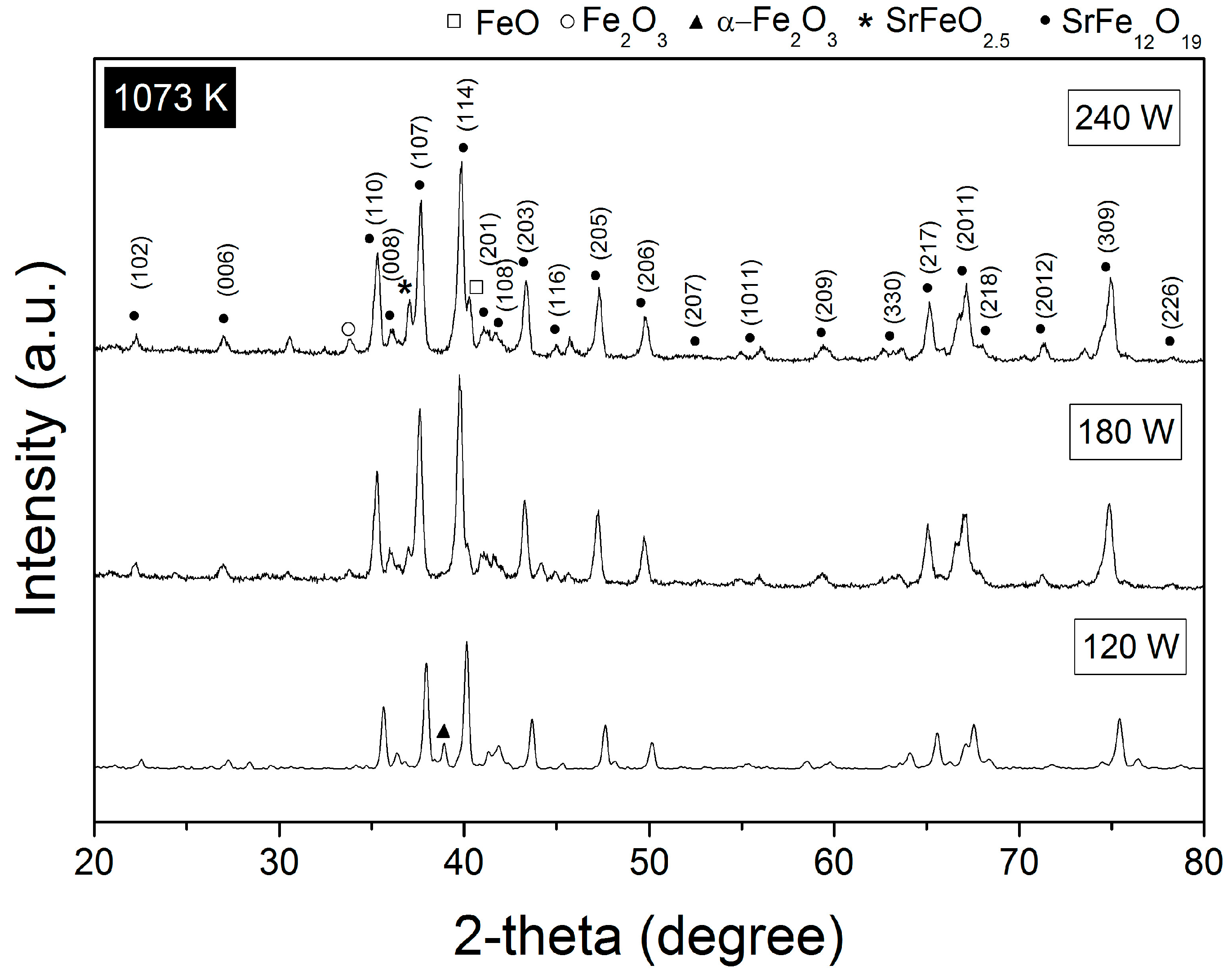
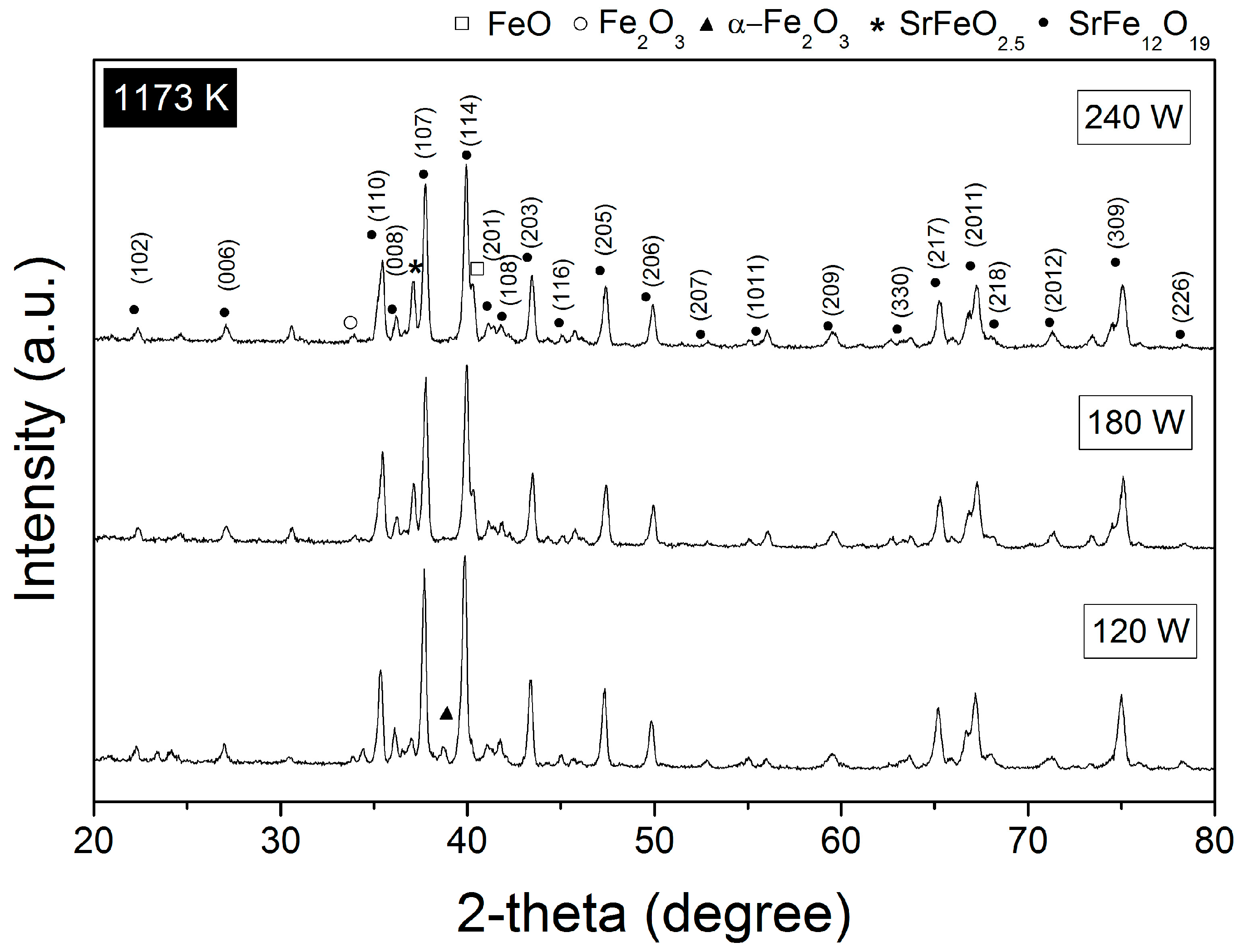
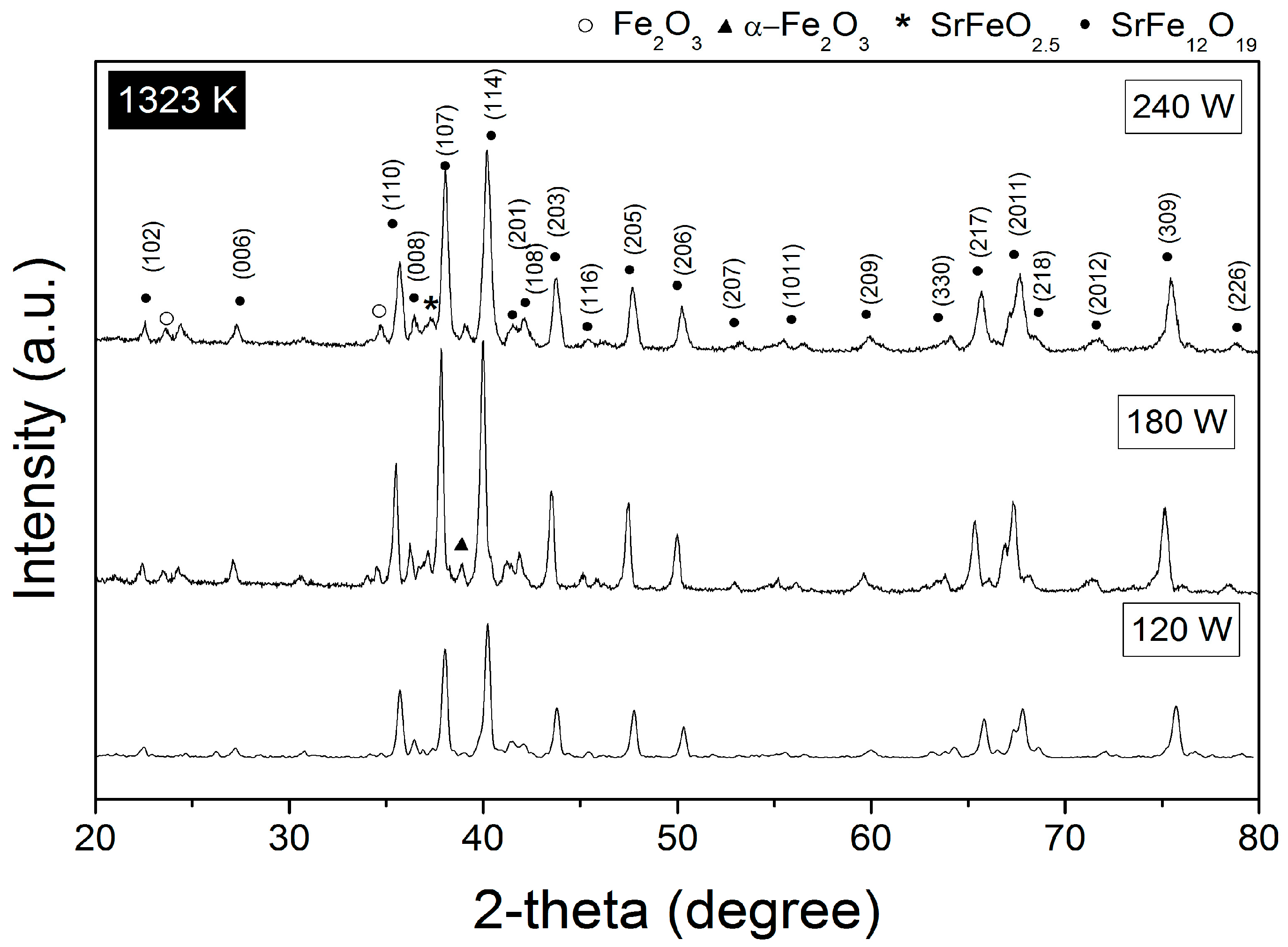
3.1. Crystal Structure
3.2. Morphology Analysis
3.3. Magnetic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chong, S.V.; Williams, G.V.M.; Kennedy, J.; Fang, F.; Tallon, J.L.; Kadowaki, K. Large low-temperature magnetoresistance in SrFe2As2 single crystals. EPL Europhys. Lett. 2013, 104, 17002. [Google Scholar] [CrossRef]
- Töpfer, J.; Seifert, D.; Le Breton, J.-M.; Langenhorts, F.; Chlan, V.; Kouril, K.; Stepánková, H. Hexagonal ferrites of X-, W-, and M-type in the system Sr-Fe-O: A comparative study. J. Solid State Chem. 2015, 226, 133–141. [Google Scholar] [CrossRef]
- Fang, C.M.; Kools, F.; Metselar, R.; de With, G.; de Groot, R.A. Magnetic and electronic propieties of strontium hexaferrite SrFe12O19 from first-principles calculations. J. Phys. Condens. Matter 2003, 15, 6229–6237. [Google Scholar] [CrossRef]
- Zi, Z.F.; Sun, Y.P.; Zhu, X.B.; Yang, Z.R.; Dai, J.M.; Song, W.H. Structural and magnetic properties of SrFe12O19 hexaferrite synthetized by a modified chemical co-precipitation method. J. Magn. Magn. Mater. 2008, 320, 2746–2751. [Google Scholar] [CrossRef]
- Xia, A.; Zuo, C.; Chen, L.; Jin, C.; Lv, Y. Hexagonal SrFe12O19 ferrites: Hydrothermal synthesis and their properties. J. Magn. Magn. Mater. 2013, 332, 186–191. [Google Scholar] [CrossRef]
- Kostishyn, V.G.; Panina, L.V.; Kozhitov, L.V.; Timofeev, A.V.; Kovalev, A.N. Synthesis and multiferroic properties of M-type SrFe12O19 hexaferrite ceramics. J. Alloys Compd. 2015, 645, 297–300. [Google Scholar] [CrossRef]
- Bolarín-Miró, A.M.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.A.; Díaz-De la Torre, S.; Valenzuela, R. Synthesis of M-type SrFe12O19 by mechanosynthesis assisted by spark plasma sintering. J. Alloys Compd. 2015, 643, S226–S230. [Google Scholar] [CrossRef]
- Ganjali, M.; Ganjali, M.; Eskandari, A.; Aminzare, M. Effect of Heat Treatment on Structural and Magnetic Properties of Nanocrystalline SrFe12O19 Hexaferrite Synthesized by Co-Precipitation Method. J. Adv. Mater. Process. 2013, 4, 41–48. [Google Scholar] [CrossRef]
- Singhal, S.; Namgyal, T.; Singh, J.; Chandra, K.; Bansal, S. A comparative study on the magnetic properties of MFe12O19 and MAlFe11O19 (M = Sr, Ba and Pb) hexafererites with different morphologies. Ceram. Int. 2011, 37, 1833–1837. [Google Scholar] [CrossRef]
- Rashid, A.U.; Southern, P.; Darr, J.A.; Awan, S.; Manzoor, S. Strontium hexaferrite (SrFe12O19) based composites for hypertermia applications. J. Magn. Magn. Mater. 2013, 344, 134–139. [Google Scholar] [CrossRef]
- Choudhury, H.A.; Choudhary, A.; Sivakumar, M.; Moholkar, V.S. Mechanistic investigation of the sonochemical synthesis of zinc ferrite. Ultrason. Sonochem. 2013, 20, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.M.H.; Trinh, V.D.; Bui, D.H.; Phan, M.H.; Huynh, D.G. Sol-gel hydrothermal synthesis of strontium hexaferrite nanoparticles and relation between their crystal structure and high coercivity properties. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025015. [Google Scholar] [CrossRef]
- Tenorio Gonzalez, F.N.; Bolarín Miró, A.M.; Sánchez De Jesús, F.; Cortés Escobedo, C.A. Mechanism and microstructural evolution of polyol mediated synthesis of nanostructure M-type SrFe12O19. J. Magn. Magn. Mater. 2016, 407, 188–194. [Google Scholar] [CrossRef]
- Hessien, M.M.; Rashad, M.M.; El-Barawy, K. Controlling the composition properties of strontium hexaferrite synthetized by co-precipitation method. J. Magn. Magn. Mater. 2008, 320, 336–343. [Google Scholar] [CrossRef]
- Viet Nga, T.T.; Duong, N.P.; Loan, T.T.; Hien, T.D. Key step in the synthesis of ultrafine strontium ferrite powders (SrFe12O19) by sol-gel method. J. Alloys Compd. 2014, 610, 630–634. [Google Scholar] [CrossRef]
- Kandjani, A.E.; Tabriz, M.F.; Pourabbas, B. Sonochemical synthesis of ZnO nanoparticles: The effect of temperature and sonocation power. Mater. Res. Bull. 2008, 43, 645–654. [Google Scholar] [CrossRef]
- Ohayon, E.; Gedanken, A. The application of ultrasound radiation to the synthesis of nanocystalline metal oxide in a non-aqueous solvent. Ultrason. Sonochem. 2010, 17, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Palomino, R.L.; Bolarín Miró, A.M.; Tenorio, F.N.; Sánchez De Jesús, F.; Cortés Escobedo, C.A.; Ammar, S. Sonochemical assisted synthesis of SrFe12O19 nanoparticles. Ultrason. Sonochem. 2016, 29, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Kaviyarasu, K.; Geetha, N.; Kanimozhi, K.; María Magdalane, C.; Sivaranjani, S.; Ayeshamariam, A.; Keneddy, J.; Maaza, M. In vitro citotoxicity effect and antibacterial performance of human lung epothelial cells A549 activity of Zinc oxide doped TiO2 nanocrystals: Investigation of bio-medical application by chemical method. Mater. Sci. Eng. C 2017, 74, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kaviyarasu, K.; Magdalane, C.M.; Kanimozhi, K.; Kennedy, J.; Siddhardha, B.; Reddy, E.S.; Rotte, N.K.; Sharma, C.S.; Thema, F.T.; Letsholathebe, D.; et al. Elucidation of photocatalysis, photoluminicense and antibacterial studies of ZnO thin films by spin coating method. J. Photochem. Photobiol. B 2017, 173, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Hassanjani-Roshan, A.; Vaezi, M.R.; Shokuhfar, A.; Rajabali, Z. Synthesis of iron oxide nanoparticles via sonochemical method and their characterization. Particuology 2011, 9, 95–99. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A Friendly Program for Material Analysis Using Diffraction. IUCr Newsl. CPD 1999, 21, 14–15. [Google Scholar]
- COD, Crystallography Open Database. Available online: http://www.crystallography.net/cod/search.html (accessed on 17 January 2018).
- Israr, F.; Kim, D.K.; Kim, Y.; Oh, S.J.; Ng, K.C.; Chun, W. Synthesis of porous Cu-BTC with ultrasonic treatment: Effects of ultrasonic power and solvent condition. Ultrason. Sonochem. 2016, 29, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.V.; Honold, E.; Skau, E.L. Topochemical mechanims involved in the preparation and deacetylation of partially acetylated cottons. Text. Res. J. 1958, 28, 861–873. [Google Scholar] [CrossRef]
- Nemury, A.; Weiss, M.; Gainetdinov, I.; Boldyrev, V.; Schöllhorn, R. Room temperature electrochemical redox reactions of the defect Perovskite SrFeO2.5−x. Chem. Mater. 1998, 10, 2403–2411. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Guo, H.; Wang, J.; He, Y.; Jiao, Z.; Wu, M. Iron oxide (III) nanoparticles fabricated by electron beam irradiation method. Mater. Sci. 2007, 25, 1143–1148. [Google Scholar]
- Hazen, R.M.; Jeanloz, R. Wustite (Fe1−xO): A review of its defect structure and physical properties. Rev. Geophys. Spa Phys. 1984, 22, 37–46. [Google Scholar] [CrossRef]
- Bennett, L.H.; Torre, E.D. Analysis of wasp-waist hysteresis loops. J. Appl. Phys. 2005, 97, 10E502. [Google Scholar] [CrossRef]
- Eckert, O. Ferrites with constricted loops and thermal magnetic treatment. Proc. IEEE 1956, 104, 428–432. [Google Scholar] [CrossRef]
- Herzer, G. Grain size dependence of coercivity and permeability in nanocrystalline ferromagnets. IEEE Trans. Magn. 1990, 26, 1397–1402. [Google Scholar] [CrossRef]
- Xu, Y.F.; Ma, Y.Q.; Xu, S.T.; Zan, F.L.; Zheng, G.H.; Dai, Z.X. Effects of vacancy and exchange-coupling between grains on magnetic properties of SrFe12O19 and α-Fe2O3 composites. Mater. Res. Bull. 2014, 57, 13–18. [Google Scholar] [CrossRef]
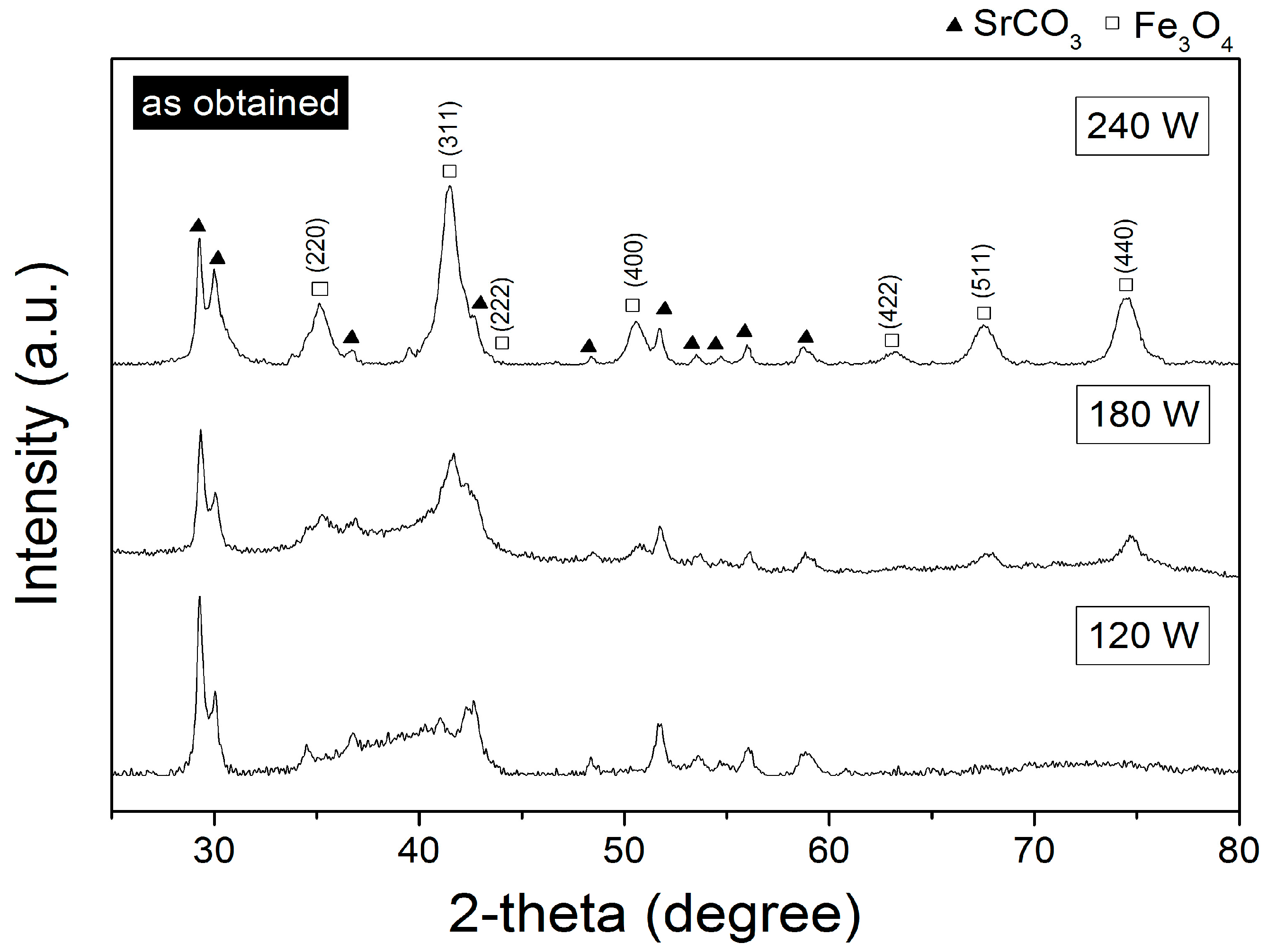


| Power | Phases, Space Group | Crystallography Open Database (COD) ID | vol % | Crystallite Size (nm) | Microstrain (adim) | Index of Crystallinity (%) |
|---|---|---|---|---|---|---|
| 120 W | Fe3O4, Fd-3m | 9005816 | 85.96 ± 0.95 | 12.4 ± 1.4 | 0.030024 | 24.13 |
| SrCO3, Pmcn | 9008198 | 14.04 ± 0.0 | 318.6 ± 7.7 | 0.005322 | 92.54 | |
| 180 W | Fe3O4, Fd-3m | 9005816 | 86.95 ± 0.5 | 6.9 ± 1.8 | 0.002524 | 63.99 |
| SrCO3, Pmcn | 9008198 | 13.05 ± 0.0 | 211.8 ± 1.0 | 0.004980 | 85.08 | |
| 240 W | Fe3O4, Fd-3m | 9005816 | 88.97 ± 0.13 | 5.7 ± 0.8 | 0.000049 | 95.08 |
| SrCO3, Pmcn | 9008198 | 11.02 ± 0.0 | 115.3 ± 1.8 | 0.000499 | 65.50 |
| Power | Phases, Space Group | Crystallography Open Database (COD) ID | vol % | Crystallite Size (nm) | Microstrain (adim) |
|---|---|---|---|---|---|
| Annealed at 1073 K | |||||
| 120 W | SrFe12O19, P63/mmc | 1006000 | 90.34 ± 0.0 | 81.7 ± 2.2 | 0.000958 |
| α-Fe2O3, R-3c | 9015065 | 9.66 ± 0.64 | 7.1 ± 0.6 | 0.000304 | |
| 180 W | SrFe12O19, P63/mmc | 1006000 | 96.78 ± 1.1 | 61.09 ± 1.3 | 0.001088 |
| α-Fe2O3, R-3c | 9015065 | 1.24 ± 0.02 | 9.6 ± 1.2 | 0.006622 | |
| SrFeO2.5, Pm-3m | 1502549 | 1.98 ± 2.1 | 9.9 ± 0.1 | 0.000603 | |
| 240 W | SrFe12O19, P63/mmc | 1006000 | 95.58 ± 0.71 | 55.1 ± 1.7 | 0.001013 |
| α-Fe2O3, R-3c | 9015065 | 1.54 ± 0.41 | 5.4 ± 0.7 | 0.001219 | |
| SrFeO2.5, Pm-3m | 1502549 | 2.41 ± 0.0 | 4.9 ± 0.63 | 0.000501 | |
| Fe2O3, R-3c | 1011240 | 0.30 ± 0.6 | 9.7 ± 1.8 | 0.002212 | |
| FeO, Pm-3m | 2106936 | 0.17 ± 0.47 | 4.2 ± 0.15 | 0.001980 | |
| Annealed at 1173 K | |||||
| 120 W | SrFe12O19, P63/mmc | 1006000 | 97.24 ± 0.0 | 67.9 ± 1.7 | 0.000945 |
| α-Fe2O3, R-3c | 9015065 | 2.24 ± 0.04 | 9.4 ± 0.45 | 0.001205 | |
| SrFeO2.5, Pm-3m | 1502549 | 0.52 ± 0.19 | 11.7 ± 1.6 | 0.002191 | |
| 180 W | SrFe12O19, P63/mmc | 1006000 | 95.11 ± 0.5 | 75.5 ± 2.3 | 0.000839 |
| α-Fe2O3, R-3c | 9015065 | 1.22 ± 0.01 | 8.9 ± 0.49 | 0.002454 | |
| SrFeO2.5, Pm-3m | 1502549 | 3.67 ± 0.59 | 9.9 ± 0.26 | 0.001775 | |
| 240 W | SrFe12O19, P63/mmc | 1006000 | 92.75 ± 0.0 | 77.2 ± 4.1 | 0.000838 |
| α-Fe2O3, R-3c | 9015065 | 1.23 ± 0.04 | 3.3 ± 0.80 | 0.001243 | |
| SrFeO2.5, Pm-3m | 1502549 | 4.10 ± 0.30 | 8.7 ± 0.31 | 0.001365 | |
| Fe2O3, R-3c | 1011240 | 0.65 ± 0.5 | 4.7 ± 0.17 | 0.002753 | |
| FeO, Pm-3m | 2106936 | 0.25 ± 0.2 | 10 ± 0.9 | 0.023388 | |
| Annealed at 1323 K | |||||
| 120 W | SrFe12O19, P63/mmc | 1006000 | 98.8 ± 1.95 | 63.4 ± 1.58 | 0.000922 |
| α-Fe2O3, R-3c | 9015065 | 1.2 ± 0.02 | 9.9 ± 0.15 | 0.011751 | |
| 180 W | SrFe12O19, P63/mmc | 1006000 | 91.92 ± 1.47 | 73.3 ± 1.29 | 0.001342 |
| α-Fe2O3, R-3c | 9015065 | 7.60 ± 0.02 | 10.1 ± 0.26 | 0.010153 | |
| SrFeO2.5, Pm-3m | 1502549 | 0.48 ± 0.27 | 10.0 ± 0.11 | 0.000604 | |
| 240 W | SrFe12O19, P63/mmc | 1006000 | 96.62 ± 9.42 | 84.7 ± 1.35 | 0.001013 |
| Fe2O3, R-3c | 1011240 | 3.38 ± 0.34 | 9.8 ± 0.26 | 0.000409 | |
| SOP (W) | Annealing Temperature (K) | Ms (Am2/Kg) | Mr (Am2/Kg) | Hc (A/m) | Mr/Ms (adim) |
|---|---|---|---|---|---|
| 120 | 1073 | 46.13 | 24.65 | 3.94 × 105 | 0.53 |
| 1173 | 52.02 | 28.01 | 3.35 × 105 | 0.53 | |
| 1323 | 67.15 | 17.03 | 4.69 × 104 | 0.25 | |
| 180 | 1073 | 47.71 | 15.23 | 2.31 × 104 | 0.31 |
| 1173 | 45.60 | 24.72 | 3.03 × 105 | 0.54 | |
| 1323 | 51.71 | 23.93 | 1.36 × 105 | 0.46 | |
| 240 | 1073 | 44.72 | 22.62 | 3.91 × 105 | 0.50 |
| 1173 | 45.02 | 23.02 | 2.49 × 105 | 0.51 | |
| 1323 | 52.01 | 24.24 | 1.47 × 105 | 0.46 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino Resendiz, R.L.; Sánchez De Jesús, F.; Cortés Escobedo, C.A.; Hernández Cruz, L.E.; Bolarín Miró, A.M. Effect of Sonication Output Power on the Crystal Structure and Magnetism of SrFe12O19 Nanoparticles. Crystals 2018, 8, 45. https://doi.org/10.3390/cryst8010045
Palomino Resendiz RL, Sánchez De Jesús F, Cortés Escobedo CA, Hernández Cruz LE, Bolarín Miró AM. Effect of Sonication Output Power on the Crystal Structure and Magnetism of SrFe12O19 Nanoparticles. Crystals. 2018; 8(1):45. https://doi.org/10.3390/cryst8010045
Chicago/Turabian StylePalomino Resendiz, Roberto L., Félix Sánchez De Jesús, Claudia A. Cortés Escobedo, Leticia E. Hernández Cruz, and Ana M. Bolarín Miró. 2018. "Effect of Sonication Output Power on the Crystal Structure and Magnetism of SrFe12O19 Nanoparticles" Crystals 8, no. 1: 45. https://doi.org/10.3390/cryst8010045





