In Situ Random Microseeding and Streak Seeding Used for Growth of Crystals of Cold-Adapted β-d-Galactosidases: Crystal Structure of βDG from Arthrobacter sp. 32cB
Abstract
:1. Introduction
2. Materials and Methods
2.1. ArthβDG Production
2.2. Purification of ArthβDG
2.3. ParβDG and ArthβDG Crystallization
2.3.1. Streak Seeding
2.3.2. Seed Stock Preparation
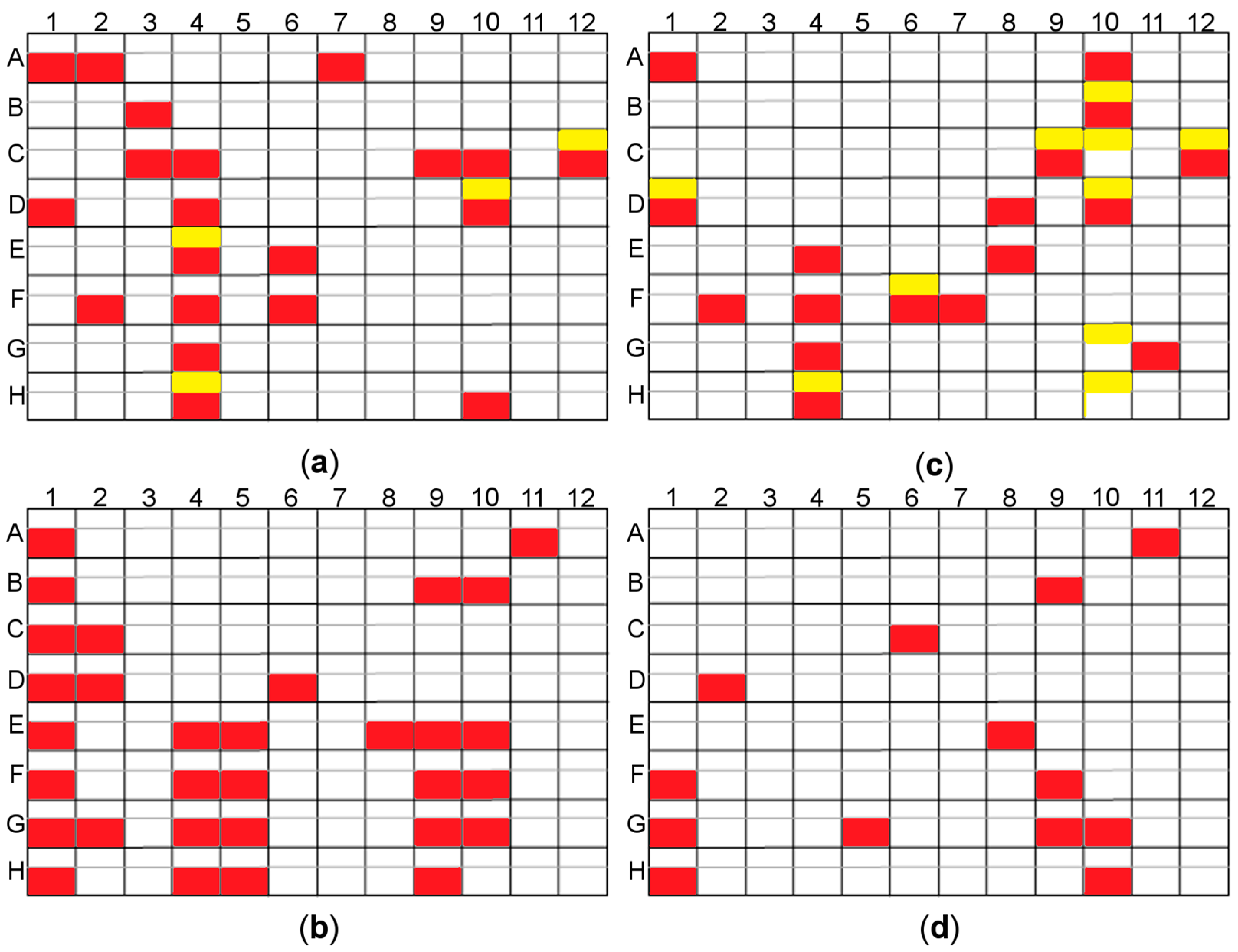
2.3.3. Random Microseed Matrix Screening Crystallization
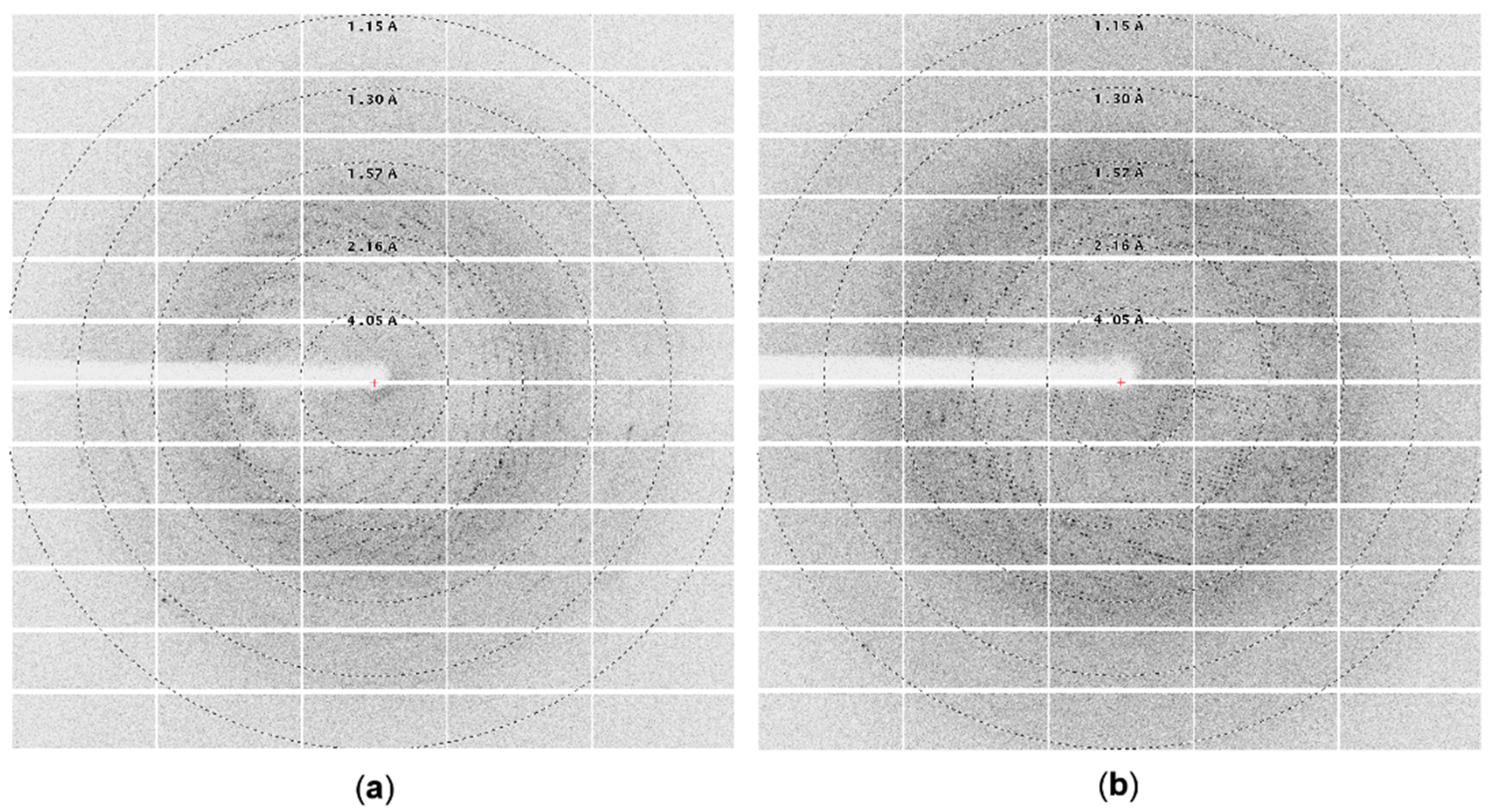
2.4. Data Collection and Processing
2.5. Structure Solution and Refinement
3. Results and Discussion
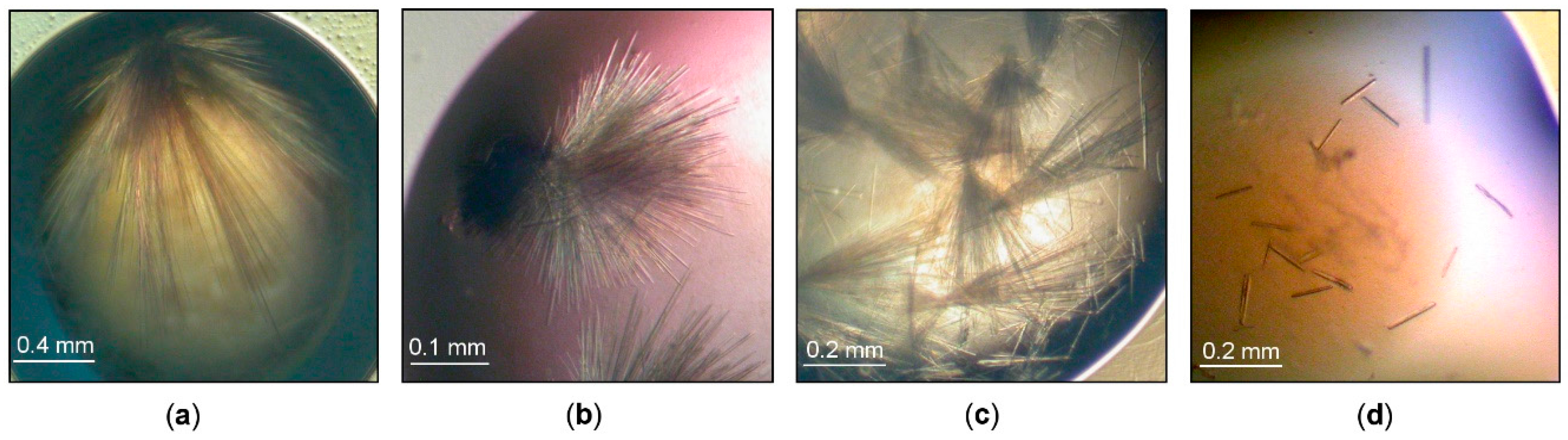
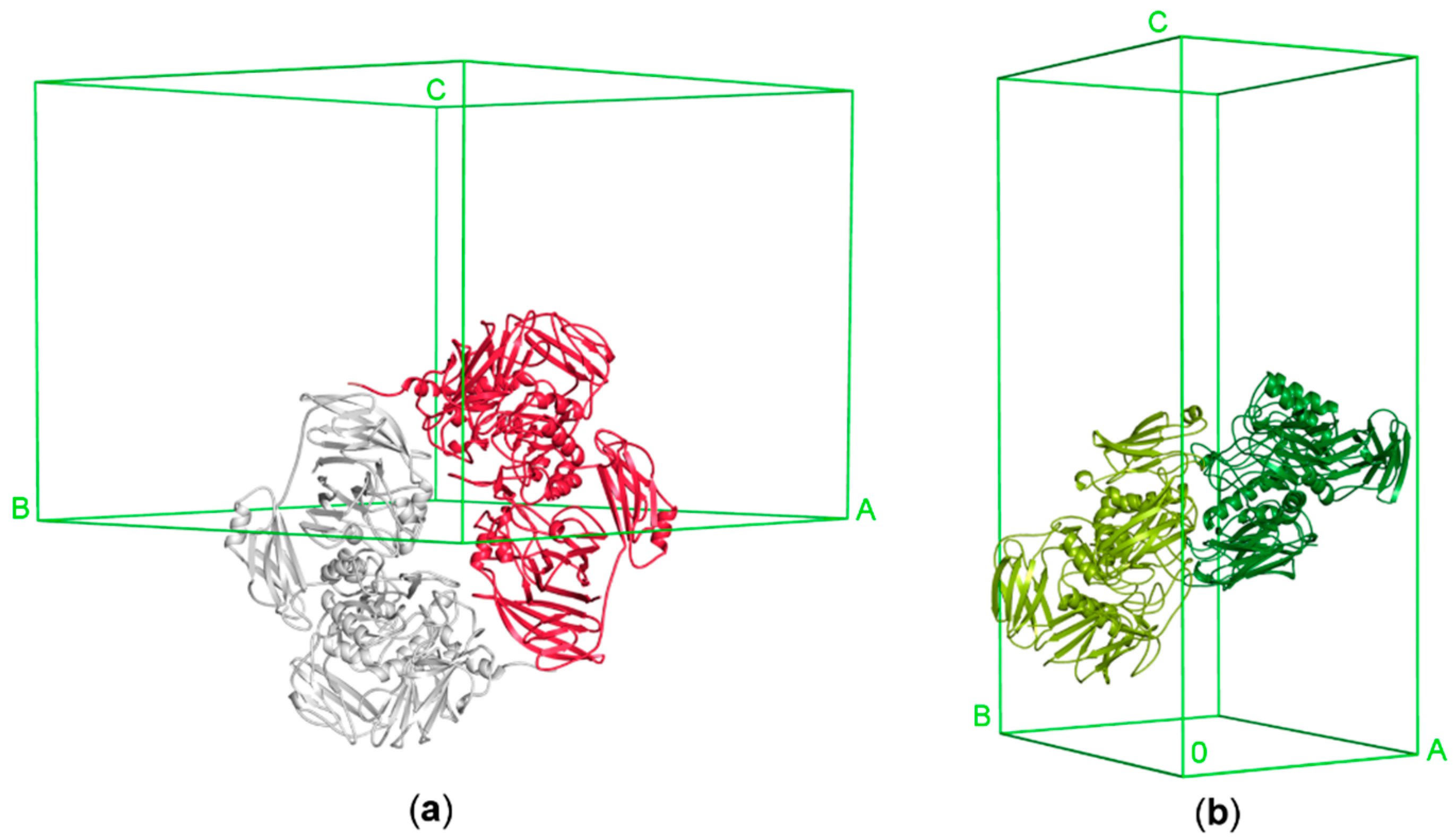
3.1. Crystallization of Cold-Adapted βDGs
3.1.1. Crystallization of Cold-Adapted ParβDG
3.1.2. Crystallization of Cold-Adapted ArthβDG
3.2. Structure of ArthβDG
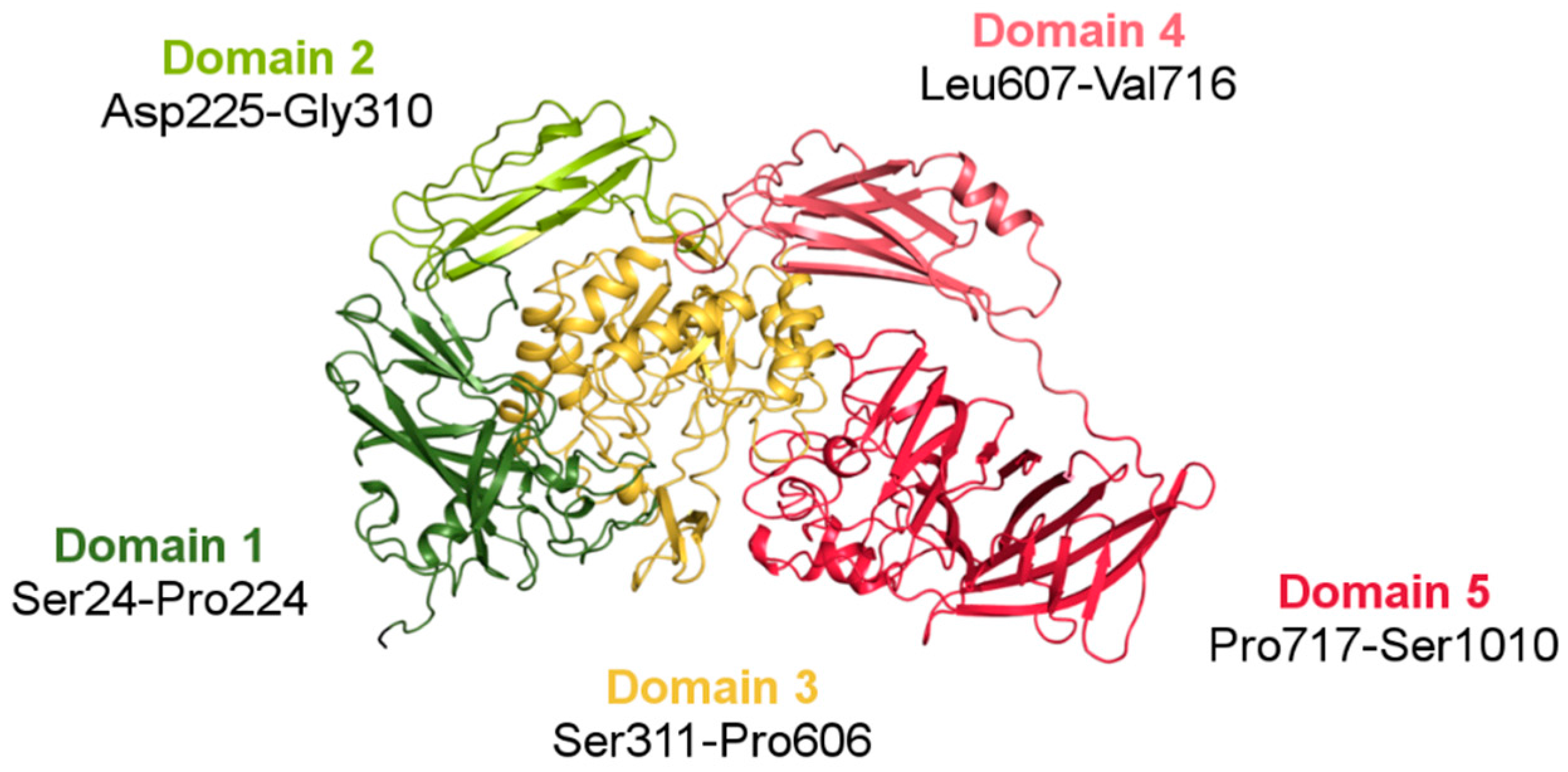
3.2.1. Overall Fold
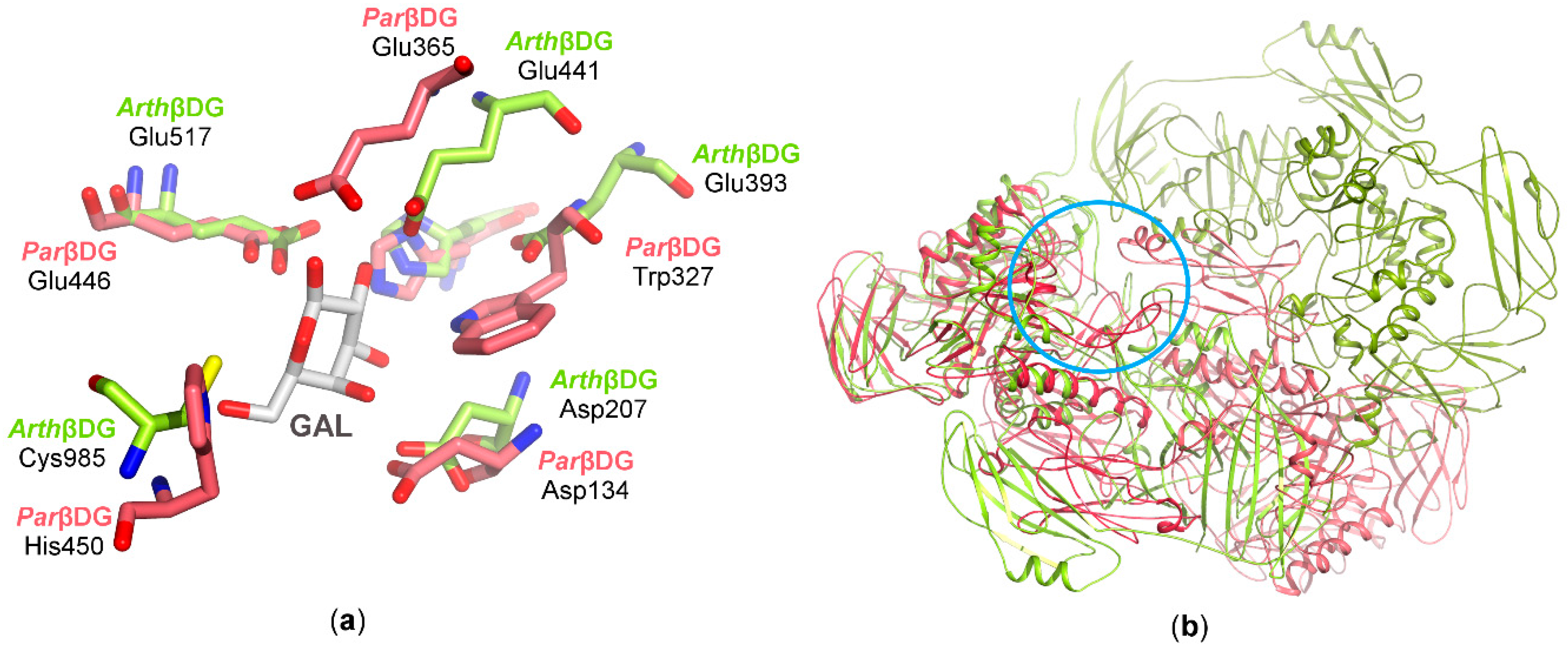
3.2.2. Architecture of the Functional Dimer
3.2.3. Catalytic Center
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pritzwald-Stegmann, B.F. Lactose and some of its derivatives. Int. J. Dairy Technol. 1986, 39, 91–97. [Google Scholar] [CrossRef]
- Khan, M.; Husain, Q.; Bushra, R. Immobilization of β-galactosidase on surface modified cobalt/multiwalled carbon nanotube nanocomposite improves enzyme stability and resistance to inhibitor. Int. J. Biol. Macromol. 2017, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Encapsulation of lactase in Ca(II)-alginate beads: Effect of stabilizers and drying methods. Food Res. Int. 2017, 1000, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Borghini, R.; Donato, G.; Alvaro, D.; Picarelli, A. New insights in IBS-like disorders: Pandora’s box has been opened; a review. Gastroenterol. Hepatol. Bed Bench 2017, 10, 79–89. [Google Scholar] [PubMed]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile organic compounds in feces associate with response to dietary intervention in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2017, S1542–S3565, 31201–31216. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.; et al. A diet low in fodmaps reduces symptoms in patients with irritable bowel syndrome and a probiotic restores Bifidobacterium species: A randomized controlled trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Cozma-Petrut, A.; Loghin, F.; Miere, D.; Dumitraşcu, D.L. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J. Gastroenterol. 2017, 23, 3771–3783. [Google Scholar] [CrossRef] [PubMed]
- Yuce, O.; Kalayci, A.G.; Comba, A.; Eren, E.; Caltepe, G. Lactose and fructose intolerance in Turkish children with chronic abdominal pain. Indian Pediatr. 2016, 53, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, K.; Umławska, W.; Iwańczak, B. Prevalence of lactose malabsorption and lactose intolerance in pediatric patients with selected gastrointestinal diseases. Adv. Clin. Exp. Med. 2015, 24, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka-Wos, A.; Cieslinski, H.; Wanarska, M.; Kozlowska-Tylingo, K.; Hildebrandt, P.; Kur, J. A novel cold-active β-d-galactosidase from the Paracoccus sp. 32d-gene cloning, purification and characterization. Microb. Cell Fact. 2011, 10, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Bialkowska, A.M.; Cieslinski, H.; Nowakowska, K.M.; Kur, J.; Turkiewicz, M. A new beta-galactosidase with a low temperature optimum isolated from the Antarctic Arthrobacter sp. 20B: Gene cloning, purification and characterization. Arch. Microbiol. 2009, 191, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Charlto, T.; Ertan, H.; Mohd Omar, S.; Siddiqui, K.S.; Williams, T.J. Biotechnological uses of enzymes from psychrophiles. Microb. Biotechnol. 2011, 4, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Harju, M. Milk sugars and minerals as ingredients. Int. J. Dairy Technol. 2001, 54, 61–63. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S. Biological functions of oligosaccharides in human milk. Acta Paediatr. 1993, 82, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Boehm, G.; Fanaro, S.; Jelinek, J.; Stahl, B.; Marini, A. Prebiotic concept for infant nutrition. Acta Paediatr. 2003, 92, 64–67. [Google Scholar] [CrossRef]
- Chierici, R.; Fanaro, S.; Saccomandi, D.; Vigi, V. Advances in the modulation of the microbial ecology of the gut in early infancy. Acta Paediatr. 2003, 92, 56–63. [Google Scholar] [CrossRef]
- Bujacz, A.; Jędrzejczak-Krzepkowska, M.; Bielecki, S.; Redzynia, I.; Bujacz, G. Crystal structures of the apo form of β-fructofuranosidase from Bifidobacterium longum and its complex with fructose. FEBS J. 2011, 278, 1728–1744. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Endo, A.; Scalabrin, D. Early gut colonization with Lactobacilli and Staphylococcus in infants: The hygiene hypothesis extended. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.L.; Wilbey, R.A.; Grandison, A.S.; Roseiro, L.B. Milk oligosaccharides: A review. Diary Technol. 2015, 68, 305–321. [Google Scholar] [CrossRef]
- Lee, L.Y.; Bharani, R.; Biswas, A.; Lee, J.; Tran, L.-A.; Pecquet, S.; Steenhout, P. Normal growth of infants receiving an infant formula containing Lactobacillus reuteri, galacto-oligosaccharides, and fructo-oligosaccharide: A randomized controlled trial. Matern. Health Neonatol. Perinatol. 2015, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Parakash, S. A comprehensive review on in vitro digestion of infant formula. Food Res. Int. 2015, 76, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial effects of human milk oligosaccharides on gut microbiota. Benef. Microbes 2014, 5, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Orrhage, K.; Nord, C.E. Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr. 1999, 88, 47–57. [Google Scholar] [CrossRef]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Donovan, S.M. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- McVeagh, P.; Miller., J.B. Human milk oligosaccharides: Only the breast. Acta Paediatr. 1997, 33, 281–286. [Google Scholar] [CrossRef]
- Wallace, T.C.; Marzorati, M.; Spence, L.; Weaver, C.M.; Williamson, P.S. New frontiers in fibers: Innovative and emerging research on the gut microbiome and bone health. J. Am. Coll. Nutr. 2017, 36, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Schoterman, M.H.; Nakatsu, C.H.; McCabe, L.D.; Wastney, M.E.; van den Heuvel, E.G.; Weaver, C.M. Galacto-oligosaccharides increase calcium absorption and gut Bifidobacteria in young girls: A double-blind cross-over trial. Br. J. Nutr. 2013, 110, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Martin, B.R.; Nakatsu, C.H.; Armstrong, A.P.; Clavijo, A.; McCabe, L.D.; McCabe, G.P.; Duignan, S.; Schoterman, M.H.; van den Heuvel, E.G. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Davoodi-Semiromi, Y.; Colee, J.C.; Culpepper, T.; Dahl, W.J.; Mai, V.; Christman, M.C.; Langkamp-Henken, B. Galactooligosaccharide supplementation reduces stress-induced gastrointestinal dysfunction and days of cold or flu: A randomized, double-blind, controlled trial in healthy university students. Am. J. Clin. Nutr. 2011, 93, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Swennen, K.; Courtin, C.M.; Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev.. Food Sci. Nutr. 2006, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Juers, D.H.; Heightman, T.D.; Vasella, A.; McCarter, J.D.; Mackenzie, L.; Withers, S.G.; Matthews, B.W. A structural view of the action of Escherichia coli (lacZ) β-galactosidase. Biochemistry 2001, 40, 14781–14794. [Google Scholar] [CrossRef] [PubMed]
- Skalova, T.; Dohnalek, J.; Spiwok, V.; Lipovova, P.; Vondrackova, E.; Petrokova, H.; Duskova, J.; Strnad, H.; Kralova, B.; Hasek, J. Cold-active beta-galactosidase from Arthrobacter sp. C2-2 forms compact 660 kDa hexamers: Crystal structure at 1.9 Å resolution. J. Mol. Biol. 2005, 353, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Faning, S.; Leahy, M.; Sheehan, M. Nucleotide and deduced amino acid sequences of Rhizobium meliloti 102F34 lacZ gene: Comparison with prokaryotic beta-galactosidases and human beta-glucuronidase. Gene 1994, 141, 91–96. [Google Scholar] [CrossRef]
- Burchhardt, G.; Bahl, H. Cloning and analysis of the beta-galactosidase-encoding gene from Clostridium thermosulfurogenes EM1. Gene 1991, 106, 13–19. [Google Scholar] [CrossRef]
- Rutkiewicz-Krotewicz, M.; Pietrzyk-Brzezinska, A.J.; Sekula, B.; Cieslinski, H.; Wierzbicka-Wos, A.; Kur, J.; Bujacz, A. Structural studies of a cold-adapted dimeric β-d-galactosidase from Paracoccus sp. 32d. Acta Crystallogr. D 2016, 72, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Szukalska, A.; Wanarska, M.; Popinigis, A.T.; Kur, J. A novel cold-active β-d-galactosidase with transglycosylation activity from the Antarctic Arthrobacter sp. 32cB-gene cloning, purification and characterization. Process. Biochem. 2014, 49, 2122–2133. [Google Scholar] [CrossRef]
- Shaw Stewart, P.D.; Kolek, S.A.; Briggs, R.A.; Chayen, N.E.; Baldock, P.F.M. Random microseeding: A theoretical and practical exploration of seed stability and seeding techniques for successful protein crystallization. Cryst. Growth Des. 2011, 11, 3432–3441. [Google Scholar] [CrossRef]
- Bujacz, G.; Wrzesniewska, B.; Bujacz, A. Cryoprotection properties of salts of organic acids: A case study for a tetragonal crystal of hew lysozyme. Acta Crystallogr. D 2010, 66, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Sparta, K.M.; Krug, M.; Heinemann, U.; Mueller, U.; Weiss, M.S. Xdsapp2.0. J. Appl. Crystallogr. 2016, 49, 1085–1092. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Cryst. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Vagin, A.A.; Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 1997, 53, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Isupov, M.N.; Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D 2001, 57, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerday, C. Psychrophily and catalysis. Biology 2013, 2, 719–741. [Google Scholar] [CrossRef] [PubMed]
- Bujacz, A.; Rutkiewicz-Krotewicz, M.; Nowakowska-Sapota, K.; Turkiewicz, M. Crystal structure and enzymatic properties of a broad substrate-specificity psychrophilic aminotransferase from the Antarcticsoil bacterium Psychrobacter sp. B6. Acta Crystallogr. D 2015, 71, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Krissnel, E.; Henrick, K. Interference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Talens-Perales, D.; Polaina, J.; Marín-Navarro, J. Enzyme Engineering for Oligosaccharide Biosynthesis. In Frontier Discoveries and Innovations in Interdisciplinary Microbiology; Springer: New Delhi, India, 2016; Chapter 2; pp. 9–31. [Google Scholar]


| Source Organism | Arthrobacter sp. 32cB |
|---|---|
| DNA source | Genomic DNA |
| Forward primer | F232cBNco (NcoI restriction site underlined) TCTACCATGGCTGTCGAAACACCGTCCGCGCTGGCGGAT |
| Reverse primer | R32cBHind (HindIII restriction site underlined) TGACAAGCTTCAGCTGCGCACCTTCAGGGTCAGTATGAAG |
| Cloning vector | pBAD/Myc-His A (Invitrogen, Carlsbad, CA, USA) |
| Expression vector | pBAD-Bgal 32cB |
| Expression host | E. coli LMG194 (Invitrogen, Carlsbad, CA, USA) |
| Diffraction Source | BL 14.2 BESSY, Berlin, Germany |
|---|---|
| Wavelength (Å) | 0.918400 |
| Temperature (K) | 100 K |
| Detector | PILATUS 3S 2M |
| Crystal-detector distance (mm) | 344.48 |
| Rotation range per image (°) | 0.1 |
| Total rotation range (°) | 180 |
| Exposure time per image (s) | 0.3 |
| Space group | P3121 |
| a, b, c (Å) | 137.78, 137.78, 127.20 |
| α, β, γ (°) | 90, 90, 120 |
| Mosaicity (°) | 0.077 |
| Resolution range (Å) | 46.7–1.8 (1.9–1.8) |
| Total No. of reflections | 1305805 |
| No. of unique reflections | 129004 |
| Completeness (%) | 99.5 (97.9) |
| Redundancy | 9.98 (9.59) |
| I/σ(I) | 14.46 (1.5) |
| Rmeas (%) | 11.3 (136.1) |
| Overall B factor from Wilson plot (Å2) | 25.6 |
| PDB ID: 6ETZ | |
|---|---|
| Resolution range (Å) | 46.73–1.80 (1.84–1.80) |
| Completeness (%) | 99.5 |
| No. of reflections, working set | 126888 (8872) |
| No. of reflections, test set | 2100 (146) |
| Final Rcryst | 0.161 (0.303) |
| Final Rfree | 0.198 (0.314) |
| Cruickshank DPI | 0.0913 |
| No. of non-H atoms: | |
| Protein | 7727 |
| Ion | 1 |
| Ligand | 23 |
| Water | 1183 |
| Total | 8934 |
| R.m.s. deviations | |
| Bonds (Å) | 0.019 |
| Angles (°) | 1.874 |
| Ramachandran plot: | |
| Most favored (%) | 98 |
| Allowed (%) | 2 |
| Protein | Composition | Surface Area [Å2] | Buried Area [Å2] | ΔGint [kcal/mol] | ΔGdiss [kcal/mol] |
|---|---|---|---|---|---|
| ParβDG | dimer | 48 760 | 7 180 | −17.5 | 24.8 |
| ArthβDG | dimer | 68 700 | 6 150 | −28.9 | 6.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkiewicz-Krotewicz, M.; Pietrzyk-Brzezinska, A.J.; Wanarska, M.; Cieslinski, H.; Bujacz, A. In Situ Random Microseeding and Streak Seeding Used for Growth of Crystals of Cold-Adapted β-d-Galactosidases: Crystal Structure of βDG from Arthrobacter sp. 32cB. Crystals 2018, 8, 13. https://doi.org/10.3390/cryst8010013
Rutkiewicz-Krotewicz M, Pietrzyk-Brzezinska AJ, Wanarska M, Cieslinski H, Bujacz A. In Situ Random Microseeding and Streak Seeding Used for Growth of Crystals of Cold-Adapted β-d-Galactosidases: Crystal Structure of βDG from Arthrobacter sp. 32cB. Crystals. 2018; 8(1):13. https://doi.org/10.3390/cryst8010013
Chicago/Turabian StyleRutkiewicz-Krotewicz, Maria, Agnieszka J. Pietrzyk-Brzezinska, Marta Wanarska, Hubert Cieslinski, and Anna Bujacz. 2018. "In Situ Random Microseeding and Streak Seeding Used for Growth of Crystals of Cold-Adapted β-d-Galactosidases: Crystal Structure of βDG from Arthrobacter sp. 32cB" Crystals 8, no. 1: 13. https://doi.org/10.3390/cryst8010013




