Structural Aspects of Porphyrins for Functional Materials Applications
Abstract
:1. Introduction
2. Porphine, the Parent Compound
3. Peripheral Substituents
4. Metalation of the Macrocycle Core
5. Role of Covalent Inter-Molecular Bonding
6. Electronic Properties as Related to Structure
6.1. Properties at the Molecular Level
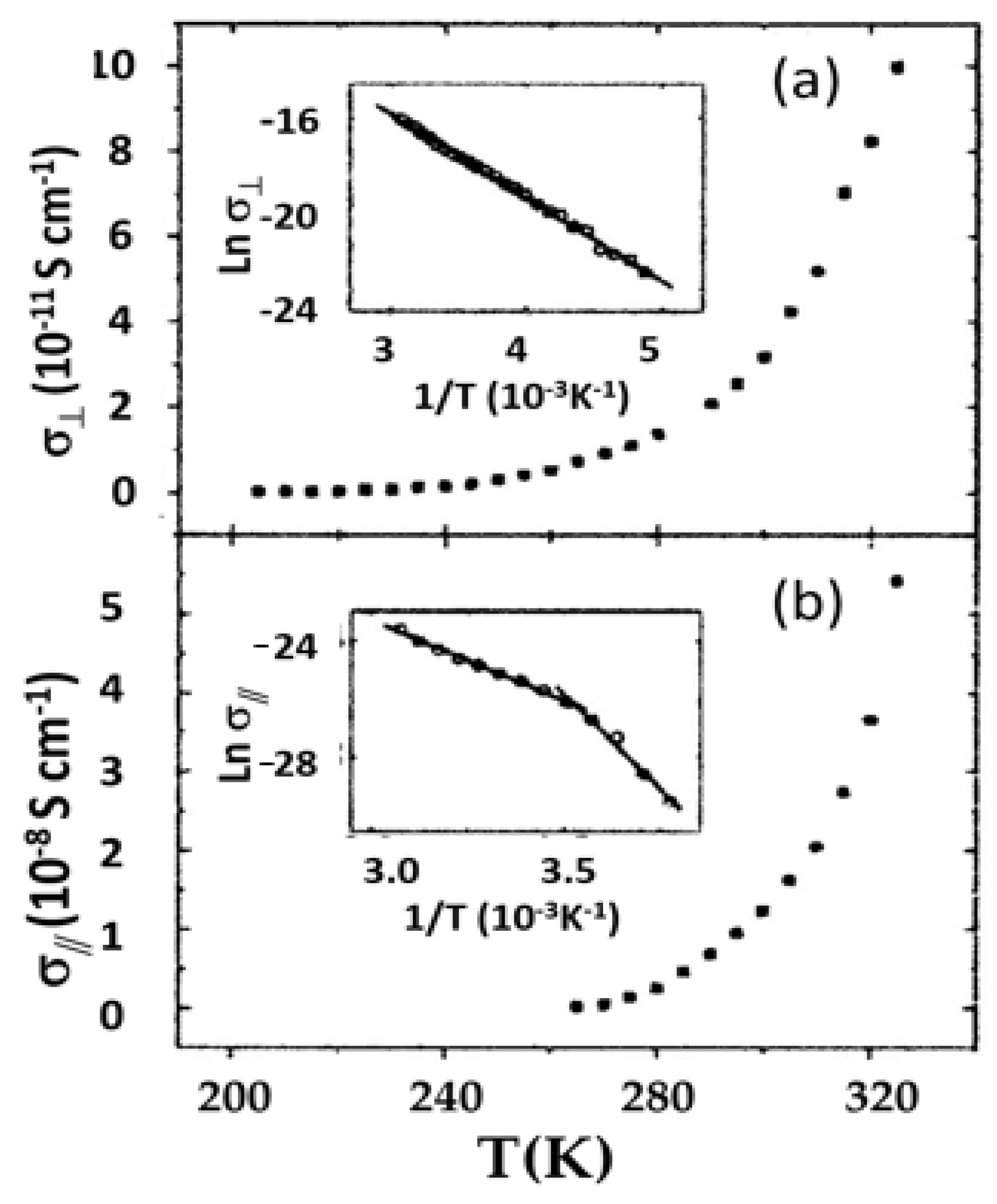
6.2 Conductivity in Single Crystals
6.3. Thin-Film Conductivities
6.4. Conductivity in Bulk-Polycrystalline Porphyrins
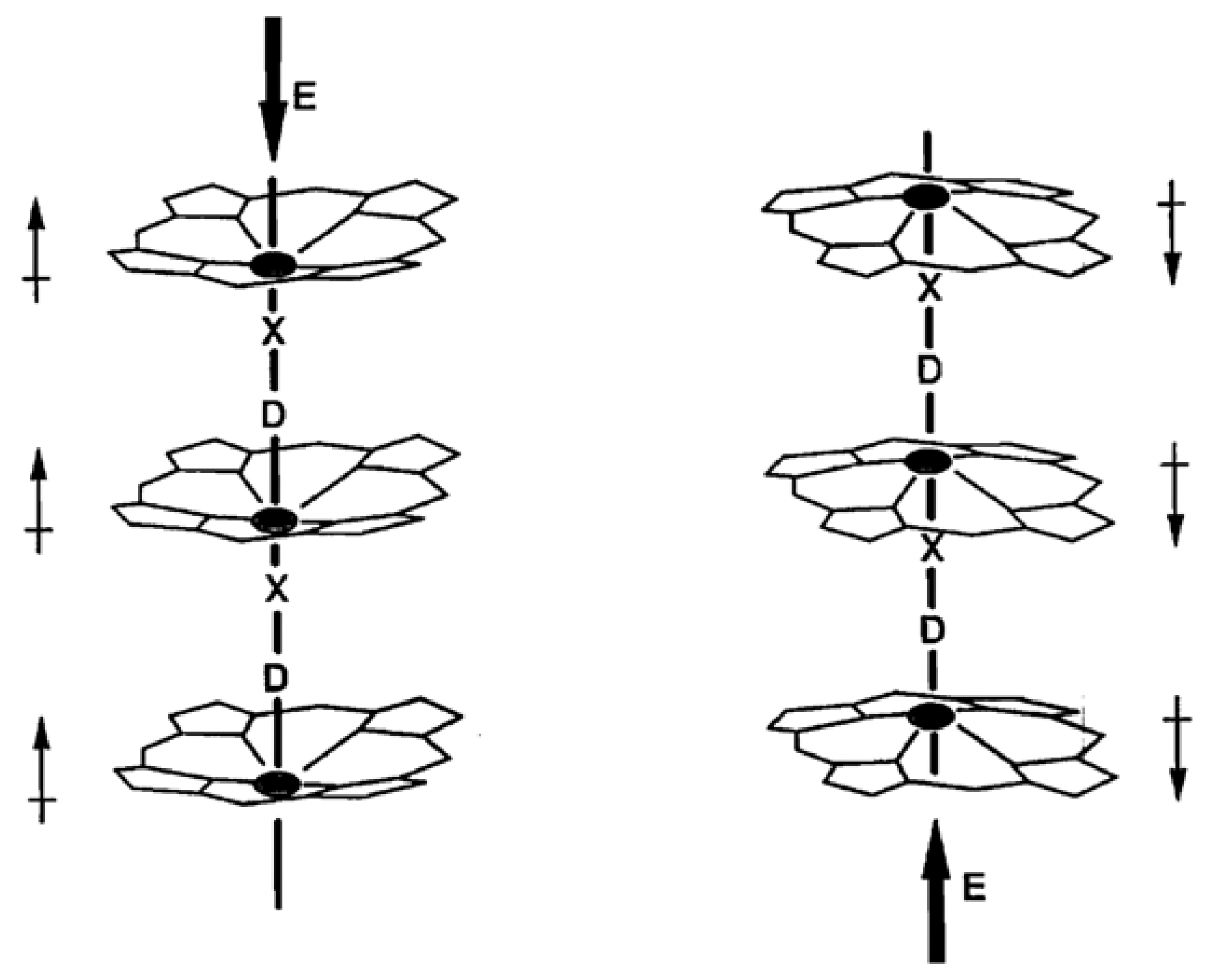
6.5. Ferroelectric Porphyrins
7. Structural Examples of Complex Multiporphyrins
7.1. Ethyne-Bridged Multiporphyrins
7.2. Cofacial Dimers
7.3. Directly-Linked Porphyrins.
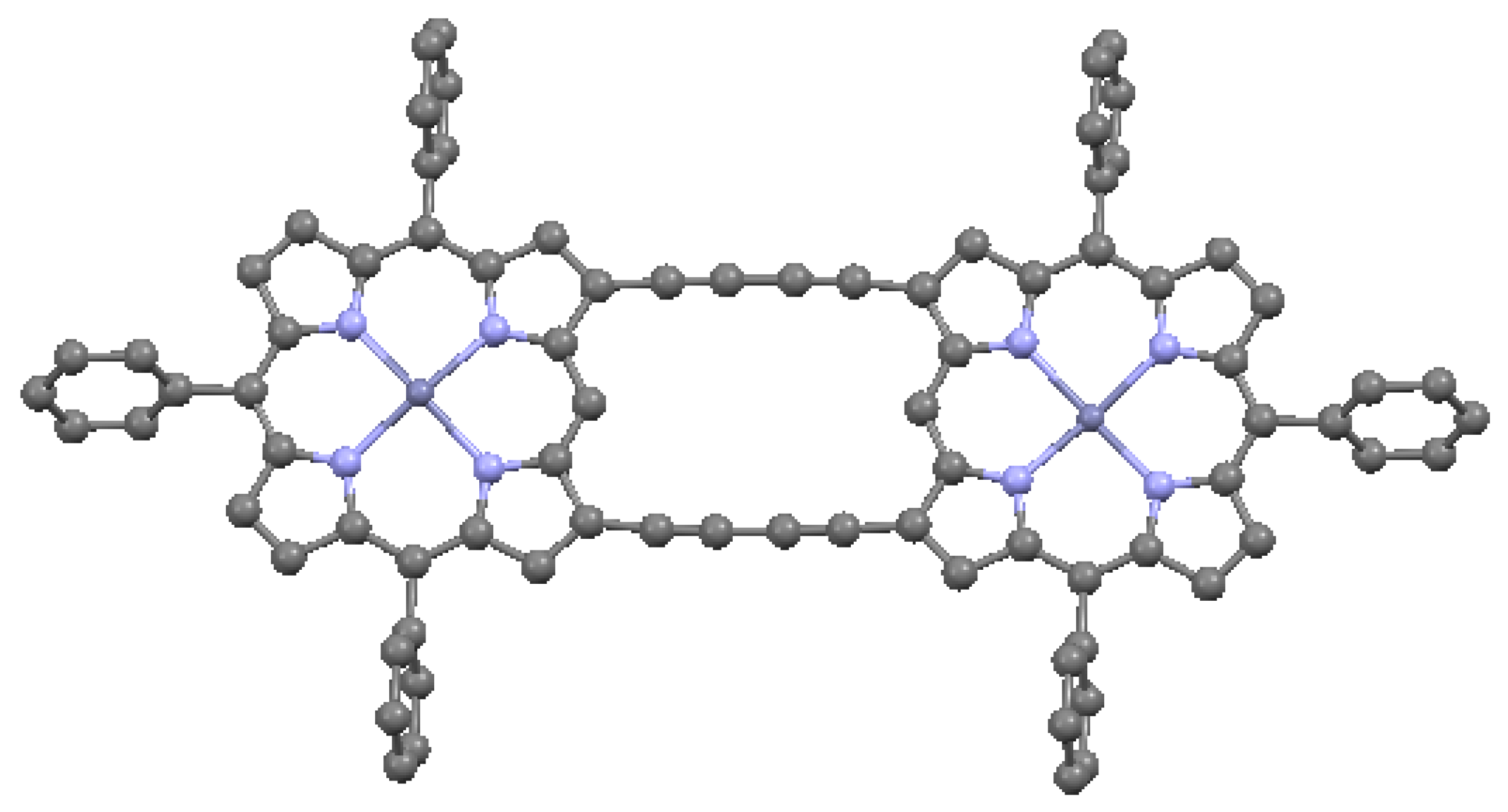
7.4. Multiply-Linked Structures
7.5. Self-Assembled Arrays
7.6. Metal-Bridged Arrays
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Porphyrin. Available online: https://en.wikipedia.org/w/index.php?title=Porphyrin&oldid=781762373 (accessed on 10 June 2017).
- Küster, W. Beitrage zur Kenntnis des Bilirubins und Hämins. Hoppe Seyler’s Z. Physiol. Chem. 1912, 82, 463–483. [Google Scholar] [CrossRef]
- Kadish, W.; Smith, K.M.; Guilard, R. (Eds.) Handbook of Porphyrin Science; World Scientific Publishing Co.: Singapore, 2010–2016; Volume 44. [Google Scholar]
- Moore, M.W. An historical introduction to porphyrin and chlorophyll synthesis. In Tetrapyrroles: Birth, Life, and Death; Warren, M., Smith, A., Eds.; Landes Bioscience and Springer Science+Business Media: Berlin, Germany, 2009; pp. 1–28. [Google Scholar]
- Suslick, K.S.; Rakow, N.A.; Kosal, M.E.; Chou, J.H. The materials chemistry of porphyrins and metalloporphyrins. J. Porphyr. Phthalocyanines 2000, 4, 407–413. [Google Scholar] [CrossRef]
- Cook, L.P.; Wong-Ng, W.; Brewer, G. Porphyrin-based chemistry for carbon capture and sequestration. In Advances in Materials Science for Environmental and Energy Technologies V; Ohji, T., Kanakhala, R., Matyáš, J., Marijooran, N., Pickrell, G., Wong-Ng, W., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 201–221. [Google Scholar]
- Cambridge Crystallographic Data Center. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 31 March 2017).
- International Union of Pure and Applied Chemistry. Available online: http://www.chem.qmul.ac.uk/iupac/tetrapyrrole/ (accessed on 31 March 2017).
- Saltsman, I.; Goldberg, I.; Balasz, Y.; Gross, Z. Porphine and pyrrole-substituted porphyrin from cyclocondensation of tripyrrane with mono-substituted pyrolles. Tetrahedron Lett. 2007, 48, 239–244. [Google Scholar] [CrossRef]
- Devillers, C.H.; Fleurat-Lessard, P.; Lucas, D. “Porphine,” the fully unsubstituted porphyrin: A comprehensive overview. In Handbook of Porphyrin Science; Kadish, W., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Singapore, 2014; Volume 37, pp. 75–231. [Google Scholar]
- Kaduk, J.A.; Wong-Ng, W.; Cook, L.P.; Chakraborty, B.; Lapidus, S.H.; Ribaud, L.; Brewer, G. Synchrotron X-ray investigation of α-chlorohemin, C34H32ClFeN4O4, an Fe-porphyrin. Solid State Sci. 2016, 53, 63–70. [Google Scholar] [CrossRef]
- Silvers, S.J.; Tulinsky, A. The crystal and molecular structure of triclinic tetraphenyl porphyrin. J. Am. Chem. Soc. 1967, 89, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Codding, P.W.; Tulinsky, A. Structure of tetra-n-propylporphine. Average structure for the free base macrocycle from three independent determinations. J. Am. Chem. Soc. 1972, 94, 4151–4157. [Google Scholar] [CrossRef] [PubMed]
- Lauher, J.W.; Ibers, J.A. Structure of Ocaethylporphyrin. A comparison with other free base porphyrins. J. Am. Chem. Soc. 1973, 95, 5148–5152. [Google Scholar] [CrossRef] [PubMed]
- Caughey, W.S.; Ibers, J.A. Crystal and molecular structure of the free base porphyrin, protoporphyrin IX dimethyl ester. J. Am. Chem. Soc. 1977, 99, 6639–6645. [Google Scholar] [CrossRef] [PubMed]
- Jentzen, W.; Trowska-Tyrk, I.; Scheidt, W.R.; Shetnutt, J.A. Planar solid-state and solution structures of (porphinato) nickel(II) as determined by X-ray diffraction and Raman spectroscopy. Inorg. Chem. 1996, 35, 3559–3567. [Google Scholar] [CrossRef]
- Devillers, C.H.; Dime, A.K.D.; Cattey, H.; Lucas, D. Crystallographic, spectroscopic and electrochemical characterization of pyridine adducts of magnesium(II) and zinc(II) porphine complexes. C. R. Chim. 2013, 16, 540–549. [Google Scholar] [CrossRef]
- Mavridis, A.; Tulinsky, A. Crystal and molecular structure of dimethoxy-porphinatogermanium (IV). Inorg. Chem. 1976, 15, 2723–2727. [Google Scholar] [CrossRef]
- Fleischer, E.B. The structure of porphyrins and metalloporphyrins. Acc. Chem. Res. 1970, 3, 105–112. [Google Scholar] [CrossRef]
- Arnold, D.P.; Goh, M.S.; Harper, S.R.; McMurtie, J.C. Oligoporphyrins with one- and two-atom covalent bridges: Synthesis, and reactivity, solid-state, solution and electronic structures, spectroscopy and applications. In Handbook of Porphyrin Science; Kadish, W., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Singapore, 2014; Volume 31, pp. 277–302. [Google Scholar]
- Esdaile, L.J.; Jensen, P.; McMurtie, J.C.; Arnold, D.P. Azoporphyrin: The porphrin analogue of azobenzene. Angew. Chem. Int. Ed. 2007, 46, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Johnson, A.W.; Winter, M. Bis-porphyrin derivatives. Part 1. Reaction of meso-hydroxymethyl-porphyinatometal derivatives with acids. J. Chem. Soc. Perkin Trans. 1977, 14, 1643–1647. [Google Scholar] [CrossRef]
- Senge, M.; Vicente, M.G.; Gerzevske, K.R.; Forsyth, T.P.; Smith, K.M. Models for the photosynthetic reaction center: Preparation, spectroscopy, and crystal and molecular structures of cofacial bisporphyrins linked by cis-1-2- and trans-1-2-ethene bridges and of 1,1-carbinol-bridged bisporphyrins. Inorg. Chem. 1994, 33, 5625–5638. [Google Scholar] [CrossRef]
- Crossley, M.J.; Burn, P.L. An approach to porphyrin-based molecular wires: Synthesis of a bis(porphyrin)tetraone and its conversion to a linearly conjugated tetrakisporphyrin system. J. Chem. Soc. Chem. Commun. 1991, 21, 1569–1571. [Google Scholar] [CrossRef]
- Perlovich, G.L. Thermodynamics of porphyrin sublimation. J. Porphyr. Phthalocyan. 2000, 4, 699–706. [Google Scholar] [CrossRef]
- Jurow, M.; Schuckman, A.; Batteas, J.D.; Drain, C.M. Porphyrins as molecular components of functional devices. Coord. Chem. Rev. 2010, 254, 2297–2310. [Google Scholar] [CrossRef] [PubMed]
- Aüwarter, W.; Écija, D.; Klappenberger, F.; Varth, J.V. Porphyrins at interfaces. Nat. Chem. 2015, 7, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Kubatkin, S.; Danilov, A.; Hjort, M.; Cornil, J.; Brédas, J.-L.; Stuhr-Hanson, N.; Hedegard, P.; Bjornholm, T. Single-electron transistor of a single organic molecule with access to several redox states. Nature 2003, 425, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Averin, D.V.; Likharev, K.K. Single electronics: A correlated transfer of single electrons and Cooper pairs in systems of small tunnel junctions. In Mesoscopic Phenomena in Solids; Altshuler, B.L., Lee, P.A., Webb, R.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 173–270. [Google Scholar]
- Wang, N.; Liu, H.; Zhan, J.; Cui, Y.; Xu, Z.; Ye, Y.; Kiguchi, M.; Murakoshi, K. Theoretical investigation on the electron transport path through the porphyrin molecules and chemisorption of CO. J. Phys. Chem. C 2009, 113, 7416–7423. [Google Scholar] [CrossRef]
- Galloni, P.; Vecchi, A.; Coletti, A.; Gatto, E.; Floris, B.; Conte, V. Porphyrins as active components for electrochemical and photoelectrochemical devices. In Handbook of Porphyrin Science; Kadish, W., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Singapore, 2014; Volume 33, pp. 225–415. [Google Scholar]
- Reimers, J.R.; Lu, T.X.; Crossley, M.J.; Hush, N.S. Molecular electronic properties of fused rigid porphyrin-oligomer molecular wires. Nanotechnology 1996, 7, 424–429. [Google Scholar] [CrossRef]
- Aviram, A. A view of the future of molecular electronics. Mol. Cryst. Liq. Cryst. 1993, 234, 13–28. [Google Scholar] [CrossRef]
- Yoon, D.H.; Lee, S.B.; Yoo, K.-H.; Kim, J.; Lim, J.K.; Aratani, N.; Tsuda, A.; Osuka, A.; Kim, D. Electrical conduction through linear porphyrin arrays. J. Am. Chem. Soc. 2003, 125, 11062–11064. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, C.; Esdaile, L.J.; Anderson, H.L.; Martin, S.; Bethell, D.; Higgins, S.J.; Nichols, R.J. Comparison of the conductance of three types of porphyrin-based molecular wires: β,meso,β-fused tapes, meso-butdiyne-linked and twisted meso-meso linked oligomers. Adv. Mater. 2012, 24, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin nanotubes by ionic self-assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Yang, C.-J.; Chen, W.-H.; Lin, K.-J. Towards the development of electrical conduction and lithium-ion transport in a tetragonal porphyrin wire. Angew. Chem. Int. Ed. 2003, 42, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lee, L.L.; Lin, K.J. Electrical and optical properties of porphyrin single crystals. J. Macromol. Sci. Part B Phys. 2008, 47, 955–966. [Google Scholar] [CrossRef]
- Schramm, C.J.; Stojakovic, D.R.; Hoffman, B.M.; Maarks, T.J. New low-dimensional molecular metals: Single-crystal electrical conductivity of nickel phthalocyanine iodide. Science 1978, 200, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Livshits, V.A.; Blyumenfel’d, L.A. Semiconductor properties of porphyrins. J. Struct. Chem. 1968, 8, 383–388. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nevin, W.A.; Mizunuma, S.; Awaji, H.; Yamaguchi, M. Ring-expanded porphyrins as an approach towards highly conductive molecular semiconductors. Chem. Phys. Lett. 1993, 205, 51–54. [Google Scholar] [CrossRef]
- Jones, R.; Tredgold, R.H.; Hoorfar, A. Electrical conductivity in Langmuir-Blodgett films of porphyrins: In-plane and thorough-the-film studies. Thin Solid Films 1984, 113, 115–128. [Google Scholar] [CrossRef]
- Lui, C.-Y.; Pan, H.-L.; Fox, M.A.; Bard, A.J. High-density nanosecond charge trapping in thin films of the photoconductor ZnODEP. Science 1993, 261, 897–899. [Google Scholar]
- Wood, D.W.; Andersen, T.N.; Eyring, H. Electrical properties of some porphyrins under high pressure. J. Phys. Chem. 1966, 70, 360–366. [Google Scholar] [CrossRef]
- Collman, J.P.; McDevitt, J.T.; Yee, G.T.; Leidner, C.R.; McCullough, L.G.; Little, W.A.; Torrance, J.B. Conductive polymers derived from iron, ruthenium, and osmium metalloporphyrins: The shish-kebab approach. Proc. Natl. Acad. Sci. USA 1986, 83, 4581–4585. [Google Scholar] [CrossRef] [PubMed]
- Collman, J.P.; McDevitt, J.T.; Leidner, C.R.; Yee, G.T.; Torrance, J.B.; Little, W.A. Synthetic, electrochemical, optical and conductivity studies of coordination polymers of iron, ruthenium, and osmium octaethylporphyrin. J. Am. Chem. Soc. 1987, 109, 4606–4614. [Google Scholar] [CrossRef]
- Suslick, K.S.; Chen, C.-T. Polymeric metalloporphyrins for field responsive materials. Polym. Mater. Sci. Eng. 1990, 63, 272–278. [Google Scholar]
- Chen, C.-T.; Suslick, K.S. One-dimensional coordination polymers: Applications to materials science. Coord. Chem. Rev. 1993, 128, 293–322. [Google Scholar] [CrossRef]
- Aratani, N.; Osuka, A. Synthetic strategies toward multiporphyrinic architectures. In Handbook of Porphyrin Science; Kadish, W., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Singapore, 2010; Volume 1, pp. 1–132. [Google Scholar]
- Gao, W.-Y.; Chrzanowski, M.; Ma, S. Metal-metalloporphyrinframeworks: A resurging class of functional materials. Chem. Soc. Rev. 2014, 43, 5841–5866. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.J.; Akda, H.; Cowley, A.R.; Rogers, J.E.; Slagle, J.E.; Fleitz, P.A.; Drobizhev, M.; Rebane, A.; Anderson, H.L. Synthesis, crystal structure, and nonlinear optical behavior of β-unsubstituted meso-meso-E-vinylene-linked porphyrin dimers. Org. Lett. 2005, 7, 5365–5368. [Google Scholar] [CrossRef] [PubMed]
- Imahori, H.; Fukuzumi, S. Porphyrin- and fullerene-based molecular photovoltaic devices. Adv. Funct. Mater. 2004, 14, 525–536. [Google Scholar] [CrossRef]
- Hisaki, I.; Hiroto, S.; Kim, K.S.; Noh, S.B.; Kim, D.; Shinokubo, H.; Osuka, A. Synthesis of doubly β-to-β 1,3-butadiene-bridged diporphyrins: Enforced planar structures and large two-photon absorption cross sections. Angew. Chem. Int. Ed. 2007, 46, 5125–5128. [Google Scholar] [CrossRef] [PubMed]
- Stulz, E.; Scott, S.M.; Bond, A.D.; Teat, S.J.; Sanders, J.M. Selection and amplification of mixed-metal porphyrin cages from dynamic combinatorial libraries. Chem. Eur. J. 2003, 6039–6048. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-Y.; Tashiro, K.; Hirabayashi, Y.; Kinbara, K.; Saigo, K.; Aida, T.; Sakamoto, S.; Yamaguchi, K. Cyclic dimers of metalloporphyrins as tunable hosts for fullerenes: A remarkable effect of rhodium(III). Angew. Chem. Int. Ed. 2001, 40, 1858–1861. [Google Scholar] [CrossRef]
- Deng, Y.; Chang, C.J.; Nocera, D.G. Direct observation of “Pac-Man” effect from dibenzofuran-bridged cofacial bisporphyrins. J. Am. Chem. Soc. 2000, 122, 410–411. [Google Scholar] [CrossRef]
- Tokuji, S.; Yurino, T.; Aratani, N.; Shinokubo, H.; Osuka, A. Palladium-catalyzed dimerization of meso-bromoporphyrins: Highly regioselective meso-β coupling through unprecedented remote C-H bond cleavage. Chem. Eur. J. 2009, 15, 12208–12211. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jang, S.Y.; Yamaguchi, S.; Sankar, J.; Hiroto, S.; Aratani, N.; Shin, J.Y.; Easwaramoorthi, A.; Kim, K.S.; Kim, D.; et al. 2,5-thienylene-bridged triangular and linear porphyrin trimers. Angew. Chem. Int. Ed. 2008, 47, 6004–6007. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, A.; Nakamura, T.; Sakamoto, S.; Yamaguchi, K.; Osuka, A. A self-assembled porphyrin box from meso-meso-linked bis [5-p-pyridyl-15-(3,5-di-octyloxyphenyl)porphyrinato zinc(II)]. Angew. Chem. Int. Ed. 2002, 41, 2817–2821. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Katoh, T.; Shinokubo, H.; Osuka, A. Pt(II)- and Pt(IV)-bridged cofacial diporphyrins via carbon—transition metal σ-bonds. J. Am. Chem. Soc. 2008, 130, 14440–14441. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Yamazaki, T.; Nishimura, Y.; Yamazaki, I.; Osuka, A. Three-dimensionally arranged windmill and grid porphyrin arrays by AgI-promoted meso-meso block oligomers. Chem. Eur. J. 2000, 6, 3254–3271. [Google Scholar] [CrossRef]
- Hori, T.; Peng, X.; Aratani, N.; Takagi, A.; Matsumoto, T.; Kawai, T.; Yoon, Z.S.; Yoon, M.C.; Yang, J.; Kim, D.; et al. Synthesis of nanometer-scale porphyrin wheels of variable size. Chem. Eur. J. 2008, 14, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamaguchi, S.; Osuka, A.; Shinokubo, H. Platinum(II) and Platinum(IV) porphyrin pincer complexes: Synthesis, structures, and reactivity. Organometallics 2010, 29, 3997–4000. [Google Scholar] [CrossRef]
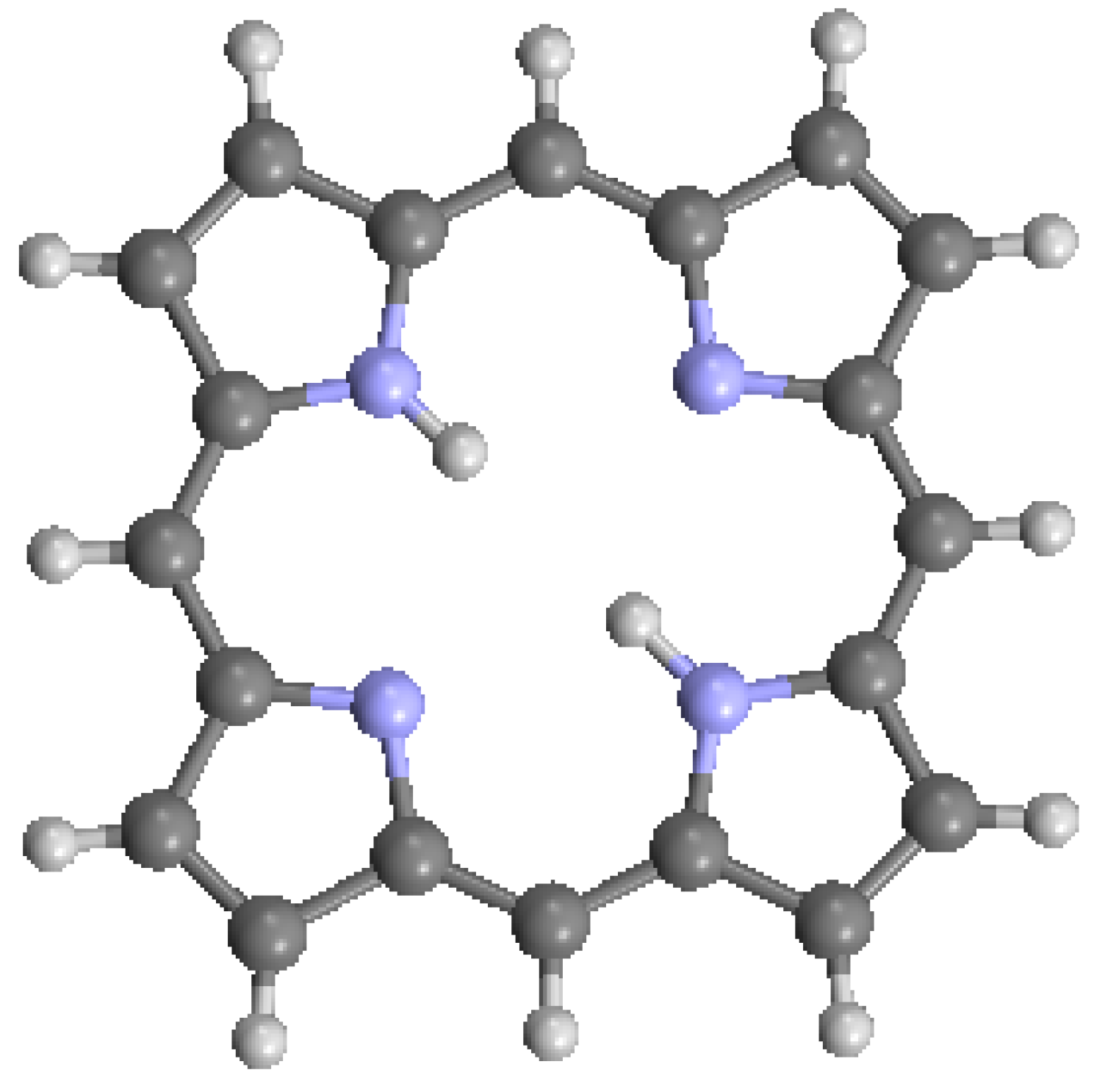
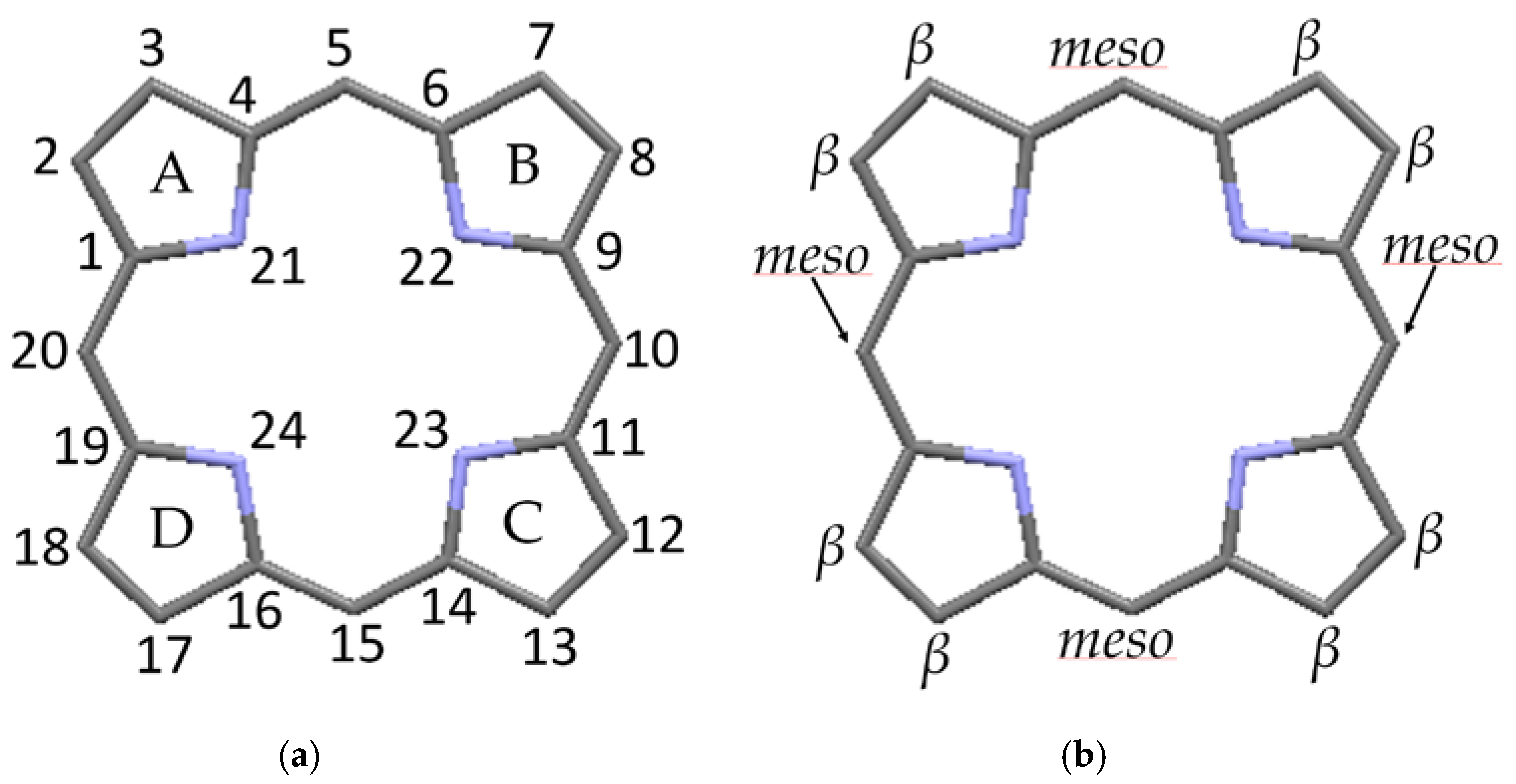
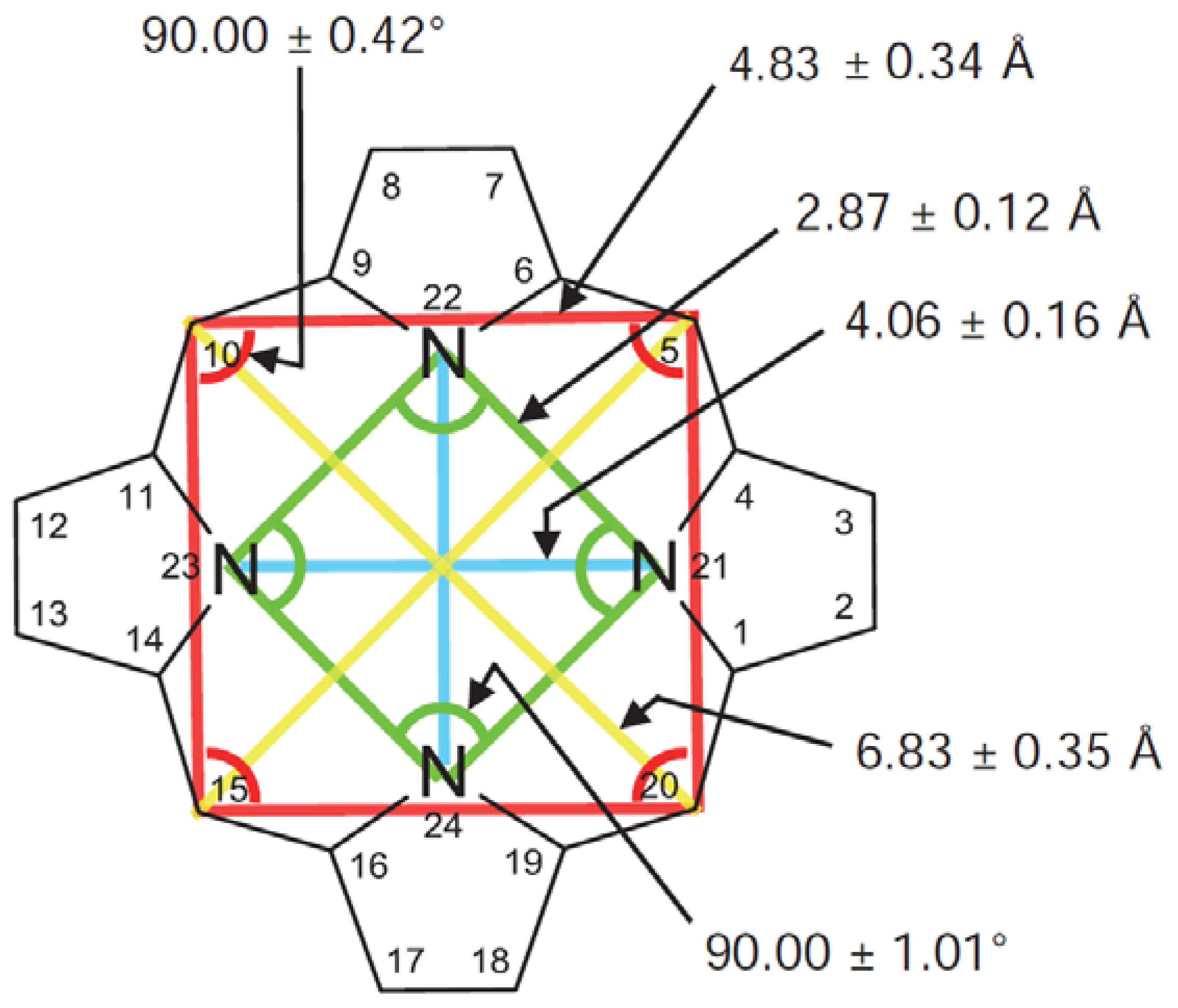
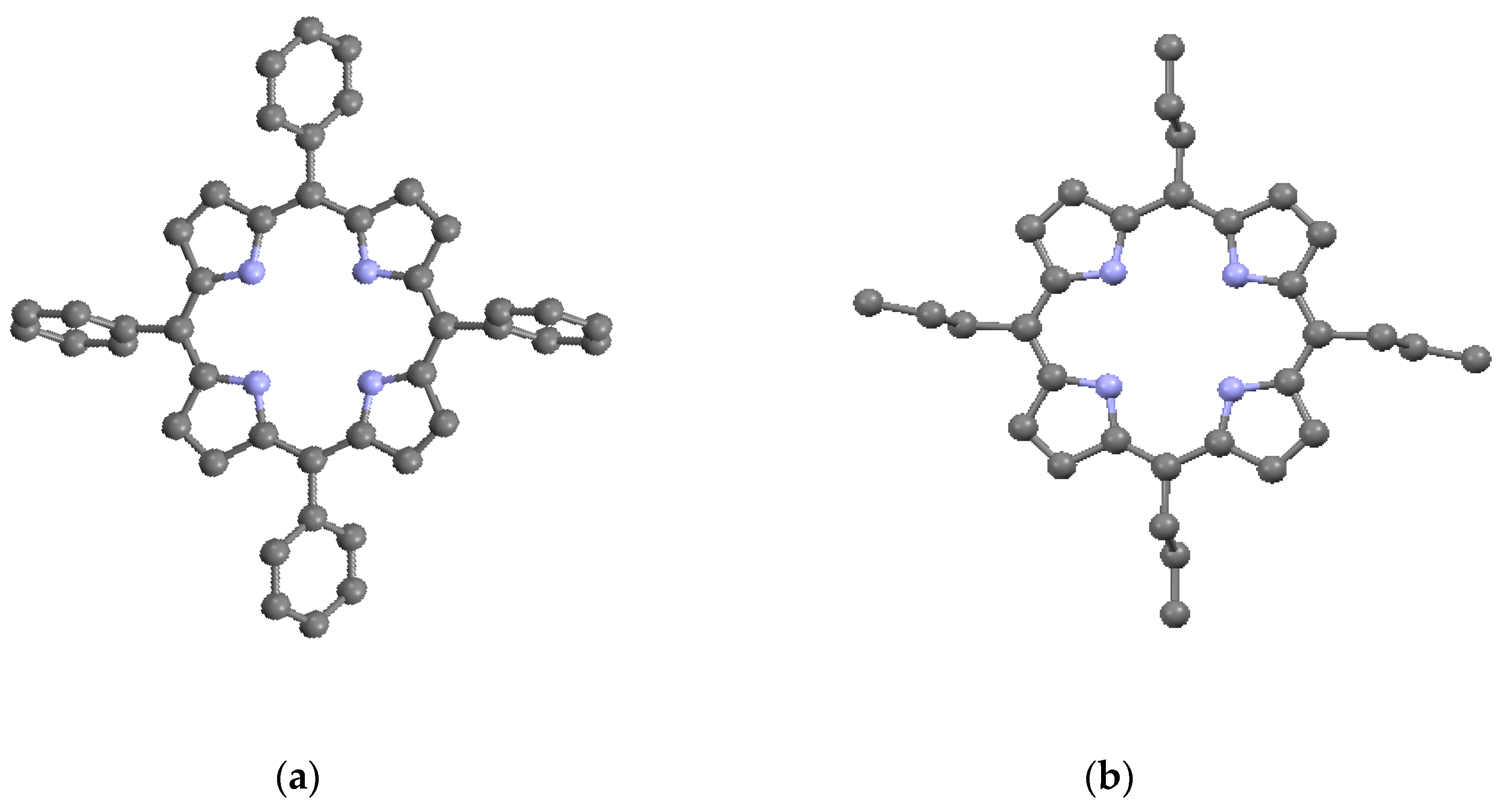
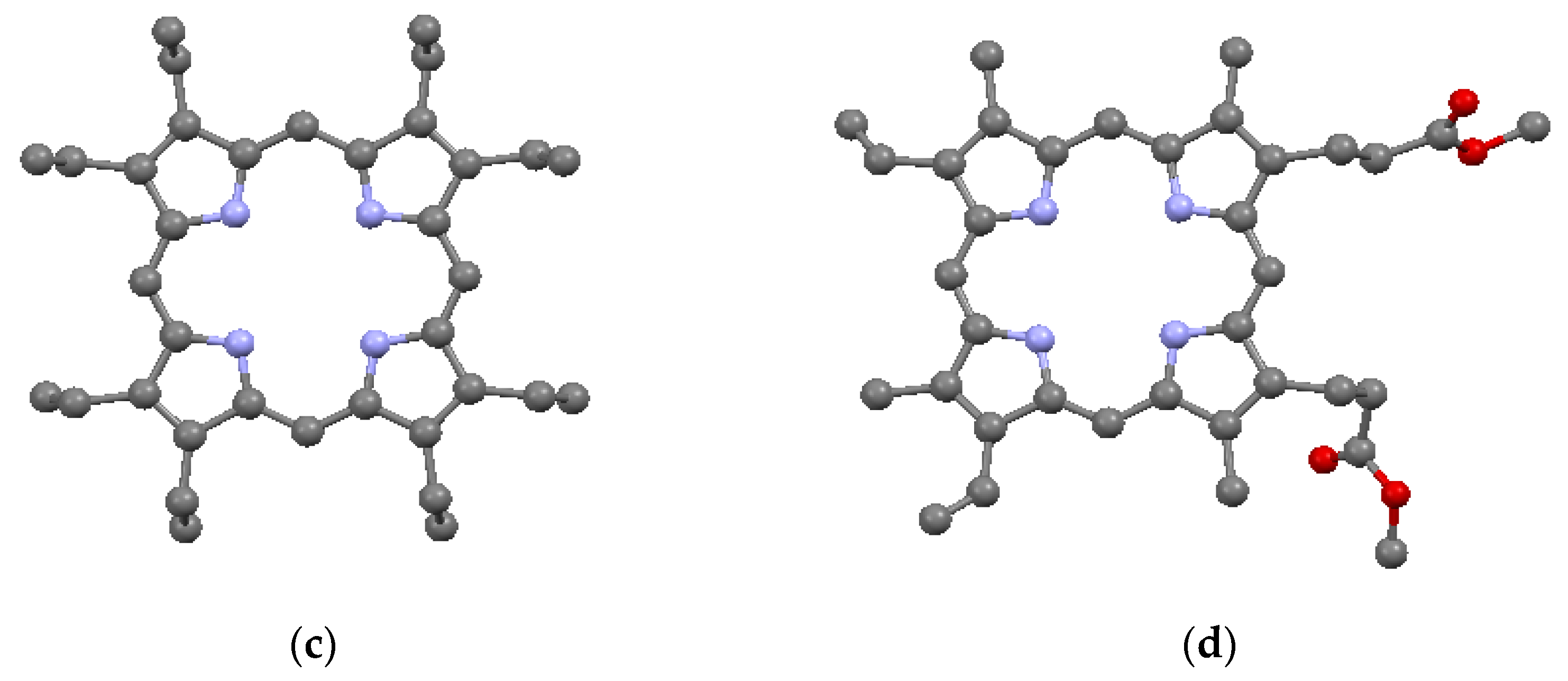
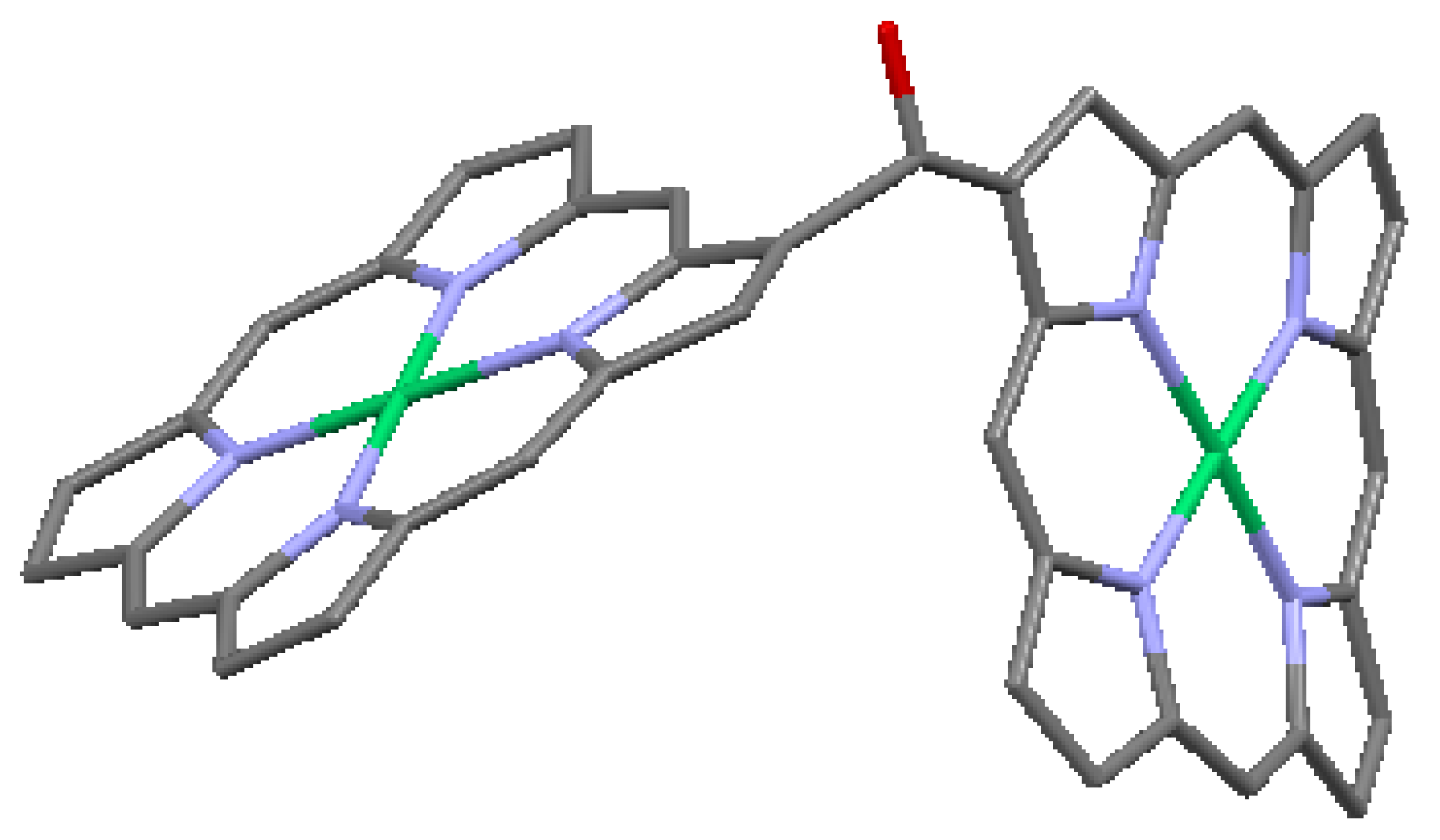
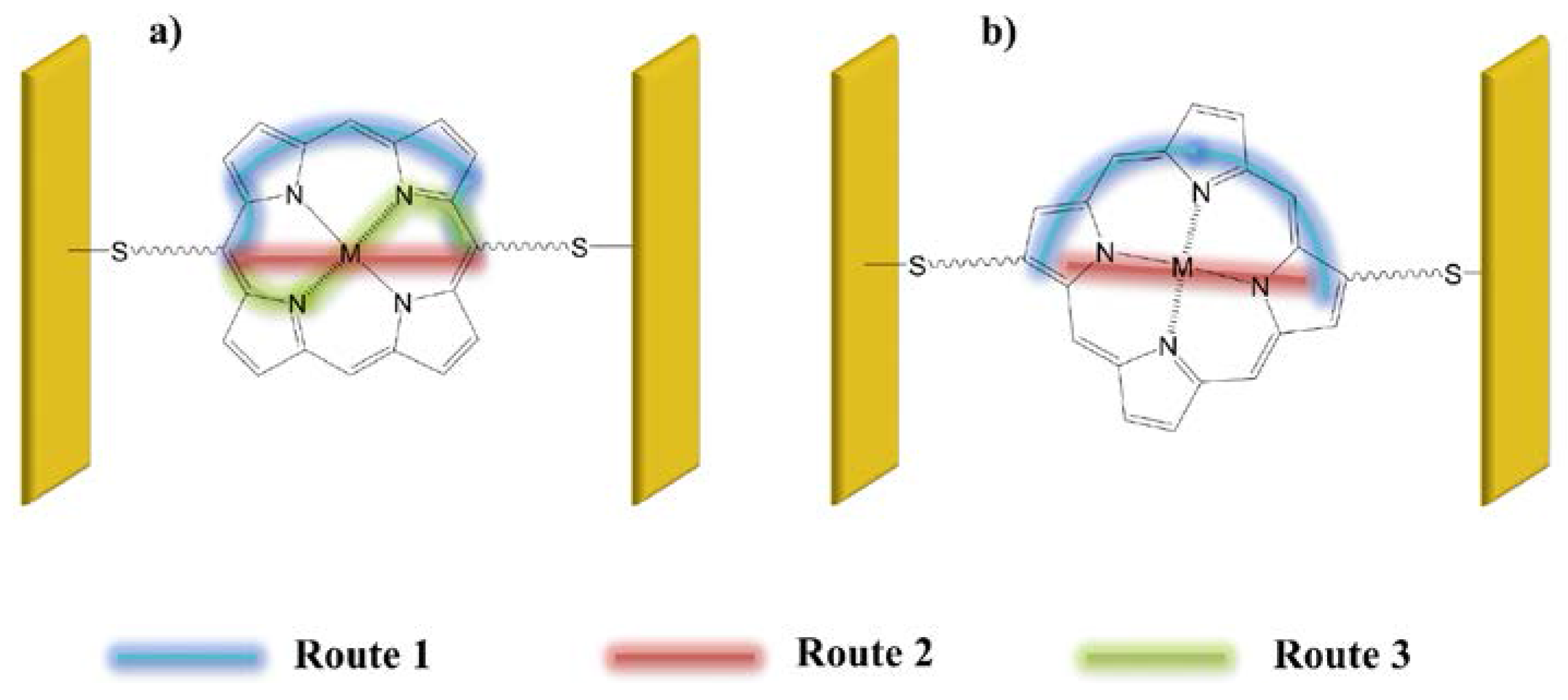
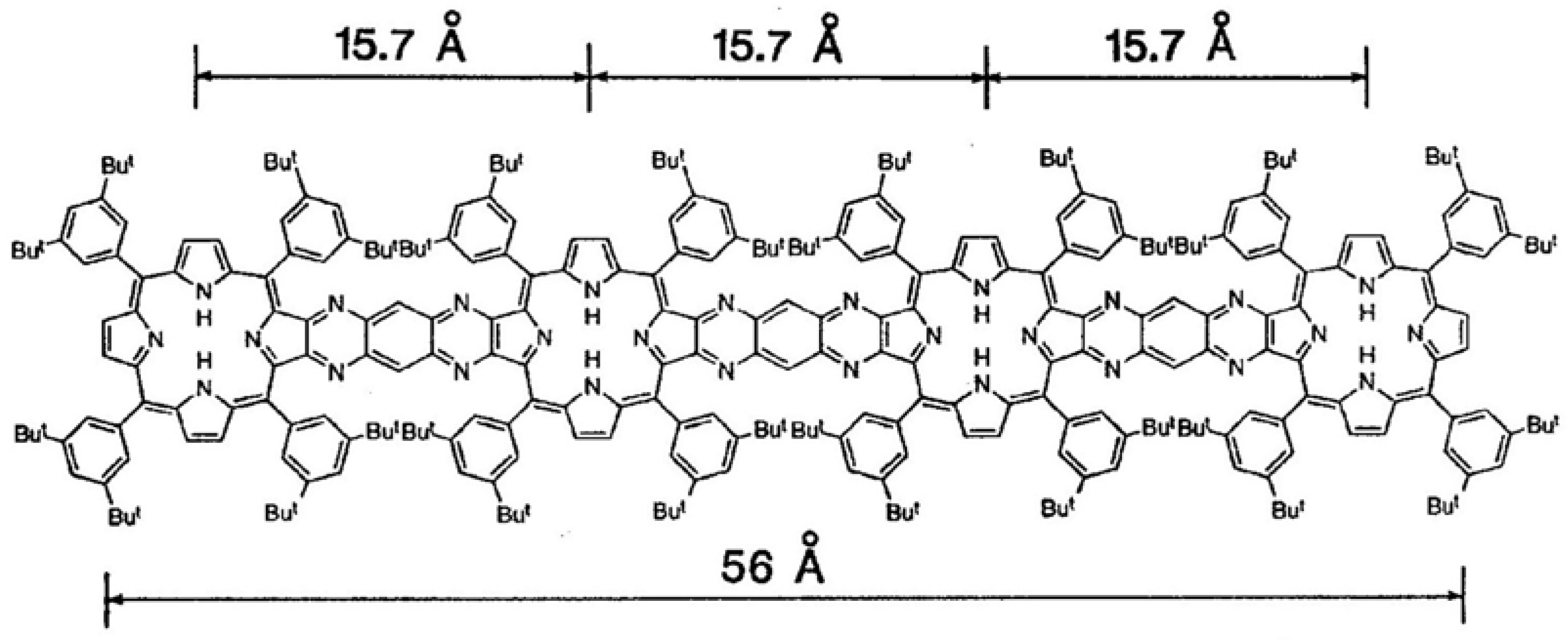
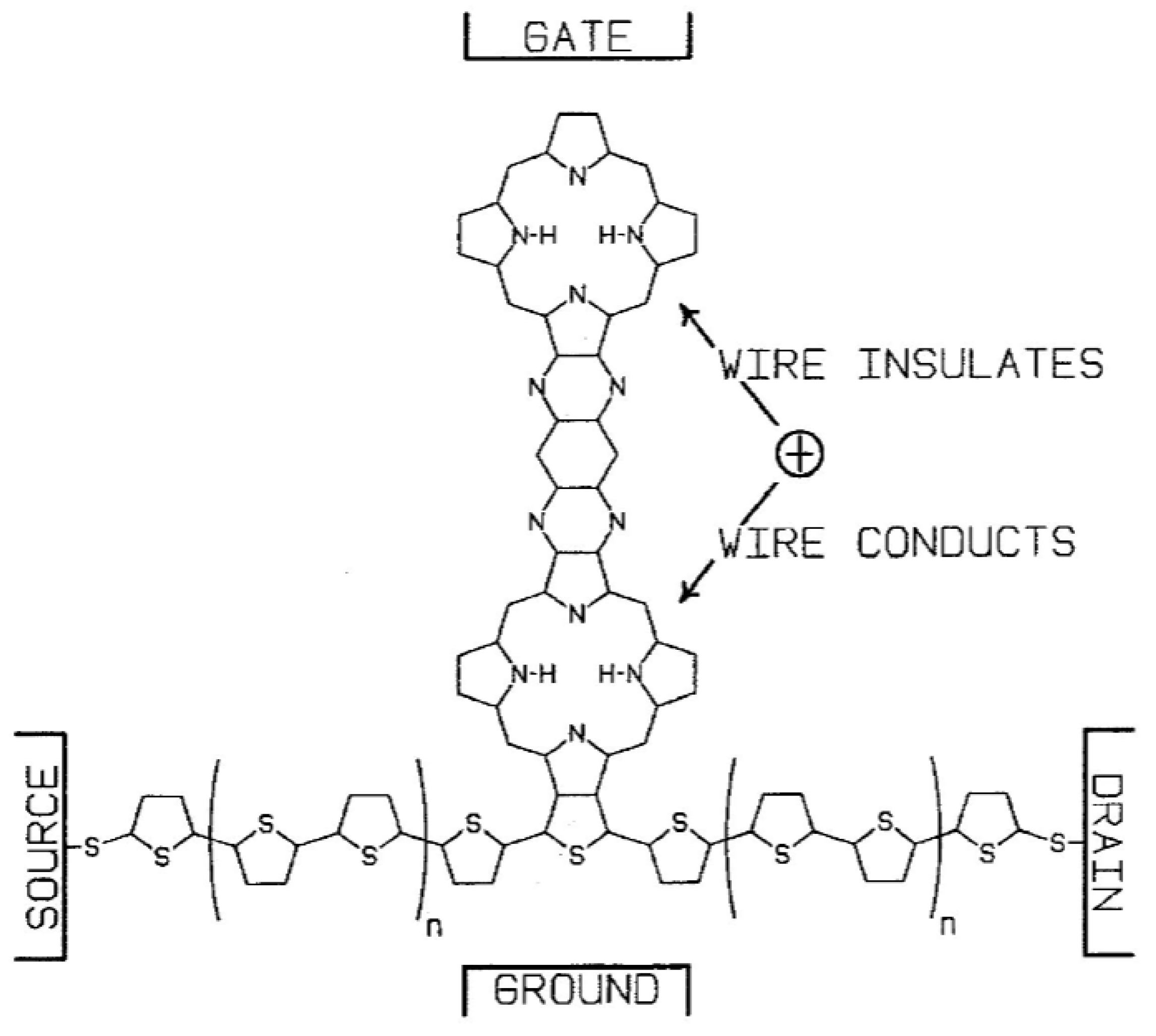
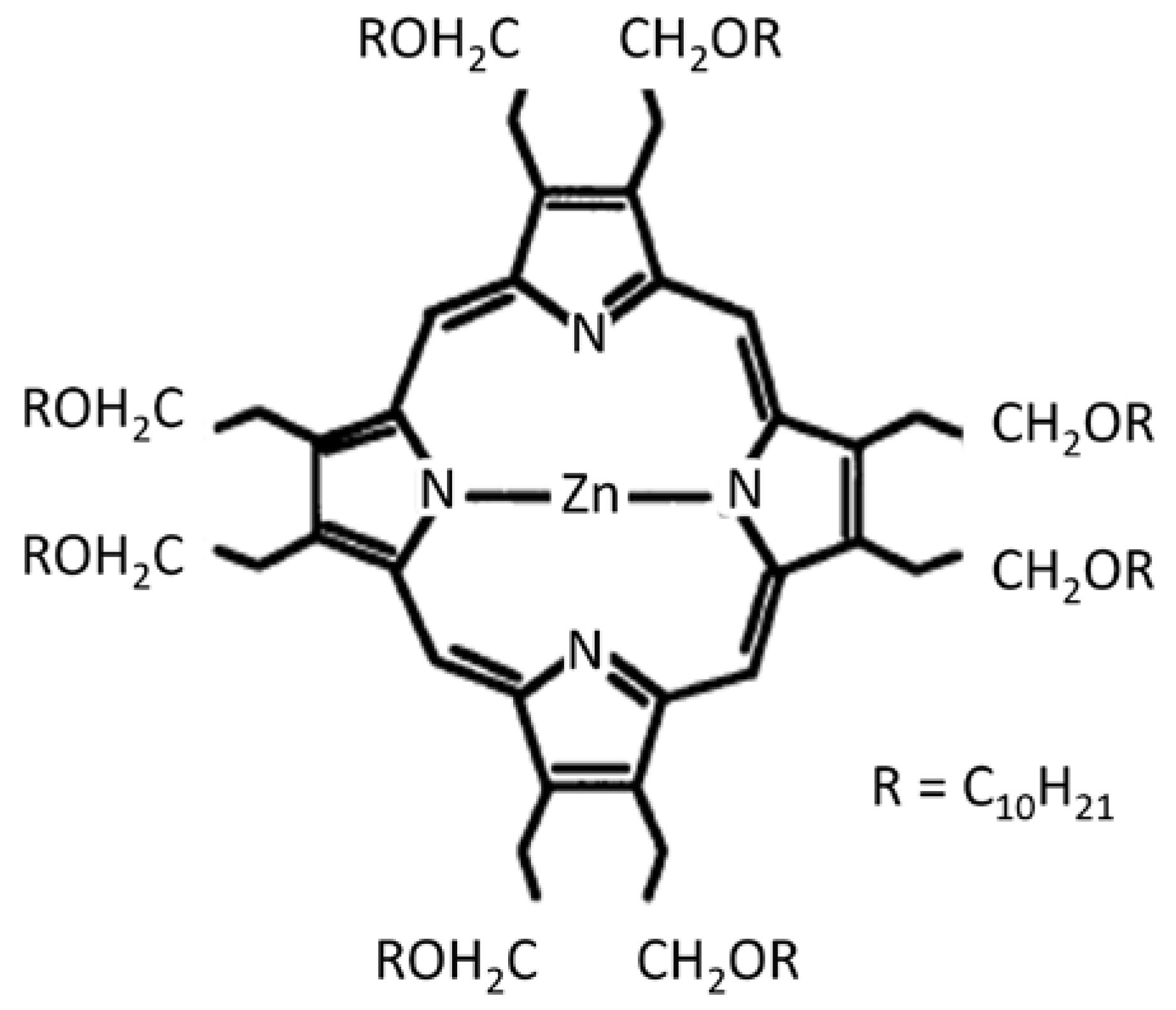
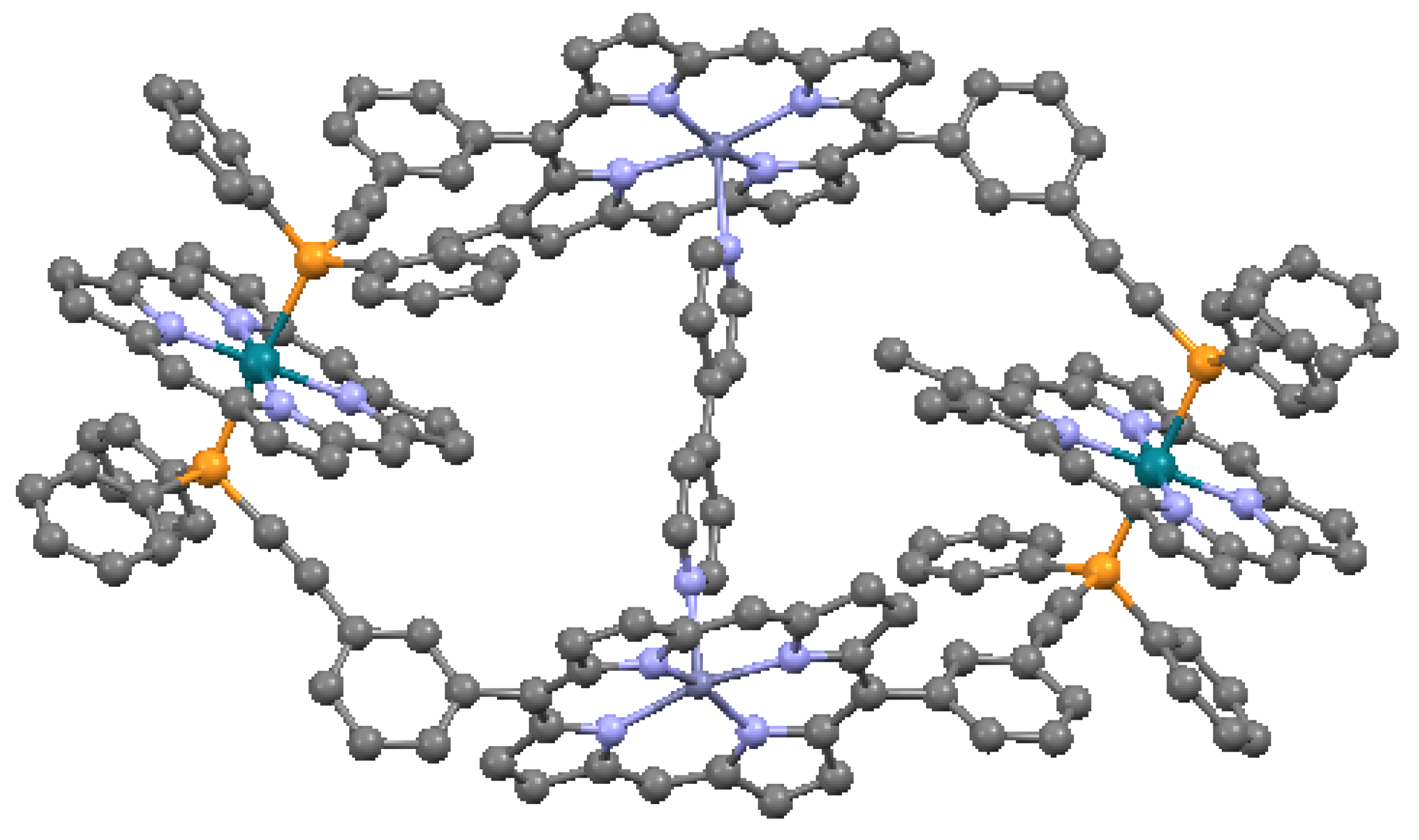
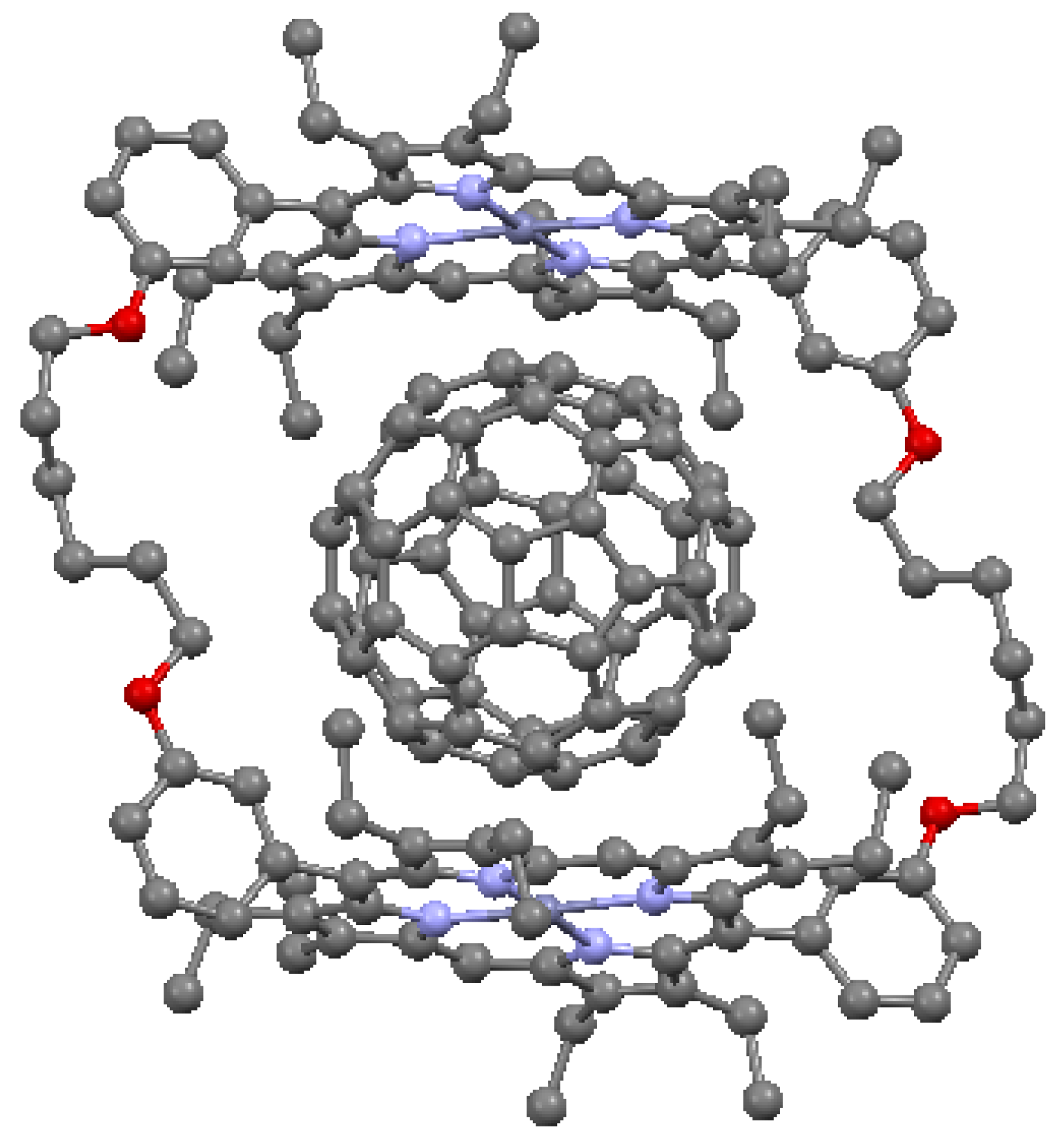
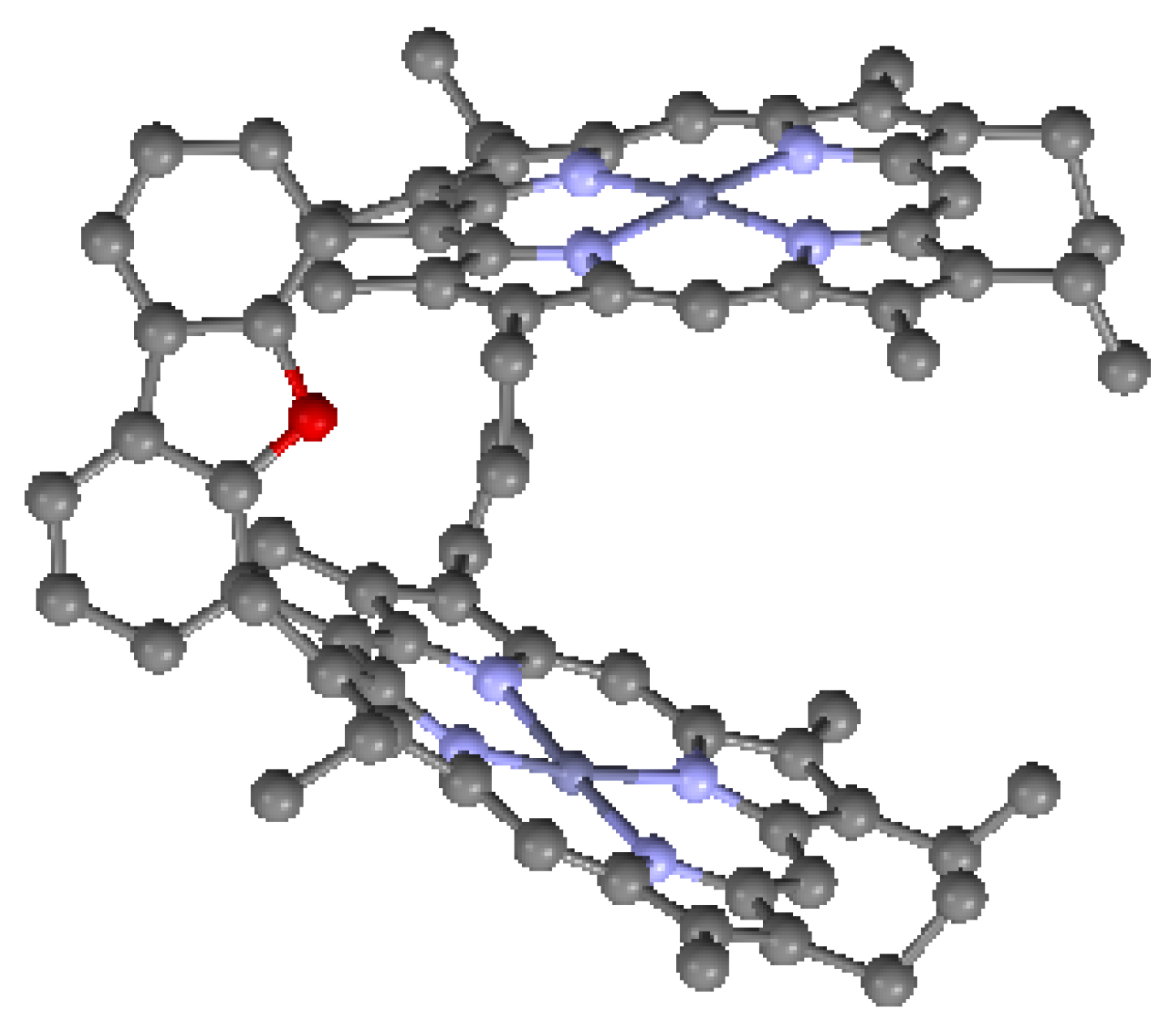
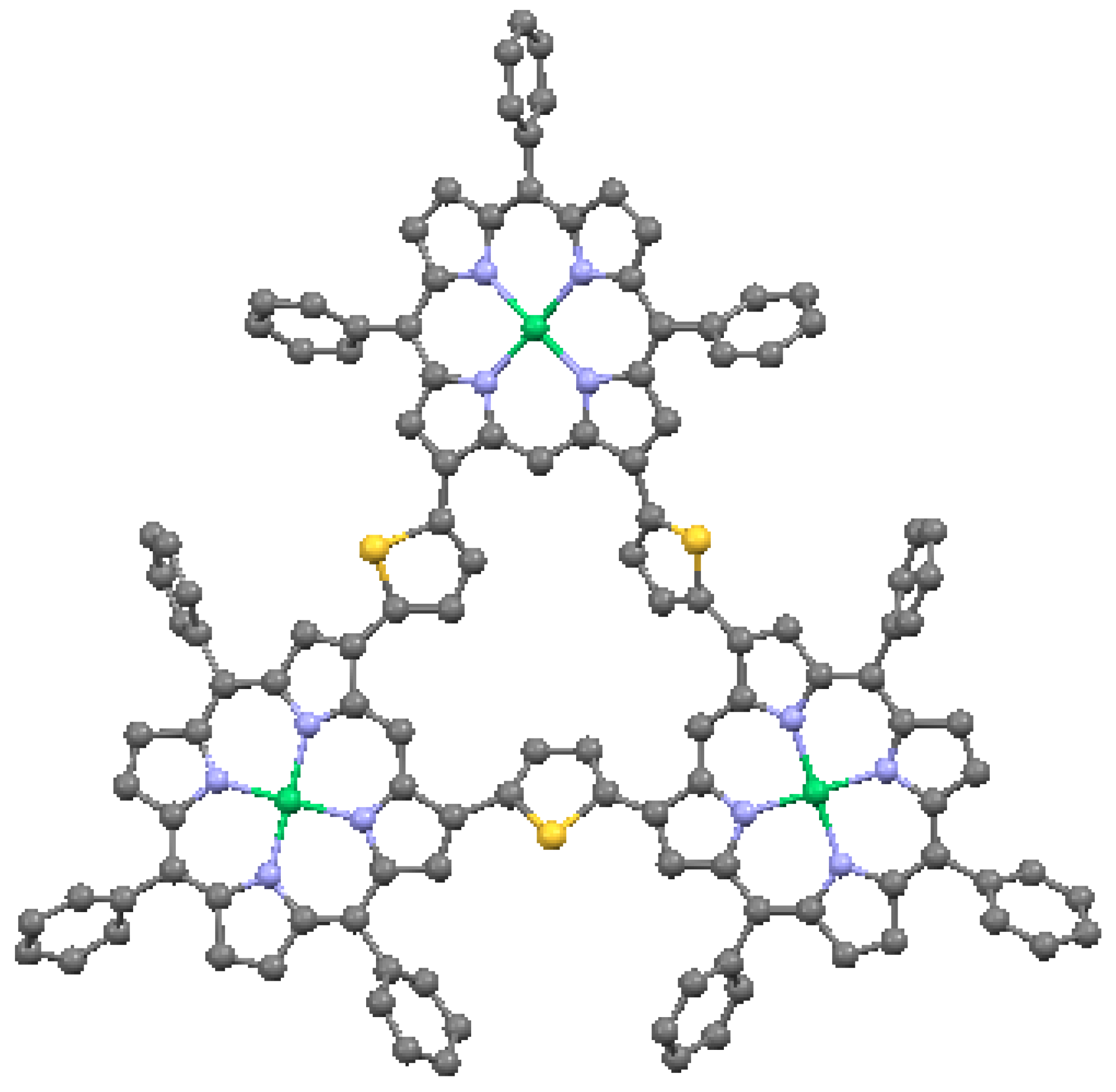
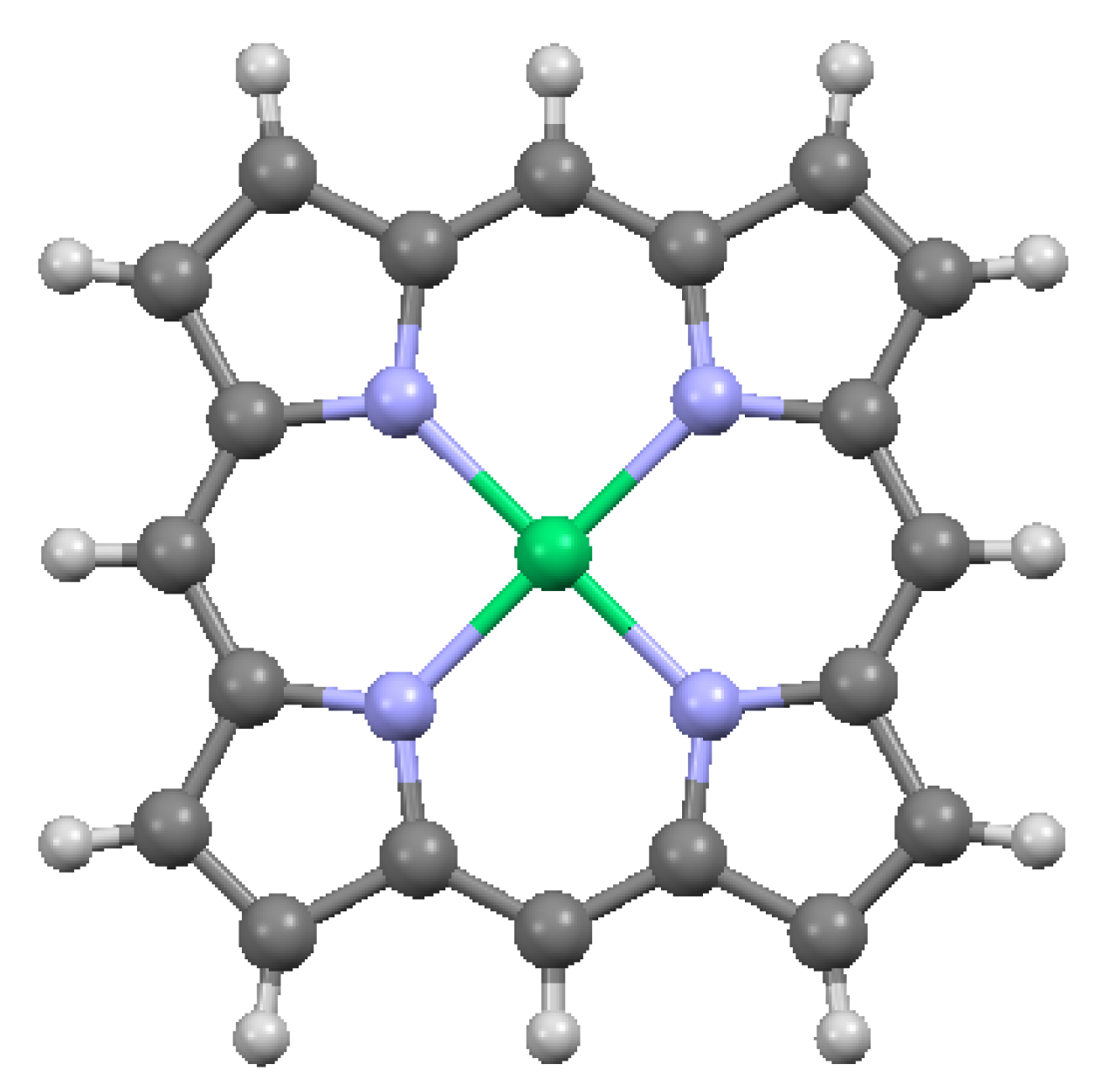
| CSD No. | 609953 (Figure 1) |
|---|---|
| Name | porphine |
| Formula | C14H20N4 |
| System | monoclinic |
| Space gr. | P 21/c |
| A (Å) | 10.2262(3) |
| B (Å) | 11.9060(5) |
| C (Å) | 12.3853(4) |
| α (deg.) | 90.0 |
| β (deg.) | 101.711(3) |
| γ (deg.) | 90.00 |
| V (Å3) | 1476.56 |
| Z | 4 |
| ρ (g/cm3) | 1.396 |
| CSD No. | 1275315 (Figure 4a) | 1051569 (Figure 4b) | 1225729 (Figure 4c) | 1238138 (Figure 4d) |
|---|---|---|---|---|
| Name | Tetraphenyl porphyrin (TPP) | Tetrapropyl porphyrin (TPrP) | Octaethyl porphyrin (OEP) | protoporphyrin IX (PP9) |
| Formula | C44H30N4 | C32H38N4 | C36H46N4 | C36H38N4O4 |
| System | triclinic | monoclinic | triclinic | triclinic |
| Space gr. | P −1 | P 21/n | P −1 | P −1 |
| a (Å) | 6.44 (1) | 5.0843 (2) | 9.791 (2) | 11.303 (5) |
| b (Å) | 10.42 (1) | 11.6074 (6) | 10.771 (2) | 22.553 (10) |
| c (Å) | 12.41 (1) | 22.1695 (11) | 7.483 (2) | 6.079 (3) |
| α (deg.) | 96.06 (5) | 90.00 | 97.43 (1) | 91.38 (2) |
| β (deg.) | 99.14 (5) | 93.53 (2) | 106.85 (1) | 94.08 (2) |
| γ (deg.) | 101.12 (5) | 90.00 | 93.25 (1) | 81.96 (1) |
| V (Å3) | 798.747 | 1305.86 (11) | 745.23 | 1530.36 |
| Z | 1 | 2 | 1 | 2 |
| ρ (g/cm3) | 1.278 | 1.217 | 1.192 | 1.282 |
| Temp. | 295 | 293 (2) | 295 | 295 |
| Ref. | [7,12] | [7,13] | [7,14] | [7,15] |
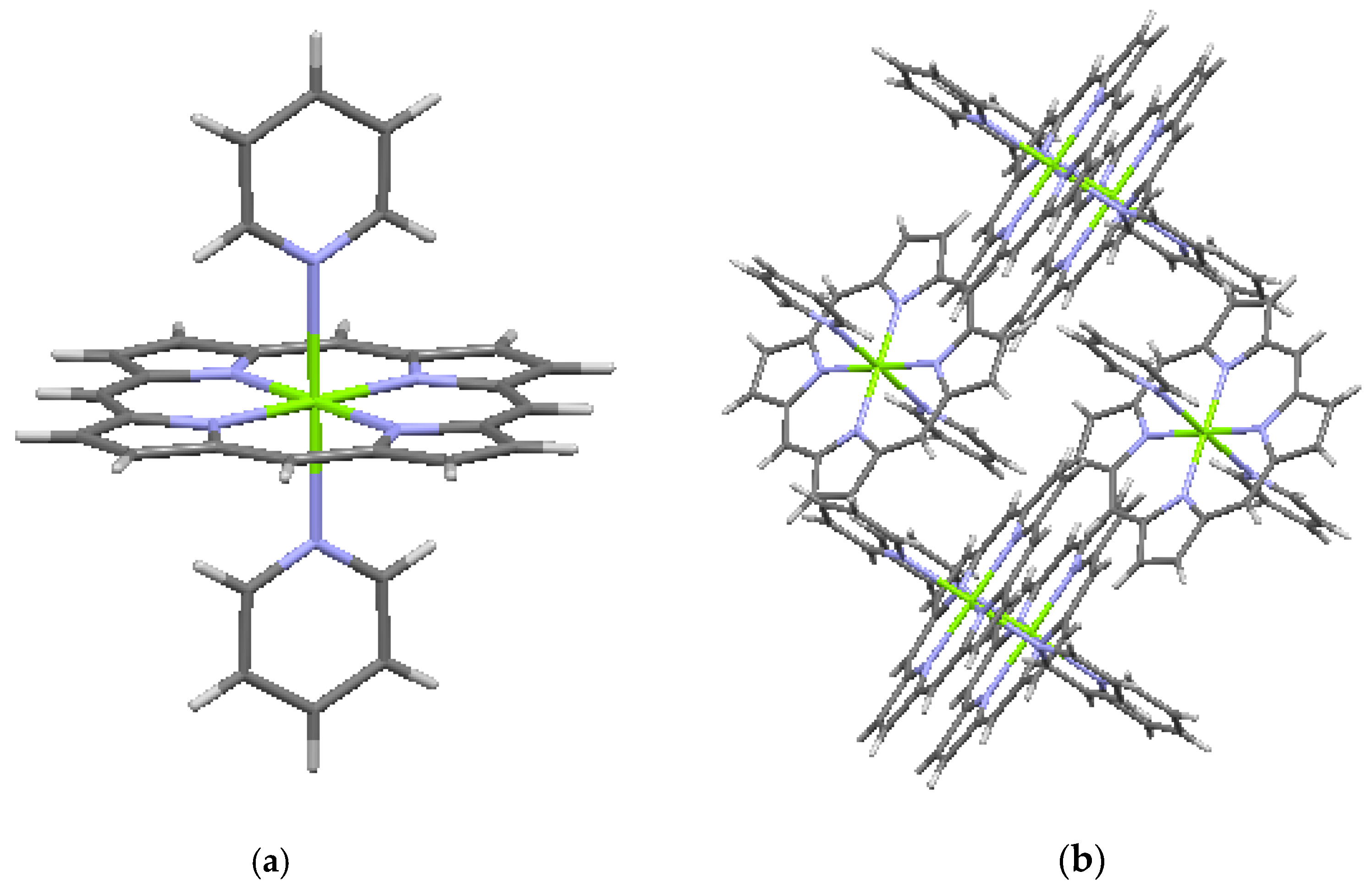
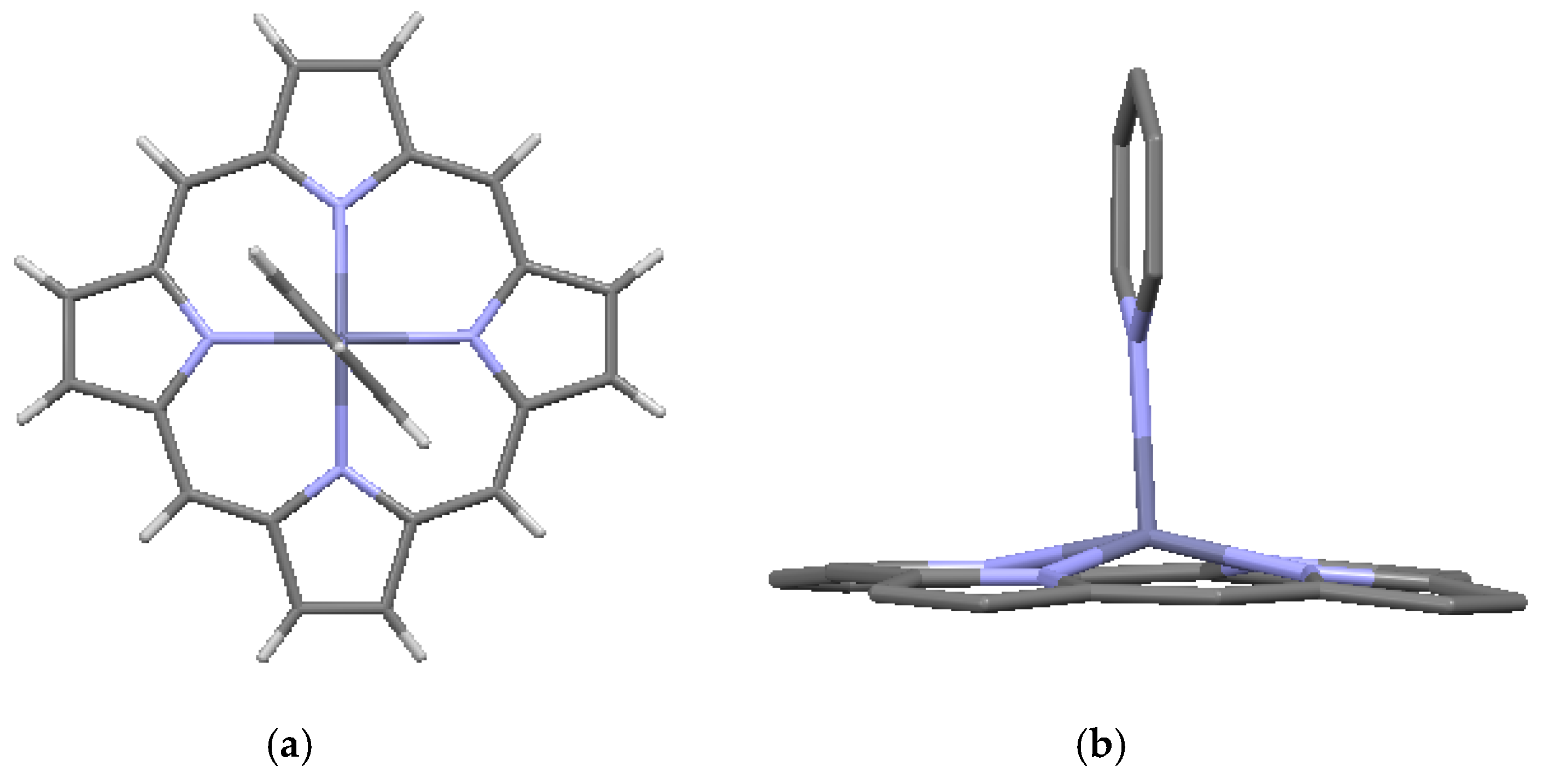
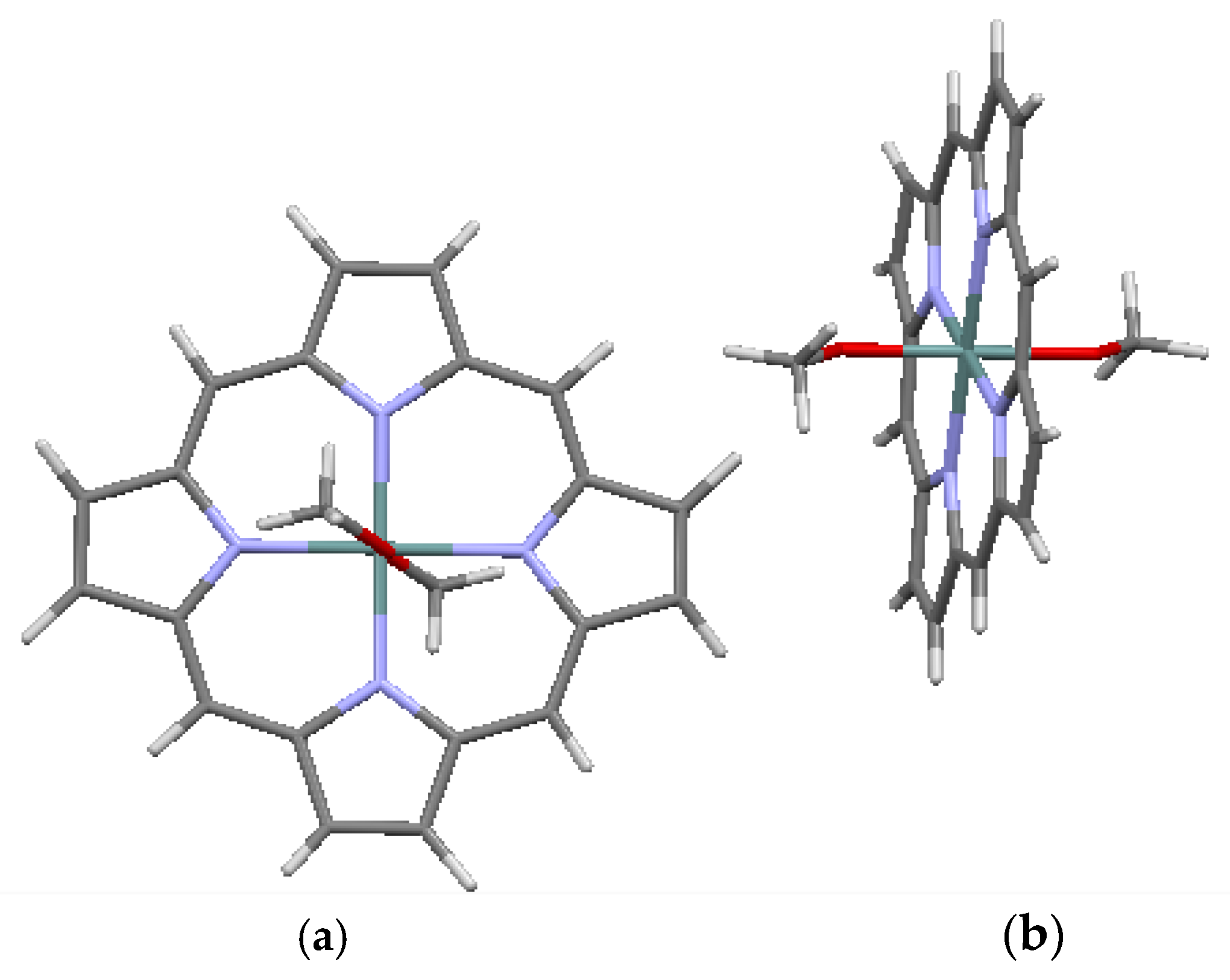
| CSD No. | 1268870 (Figure 5) | 912742 (Figure 6a,b) | 912743 (Figure 7a,b) | 1296339 (Figure 8a,b) |
|---|---|---|---|---|
| Name | Ni(porphine) | Mg(porphine) (C5H5N)2 | Zn(porphine) (C5H5N) | Ge(porphine) (OCH3)2 |
| Formula | C20H12NiN4 | C20H12MgN4 (C5H5N)2 | C20H12ZnN4 (C5H5N) | C20H12GeN4 (OCH3)2 |
| System | monoclinic | monoclinic | monoclinic | monoclinic |
| Space gr. | P 21/c | C 2/c | P 21/c | P 21/c |
| A (Å) | 10.1066 (7) | 12.7579 (9) | 9.5746 (4) | 15.013 (5) |
| B (Å) | 11.945 (9) | 15.0501 (12) | 14.6945 (6) | 14.441 (5) |
| C (Å) | 12.229 (2) | 12.3850 (8) | 14.6410 (6) | 8.414 (4) |
| α (deg.) | 90.0 | 90.00 | 90.0 | 90.0 |
| β (deg.) | 101.56 (3) | 92.071 (4) | 105.542 (2) | 91.85 (2) |
| γ (deg.) | 90.0 | 90.00 | 90.0 | 90.0 |
| V (Å3) | 1446.38 | 2376.46 | 1984.44 | 1823.23 |
| Z | 4 | 4 | 4 | 4 |
| ρ (g/cm3) | 1.686 | 1.372 | 1.516 | 1.614 |
| Displ. (Å) | 0.0191 | 0.0242 | 0.0873 | 0.0157 |
| Ref. | [7,16] | [7,17] | [7,17] | [7,18] |
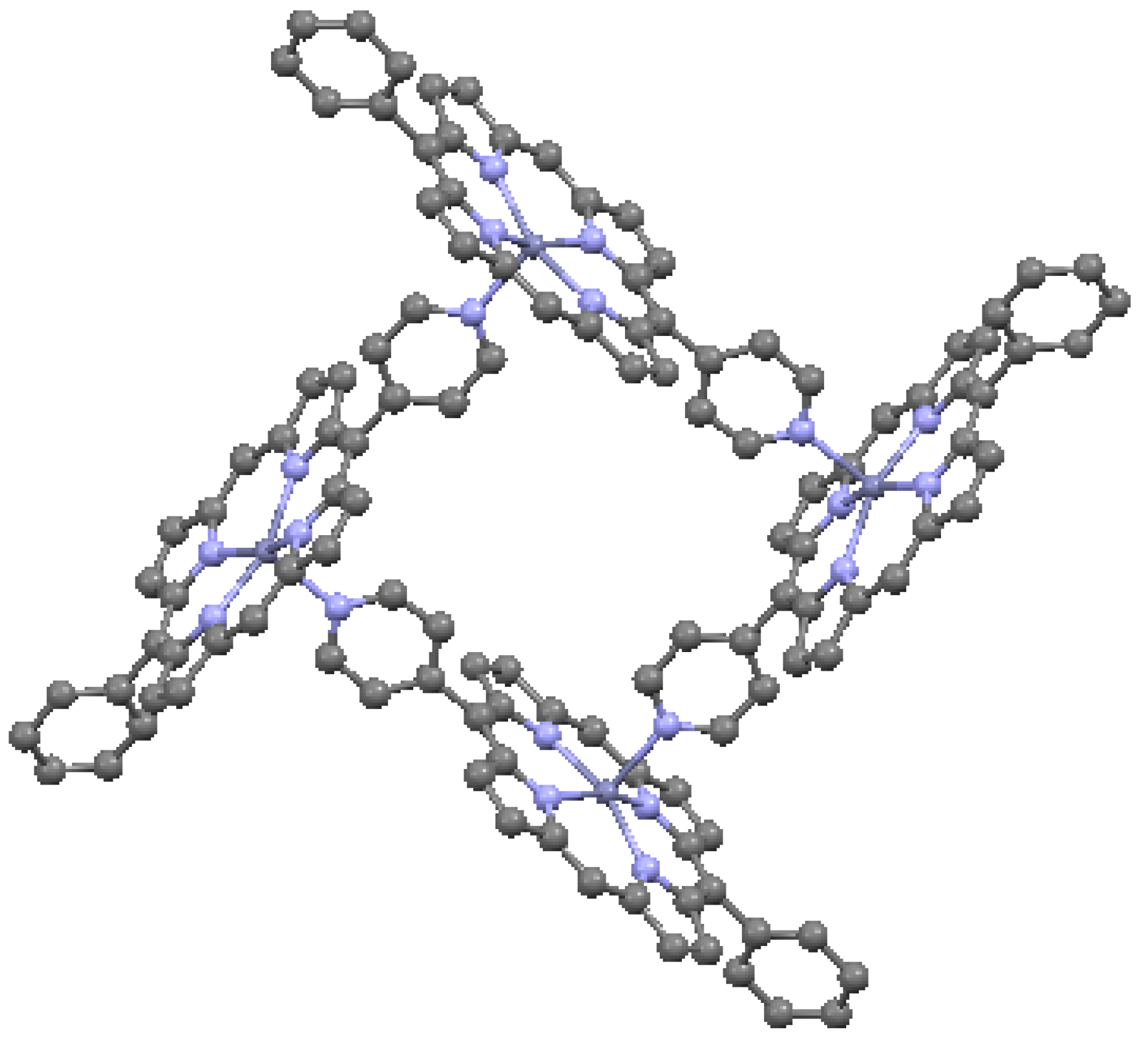
| CSD No. | 627200 (Figure 9) | 1230159 (Figure 10) |
|---|---|---|
| Formula | C81H51N11Ni2 | C67H80N8Ni2O |
| System | monoclinic | monoclinic |
| Space gr. | P 2/c | C 2/c |
| A (Å) | 13.0693 (3) | 40.31 (2) |
| B (Å) | 9.7304 (2) | 14.997 (7) |
| C (Å) | 24.1344 (5) | 21.954 (11) |
| α (deg.) | 90.00 | 90 |
| β (deg.) | 104.2710 (10) | 108.6 (4) |
| γ (deg.) | 90.00 | 90 |
| V (Å3) | 2974.45 (11) | 12578.6 |
| Z | 2 | 8 |
| ρ (g/cm3) | 1.447 | 1.309 |
| Temp. | 150 (2) | 130 |
| Ref. | [7,21] | [7,22,23] |
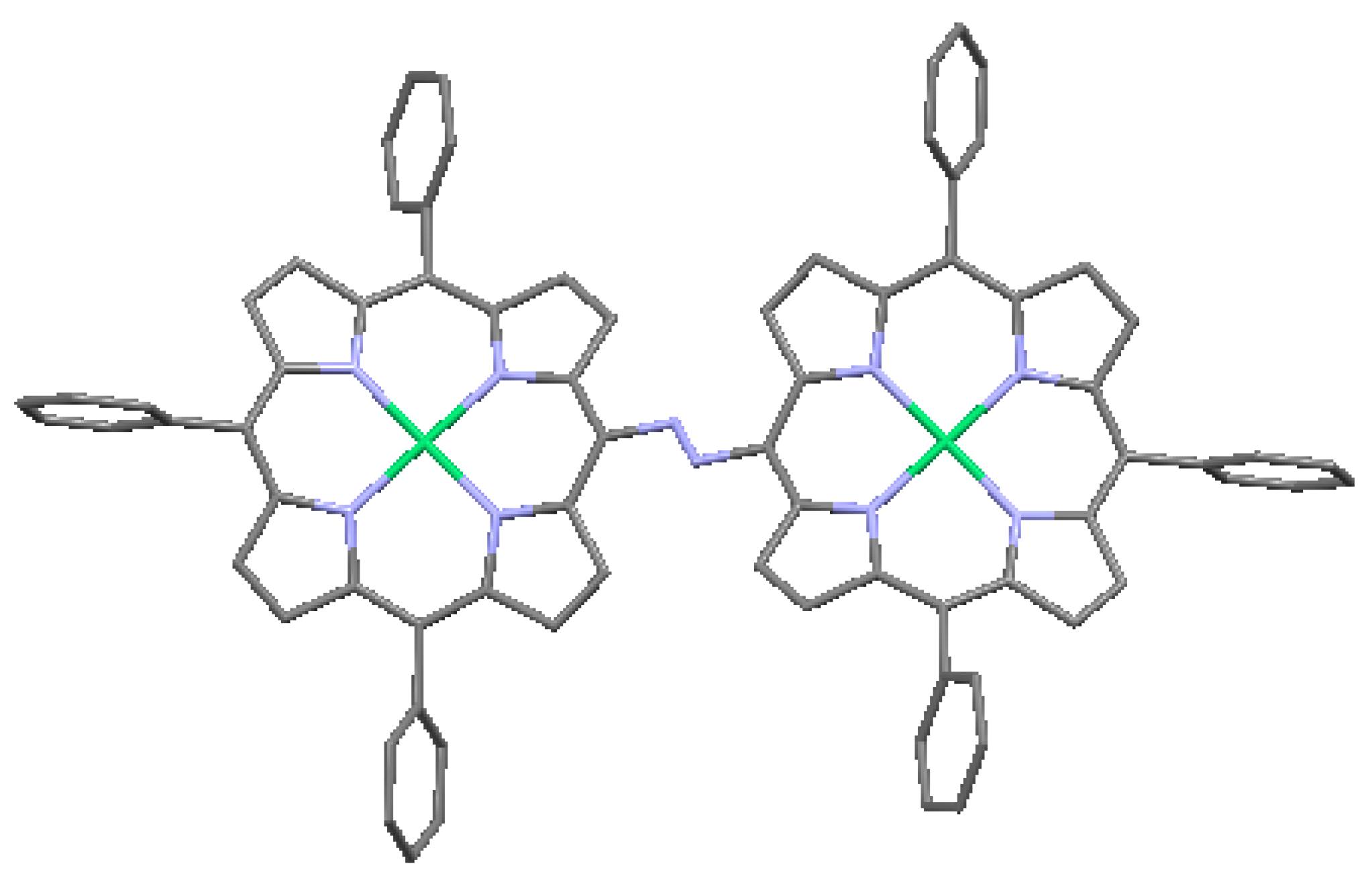
| CSD No. | 635463 (Figure 17) | 213358 (Figure 18) | 150074 (Figure 19) | 141402 (Figure 20) |
| F. wt. | 2947.42 | 5186.85 | 2449.64 | 1397.20 |
| Formula | C331H179N8O6Zn2 | C386H291CI2N18P4Rh2Zn2 | C168H124N8O4Zn2 | --- |
| System | triclinic | monoclinic | triclinic | monoclinic |
| Space gr. | P −1 | P 21/n | P −1 | C 2/c |
| A (Å) | 9.287 (5) | 25.1838 (9) | 13.6550 (11) | 23.0808 (2) |
| B (Å) | 20.381 (5) | 16.5456 (6) | 15.3346 (12) | 24.9458 (9) |
| C (Å) | 21.529 (5) | 32.5020 (11) | 16.2865 (13) | 13.4593 (5) |
| α (deg.) | 77.218 (5) | 90.00 | 109.187 (2) | 90.0 |
| β (deg.) | 80.967 (5) | 93.902 (2) | 107.904 (2) | 110.503 (2) |
| γ (deg.) | 80.500 (5) | 90.00 | 97.732 (3) | 90.0 |
| V (Å3) | 3889 (2) | 13511.6 (8) | 2957.7 (4) | 7549.5 (4) |
| Z | 1 | 2 | 1 | 4 |
| ρ (g/cm3) | 1.259 | 1.275 | 1.375 | 1.229 |
| Temp. | 90 (2) | 150 (2) | 163.2 | 183 (2) |
| Ref. | [7,53] | [7,54] | [7,55] | [7,56] |
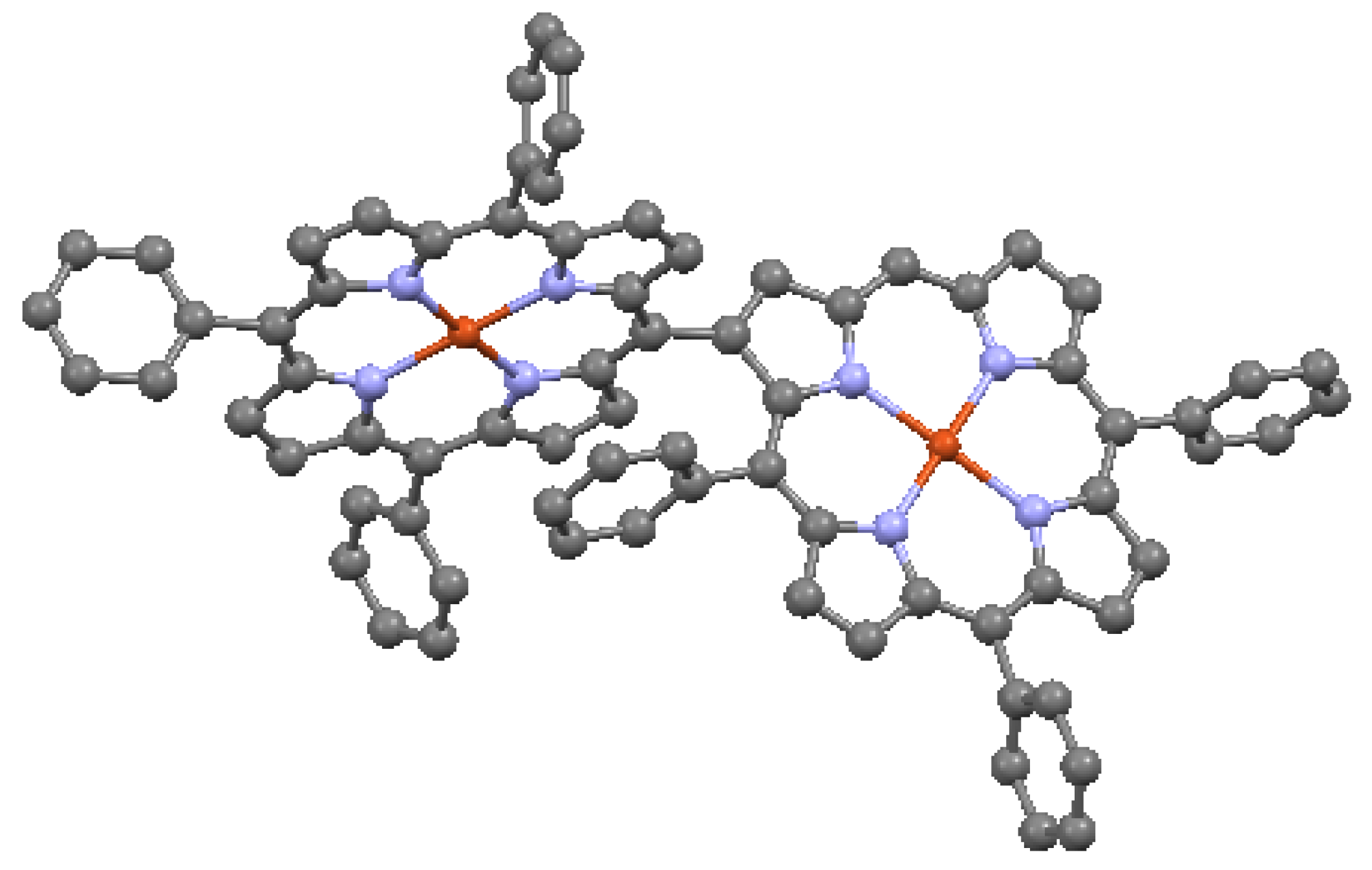
| CSD No. | 728863 (Figure 21) | 685451 (Figure 22) | 173782 (Figure 23) | 712832 (Figure 24) |
| F. wt. | 1310.84 | 3308.31 | 1825.03 | --- |
| Formula | C82H51C1Cu2N8 | C200H216C17N12Ni3S3 | C120H112N10Zn2 | --- |
| System | orthorhombic | triclinic | monoclinic | monoclinic |
| Space gr. | Pbca | P−1 | P21/n | C 2/c |
| A (Å) | 26.455 (5) | 18.846 (5) | 13.7485 (2) | 38.482 (6) |
| B (Å) | 13.095 (5) | 27.950 (5) | 18.3200 (3) | 27.205 (5) |
| C (Å) | 35.226 (5) | 27.989 (5) | 40.8739 (7) | 17.098 (5) |
| α (deg.) | 90.000 (5) | 112.376 (5) | 90 | 90.0 |
| β (deg.) | 90.000 (5) | 103.171 (5) | 84.1947 (3) | 111.967 (5) |
| γ (deg.) | 90.000 (5) | 103.126 (5) | 90 | 90.0 |
| V (Å3) | 12203 (5) | 12445 (5) | 10242.2 (2) | 15306.3 |
| Z | 8 | 2 | 8 | --- |
| ρ (g/cm3) | 1.427 | 0.883 | 2.367 | --- |
| Temp. | 90 (2) | 90 (2) | 296.2 | --- |
| Ref. | [7,57] | [7,58] | [7,59] | [7,60] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, L.P.; Brewer, G.; Wong-Ng, W. Structural Aspects of Porphyrins for Functional Materials Applications. Crystals 2017, 7, 223. https://doi.org/10.3390/cryst7070223
Cook LP, Brewer G, Wong-Ng W. Structural Aspects of Porphyrins for Functional Materials Applications. Crystals. 2017; 7(7):223. https://doi.org/10.3390/cryst7070223
Chicago/Turabian StyleCook, Lawrence P., Greg Brewer, and Winnie Wong-Ng. 2017. "Structural Aspects of Porphyrins for Functional Materials Applications" Crystals 7, no. 7: 223. https://doi.org/10.3390/cryst7070223