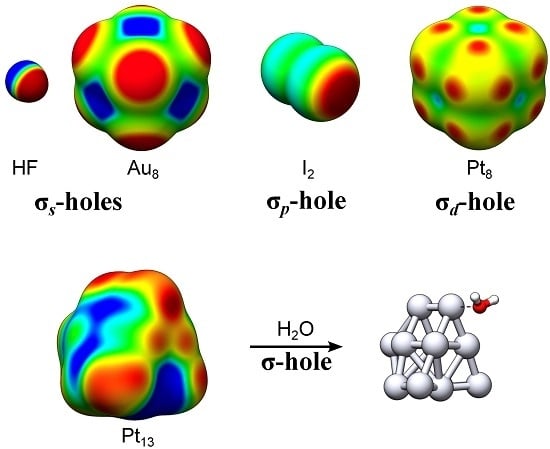
σ-Holes on Transition Metal Nanoclusters and Their Influence on the Local Lewis Acidity
Abstract
:1. Introduction
2. Theory
3. Computational Details
4. Results and Discussion
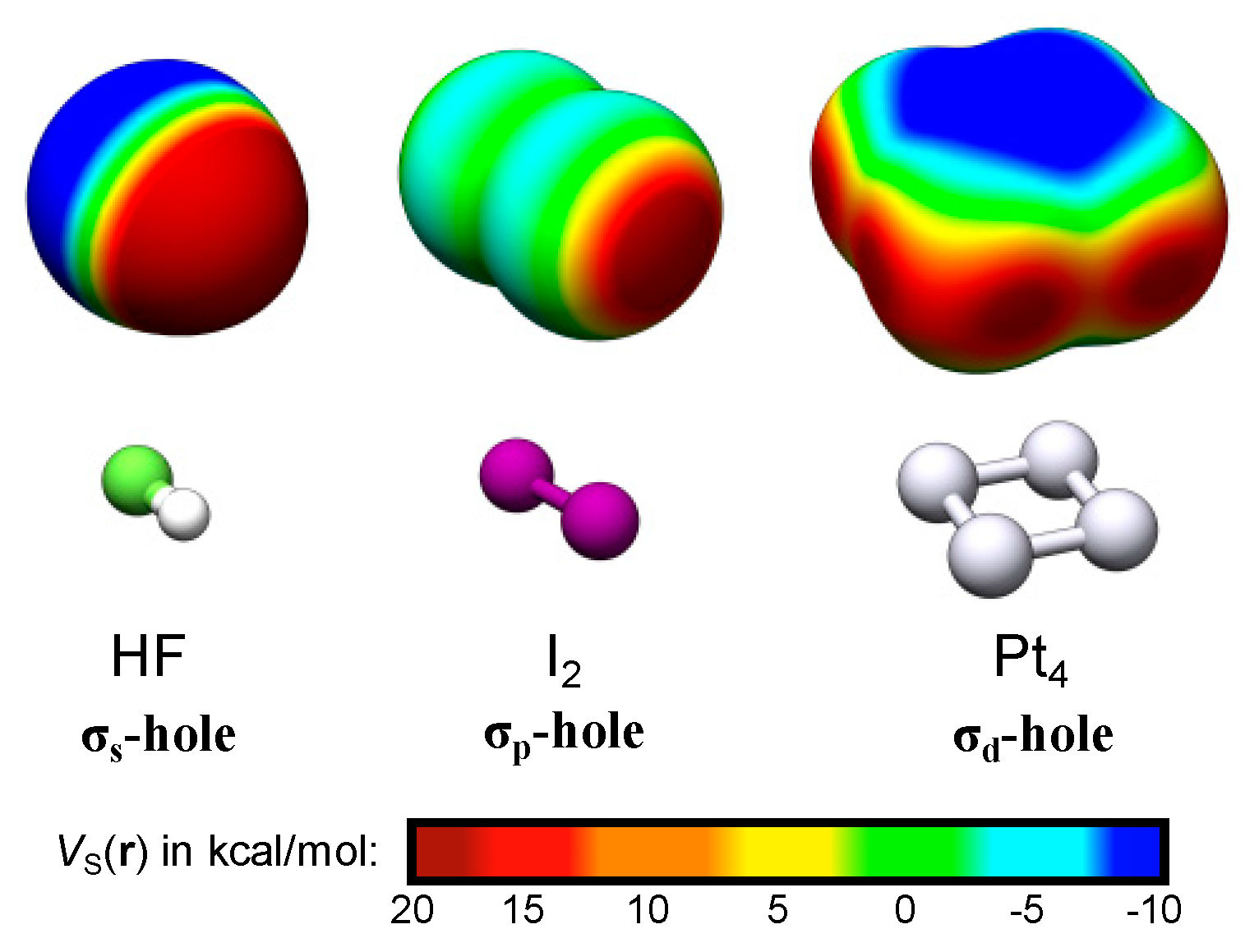
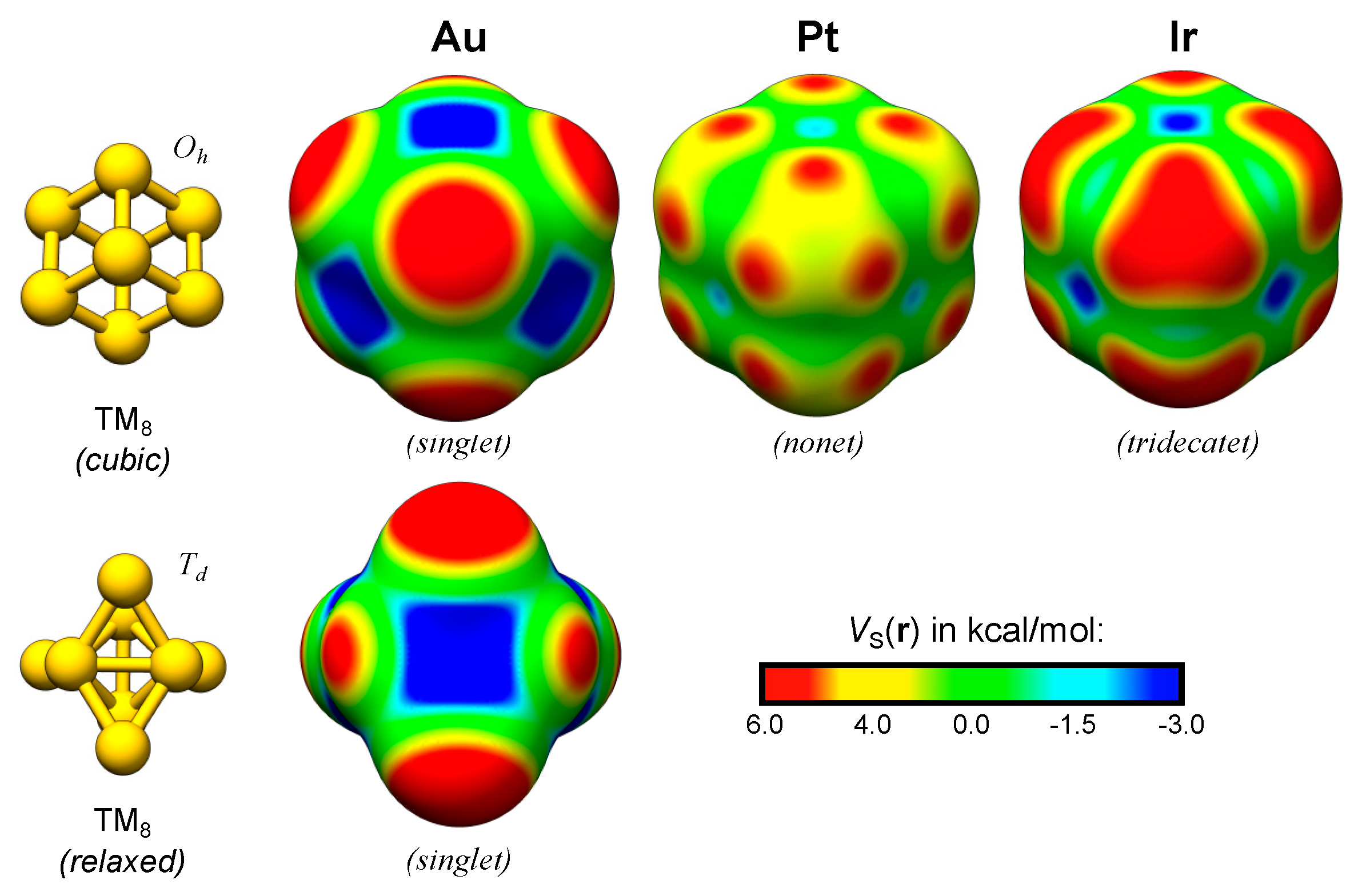
4.1. Exemplifying the Origin of the TM σs-Holes and σd-Holes—The TM2 and TM8 of Ir, Pt and Au
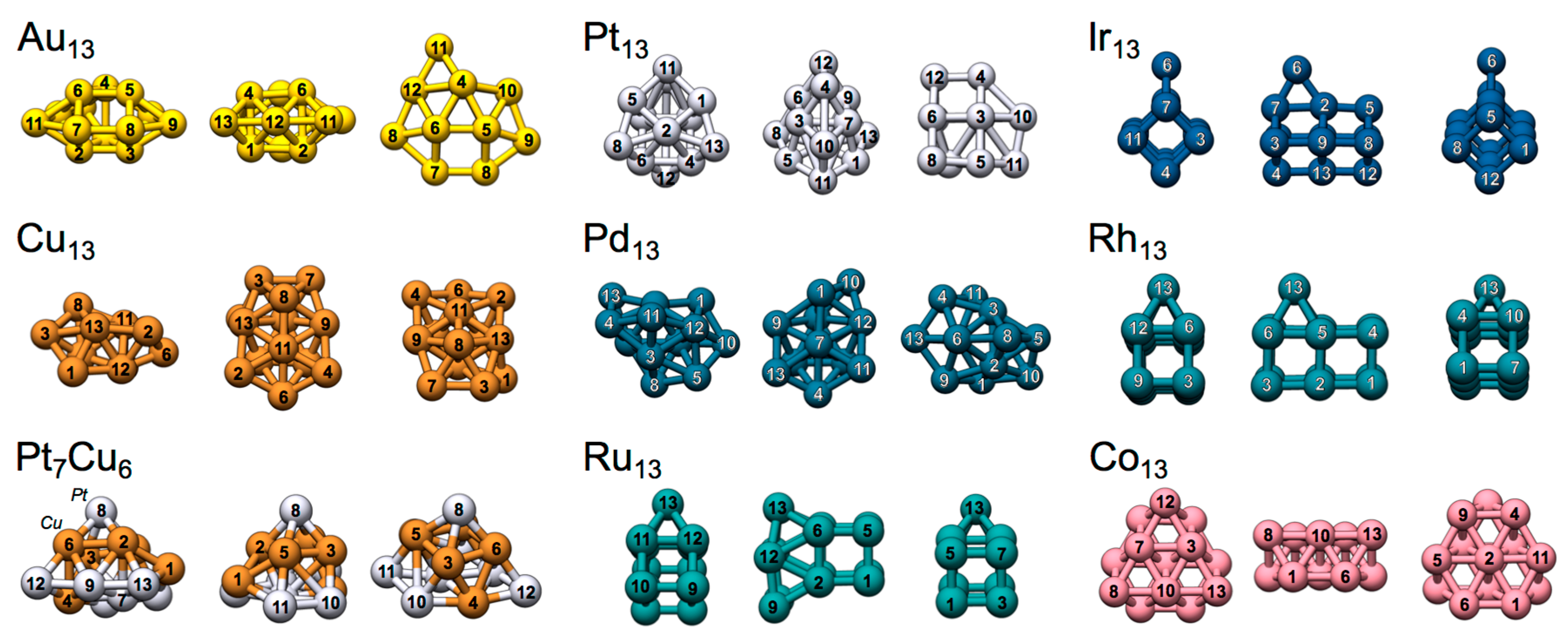
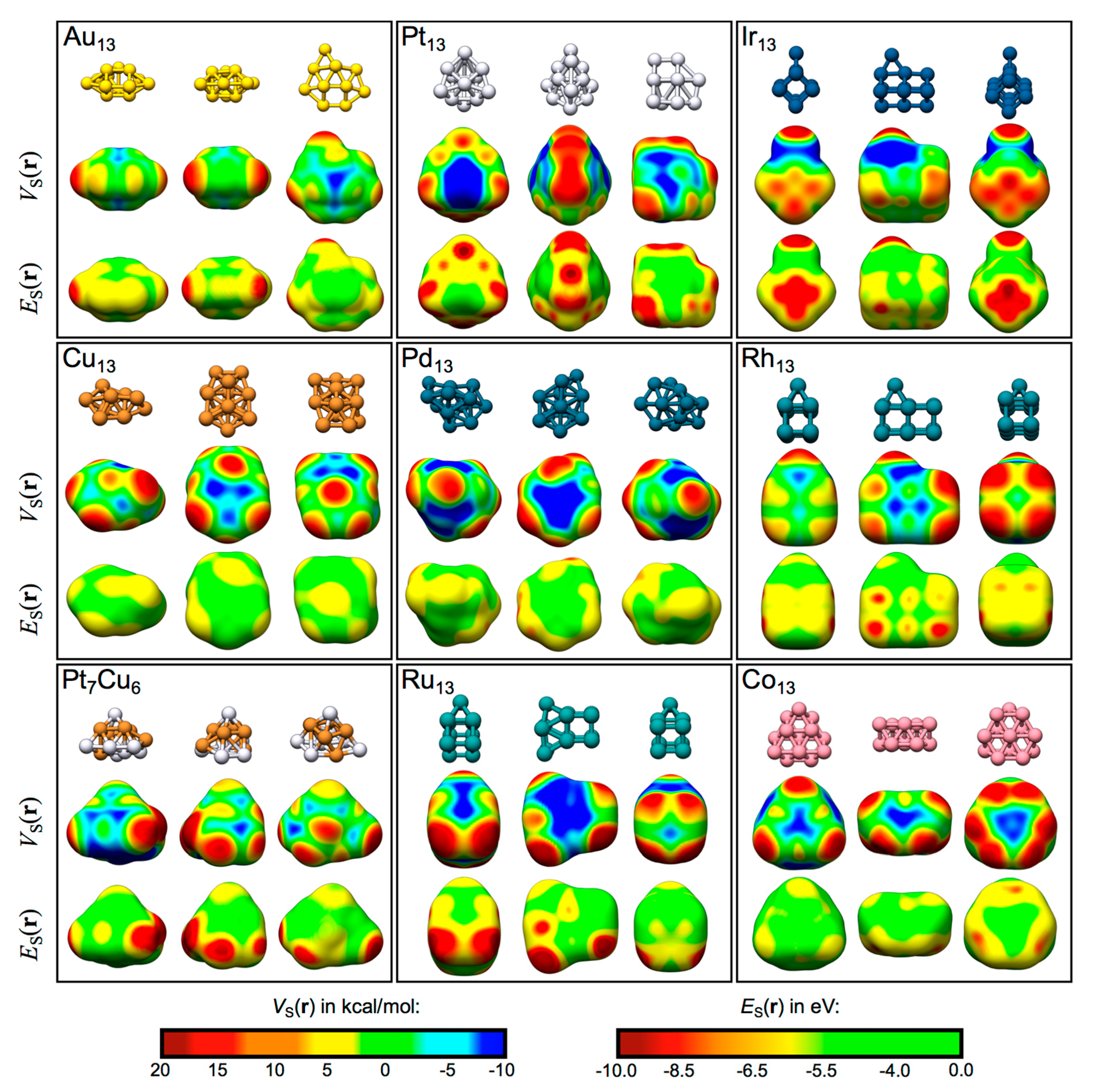
4.2. Local Lewis Acidic (and Basic) Characteristics of the TM13 Nanoclusters
4.2.1. Au13, Pt13 and Ir13
4.2.2. Rh13 and Ru13
4.2.3. Cu13 and Pt7Cu6
4.2.4. Pd13 and Co13
4.3. General Discussion
- Partially occupied d-orbitals
- Favorable electronic configuration (e.g., spin-state with low degree of spd-hybridization)
- Locally symmetric bonding arrangement (e.g., cubic)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Clark, T.; Hennemann, M.; Murray, J.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley. Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Stenlid, J.H.; Brinck, T. Unpublished work. 2017.
- Stenlid, J.H.; Johansson, A.J.; Brinck, T. Unpublished work. 2017.
- Stenlid, J.H.; Johansson, A.J.; Brinck, T. Searching for the thermodynamic limit—A DFT study of the step-wise water oxidation of the bipyramidal Cu7 cluster. Phys. Chem. Chem. Phys. 2014, 16, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Farmanzadeh, D.; Abdollahi, T. Investigation on the chemical active sites of copper nanoclusters as nanocatalyst for the adsorption of acetylene: Calibration of DFT method and basis set. Theor. Chem. Acc. 2016, 135, 1–14. [Google Scholar] [CrossRef]
- Farmanzadeh, D.; Abdollahi, T. A model for the ethylene and acetylene adsorption on the surface of Cun(n = 10–15) nanoclusters: A theoretical study. Appl. Surf. Sci. 2016, 385, 241–248. [Google Scholar] [CrossRef]
- Shields, Z.P.; Murray, J.S.; Politzer, P. Directional tendencies of halogen and hydrogen bonds. Int. J. Quantum Chem. 2010, 110, 2823–2832. [Google Scholar] [CrossRef]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Brinck, T.; Murray, J.S.; Politzer, P. Surface electrostatic potentials of halogenated methanes as indicators of directional intermolecular interactions. Int. J. Quantum Chem. 1992, 44, 57–64. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- TURBOMOLE V6.4 2012, a Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, Since 2007. Available online: http://www.turbomole.com (accessed on 28 March 2017).
- Piotrowski, M.J.; Piquini, P.; Da Silva, J.L.F. Density functional theory investigation of 3d, 4d, and 5d 13-atom metal clusters. Phys. Rev. B 2010, 81, 155446. [Google Scholar] [CrossRef]
- Chaves, A.S.; Piotrowski, M.J.; Guedes-Sobrinho, D.; Da Silva, J.L.F. Theoretical Investigation of the Adsorption Properties of CO, NO, and OH on Monometallic and Bimetallic 13-Atom Clusters: The example of Cu13, Pt7Cu6, and Pt13. J. Phys. Chem. A 2015, 119, 11565–11573. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.J.; Piquini, P.; Zeng, Z.; Da Silva, J.L.F. Adsorption of NO on the Rh13, Pd13, Ir13, and Pt13 clusters: A density functional theory investigation. J. Phys. Chem. C 2012, 116, 20540–20549. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Wei, D.; Li, L.; Li, S. Unexpected odd-even oscillation in the enhanced chemical activities of the Run (n = 2–14) Nanoclusters for H2O adsorption and splitting. J. Phys. Chem. C 2017, 121, 7188–7198. [Google Scholar] [CrossRef]
- Piotrowski, M.J.; Piquini, P.; Cândido, L.; Da Silva, J.L.F. The role of electron localization in the atomic structure of transition-metal 13-atom clusters: the example of Co13, Rh13, and Hf13. Phys. Chem. Chem. Phys. 2011, 13, 17242–17248. [Google Scholar] [CrossRef] [PubMed]
- Beret, E.C.; Ghiringhelli, L.M.; Scheffler, M. Free gold clusters: Beyond the static, monostructure description. Faraday Discuss. 2011, 152, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Oezaslan, M.; Heggen, M.; Strasser, P. Size-dependent morphology of dealloyed bimetallic catalysts: Linking the nano to the macro scale. J. Am. Chem. Soc. 2012, 134, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.; Heimel, G.; Romaner, L.; Brédas, J.-L.; Zojer, E. First-principles study of the geometric and electronic structure of Au13 clusters: Importance of the prism motif. Phys. Rev. B 2008, 77, 165411. [Google Scholar] [CrossRef]
- Sun, J.; Xie, X.; Cao, B.; Duan, H. A density-functional theory study of Au13, Pt13, Au12Pt and Pt12Au clusters. Comput. Theor. Chem. 2017, 1107, 127–135. [Google Scholar] [CrossRef]
- Amft, M.; Johansson, B.; Skorodumova, N.V. Influence of the cluster dimensionality on the binding behavior of CO and O2 on Au13. J. Chem. Phys. 2012, 136, 24312. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dick, J.E.; Bard, A.J. Advanced electrochemistry of individual metal clusters electrodeposited atom by atom to nanometer by nanometer. Acc. Chem. Res. 2016, 49, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Castleman, A.W.; Khanna, S.N. Reactivity of metal clusters. Chem. Rev. 2016, 116, 14456–14492. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Zhou, N.; López-Puente, V.; Wang, Q.; Polavarapu, L.; Pastoriza-Santos, I.; Xu, Q.-H. Plasmon-enhanced light harvesting: Applications in enhanced photocatalysis, photodynamic therapy and photovoltaics. RCS Adv. 2015, 5, 29076–29097. [Google Scholar] [CrossRef]
- Di Pietro, P.; Strano, G.; Zuccarello, L.; Satriano, C. Gold and silver nanoparticles for applications in theranostics. Curr. Top. Med. Chem. 2016, 16, 3069–3102. [Google Scholar] [CrossRef]
- Kopp, M.; Kollenda, S.; Epple, M. Nanoparticle–protein interactions: Therapeutic approaches and supramolecular chemistry. Acc. Chem. Res. 2017, 50, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Liu, P.; Qin, R.; Fu, G.; Zheng, N. Surface coordination chemistry of metal nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Brinck, T.; Carlqvist, P.; Stenlid, J.H. The local electron attraction energy and its use for predicting nucleophilic reactions and halogen bonding. J. Phys. Chem. A 2016, 120, 10023–10032. [Google Scholar] [CrossRef] [PubMed]
- Stenlid, J.H.; Brinck, T. Nucleophilic aromatic substitution reactions described by the local electron attachment energy. J. Org. Chem. 2017, 82, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.F. On the mapping of electrostatic properties from Bragg diffraction data. Chem. Phys. Lett. 1979, 65, 335–342. [Google Scholar] [CrossRef]
- Politzer, P.; Truhlar, D.G. Chemical Applications of Atomic and Molecular Electrostatic Potentials; Plenum Press: Berlin, Germany, 1981. [Google Scholar]
- Janak, J.F. Proof that ∂E/∂ni = εi in density-functional theory. Phys. Rev. B 1978, 18, 7165–7168. [Google Scholar] [CrossRef]
- Ehresmann, B.; Martin, B.; Horn, A.H.C.; Clark, T. Local molecular properties and their use in predicting reactivity. J. Mol. Model. 2003, 9, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Clark, T. The local electron affinity for non-minimal basis sets. J. Mol. Model. 2010, 16, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Bulat, F.A. Average local ionization energy: A review. J. Mol. Model. 2010, 16, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, P.; Murray, J.S.; Brinck, T.; Politzer, P. Average local ionization energies on the molecular surfaces of aromatic systems as guides to chemical reactivity. Can. J. Chem. 1990, 68, 1440–1443. [Google Scholar] [CrossRef]
- Bulat, F.A.; Levy, M.; Politzer, P. Average local ionization energies in the Hartree−Fock and Kohn−Sham theories. J. Phys. Chem. A 2009, 113, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A “chain-of-spheres” algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XVIII. Constraints and stability in Hartree–Fock theory. J. Chem. Phys. 1977, 66, 3045–3050. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Stability analysis for solutions of the closed shell Kohn–Sham equation. J. Chem. Phys. 1996, 104, 9047–9052. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. The NBO 3.1 Program Manual; Theoretical Chemistry Institute and Department of Chemistry, University of Wisconsin: Madison, WI, USA, 1990. [Google Scholar]
- Kozuch, S. Should “anion–π interactions” be called “anion–σ interactions”? A revision of the origin of some hole-bonds and their nomenclature. Phys. Chem. Chem. Phys. 2016, 18, 30366–30369. [Google Scholar] [CrossRef] [PubMed]
- Böyükata, M.; Belchior, J.C. Structural and energetic analysis of copper clusters: MD study of Cun (n = 2–45). J. Braz. Chem. Soc. 2008, 19, 884–893. [Google Scholar] [CrossRef]
- Hu, Z.; Boyd, R.J. Structure sensitivity and cluster size convergence for formate adsorption on copper surfaces: A DFT cluster model study. J. Chem. Phys. 2000, 112, 9562–9568. [Google Scholar] [CrossRef]
- Itoh, M.; Kumar, V.; Adschiri, T.; Kawazoe, Y. Comprehensive study of sodium, copper, and silver clusters over a wide range of sizes 2 ≤ N ≤ 75. J. Chem. Phys. 2009, 131, 174510. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-C.; Jiang, L.; Pang, X.-Y.; Nakamura, J. Cluster and periodic DFT calculations: The adsorption of atomic nitrogen on M(111) (M = Cu, Ag, Au) surfaces. J. Phys. Chem. B 2005, 109, 17943–17950. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, J.; Palmer, R.E. Amorphous structures of Cu, Ag, and Au nanoclusters from first principles calculations. J. Chem. Phys. 2002, 117, 9548–9551. [Google Scholar] [CrossRef]
- Stenlid, J.H.; Johansson, A.J.; Kloo, L.; Brinck, T. Aqueous solvation and surface oxidation of the cu7 nanoparticle: Insights from theoretical modeling. J. Phys. Chem. C 2016, 120, 1977–1988. [Google Scholar] [CrossRef]
- Murray, J.; Lane, P.; Clark, T.; Riley, K.; Politzer, P. σ-Holes, π-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, L. Structures of platinum clusters: Planar or spherical? J. Phys. Chem. A 2004, 108, 8605–8614. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, L.; Hirata, Y.; Pawluk, T.; Wang, L. The simple cubic structure of Ir clusters and the element effect on cluster structures. Chem. Phys. Lett. 2004, 383, 67–71. [Google Scholar] [CrossRef]
- Du, J.; Sun, X.; Chen, J.; Jiang, G. A Theoretical study on small iridium clusters: Structural evolution, electronic and magnetic properties, and reactivity predictors. J. Phys. Chem. A 2010, 114, 12825–12833. [Google Scholar] [CrossRef] [PubMed]
- Reiher, M.; Salomon, O.; Hess, B.A. Reparameterization of hybrid functionals based on energy differences of states of different multiplicity. Theor. Chem. Acc. 2001, 107, 48–55. [Google Scholar] [CrossRef]
- Slimani, A.; Yu, X.; Muraoka, A.; Boukheddaden, K.; Yamashita, K. Reparametrization approach of DFT functionals based on the equilibrium temperature of spin-crossover compounds. J. Phys. Chem. A 2014, 118, 9005–9012. [Google Scholar] [CrossRef] [PubMed]
- Young, D.C. Spin Contamination. In Computational Chemistry; John Wiley & Sons, Inc.: San Francisco, CA, USA, 2001; pp. 227–230. [Google Scholar]
- Chaves, A.S.; Piotrowski, M.J.; Da Silva, J.L.F. Evolution of the structural, energetic, and electronic properties of the 3d, 4d, and 5d transition-metal clusters (30 TMn systems for n = 2–15): A density functional theory investigation. Phys. Chem. Chem. Phys. 2017, 19, 15484–15502. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.L.F.; Piotrowski, M.J.; Aguilera-Granja, F. Hybrid density functional study of small Rhn n = 2–15 clusters. Phys. Rev. B 2012, 86, 125430. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Jensen, F.; Dorigo, A.; Houk, K.N. A spin correction procedure for unrestricted Hartree-Fock and Møller-Plesset wavefunctions for singlet diradicals and polyradicals. Chem. Phys. Lett. 1988, 149, 537–542. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Okumura, M.; Mori, W.; Maki, J.; Takada, K.; Noro, T.; Tanaka, K. Comparison between spin restricted and unrestricted post-Hartree–Fock calculations of effective exchange integrals in Ising and Heisenberg models. Chem. Phys. Lett. 1993, 210, 201–210. [Google Scholar] [CrossRef]
- Yasuda, N.; Kitagawa, Y.; Hatake, H.; Saito, T.; Kataoka, Y.; Matsui, T.; Kawakami, T.; Yamanaka, S.; Okumura, M.; Yamaguchi, K. Approximate spin projection for geometry optimization of biradical systems: Case studies of through-space and through-bond systems. In Quantum Systems in Chemistry and Physics; Springer: Dordrecht, The Netherlands, 2012; pp. 345–359. [Google Scholar]
- Heisenberg, W. Zur Theorie des Ferromagnetismus. Z. Phys. 1928, 49, 619–636. [Google Scholar] [CrossRef]
- Kambe, K. Paramagnetic susceptibilities of some polynuclear complex salts. J. Phys. Soc. Jpn. 1950, 5, 48–51. [Google Scholar] [CrossRef]


| Sym | (2S + 1) | AS | VS | ES | s-occ 1 | d-occ 1 | p-occ 1 | |
|---|---|---|---|---|---|---|---|---|
| Au13 | Cs | 2 | 10 | 10 | 9 | 0.73 | 9.90 | 0.38 |
| Cu13 | C2 | 2 | 7 | 6 | 6 | 0.63 | 9.94 | 0.44 |
| Pt13 | Cs | 3 | 7 | 15 | 15 | 0.61 | 9.08 | 0.32 |
| Pt7Cu6 | C1 | 3 | 13 | 12 | 11 | 0.56 2 | 9.56 2 | 0.34 2 |
| Pd13 | C2 | 9 | 7 | 7 | 8 | 0.37 | 9.34 | 0.29 |
| Co13 | C3 | 28 | 4 | 4 | 3 | 0.65 | 7.82 | 0.52 |
| Rh13 | Cs | 2 3 | 7 | 7 | 14 | 0.43 | 8.30 | 0.27 |
| Ir13 | Cs | 4 | 10 | 17 | 15 | 0.67 | 7.97 | 0.37 |
| Ru13 | Cs | 13 | 7 | 6 | 9 | 0.46 | 7.22 | 0.34 |
| ASfav | VS,max | σtype | ES,min | ΔEint | dTM-O | dTM-σ | dσ-O | |
|---|---|---|---|---|---|---|---|---|
| Au13 | 13 | 16.80 | σs | −8.88 | −0.37 | 2.44 | 2.20 | 0.48 |
| Cu13 | 1(2) | 19.55 | σs | −6.53 | −0.54 | 2.14 | 2.02 | 0.56 |
| Pt13 | 12 | 20.12 | σd | −11.93 | −0.80 | 2.22 | 2.13 | 0.13 |
| Pt7Cu6 | 1 1 | 35.38 | σs | −10.88 | −0.73 | 2.09 | 1.91 | 0.23 |
| Pd13 | 8(11) 2 | 13.31 | σs | −5.14 | −0.59 | 2.28 | 2.16 | 1.35 |
| Co13 | 8 3 | 21.65 | σs | −5.64 | −0.48 | 2.13 | 2.06 | 0.90 |
| Rh13 | 1(7) | 20.43 | σd | −8.74 | −0.81 | 2.23 | 2.13 | 0.27 |
| Ir13 | 6 | 33.75 | σs | −12.86 | −1.00 | 2.17 | 2.18 | 0.33 |
| Ru13 | 3(1) 4 | 29.16 | σs | −12.11 | −0.92 | 2.22 | 2.07 | 0.96 |
| ΔEint,ave | dTM-O,ave | dTM-σ,ave | dσ-O,ave | R2V | R2E | |
|---|---|---|---|---|---|---|
| Au13 | −0.29 | 2.57 | 2.21 | 0.83 | 0.84 | 0.81 |
| Cu13 | −0.47 | 2.19 | 2.06 | 0.43 | 0.85 | 0.95 |
| Pt13 | −0.47 | 2.38 | 2.20 | 0.46 | 0.67 1 | 0.82 1 |
| Pt7Cu6 | −0.47 2 | 2.30 2 | 2.09 | 0.57 2 | 0.92 2 | 0.65 2 |
| Pd13 | −0.45 | 2.34 | 2.15 | 0.82 | 0.58 | 0.25 |
| Co13 | −0.470 | 2.15 | 2.10 | 0.61 | n.a. 3 | n.a. 3 |
| Rh13 | −0.64 | 2.29 | 2.15 | 0.37 | 0.19 | 0.18 |
| Ir13 | −0.59 | 2.41 | 2.28 | 0.45 | 0.28 (0.90) 4 | 0.09 (0.75) 4 |
| Ru13 | −0.64 | 2.25 | 2.17 | 0.91 | 0.03 | 0.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenlid, J.H.; Johansson, A.J.; Brinck, T. σ-Holes on Transition Metal Nanoclusters and Their Influence on the Local Lewis Acidity. Crystals 2017, 7, 222. https://doi.org/10.3390/cryst7070222
Stenlid JH, Johansson AJ, Brinck T. σ-Holes on Transition Metal Nanoclusters and Their Influence on the Local Lewis Acidity. Crystals. 2017; 7(7):222. https://doi.org/10.3390/cryst7070222
Chicago/Turabian StyleStenlid, Joakim H., Adam Johannes Johansson, and Tore Brinck. 2017. "σ-Holes on Transition Metal Nanoclusters and Their Influence on the Local Lewis Acidity" Crystals 7, no. 7: 222. https://doi.org/10.3390/cryst7070222