Crystallization under an External Electric Field: A Case Study of Glucose Isomerase
Abstract
:1. Introduction
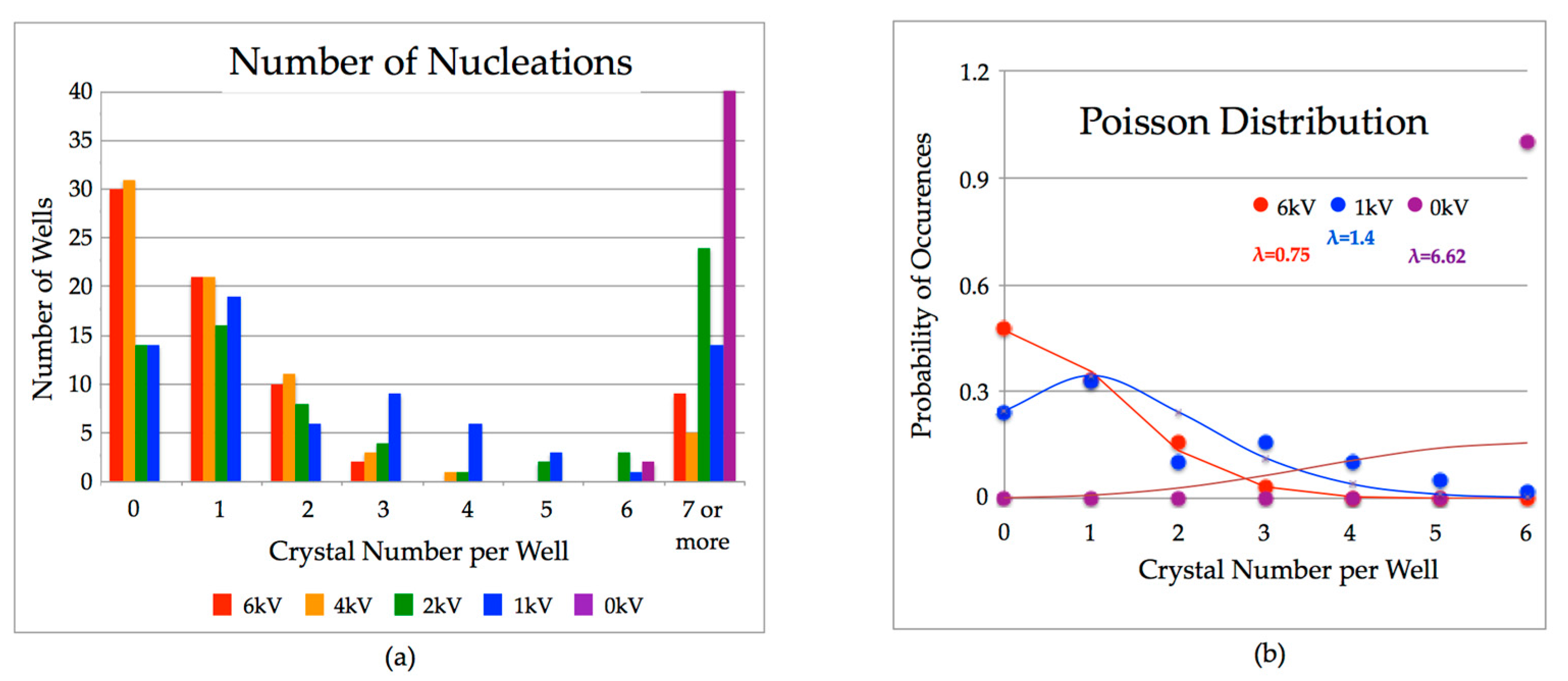
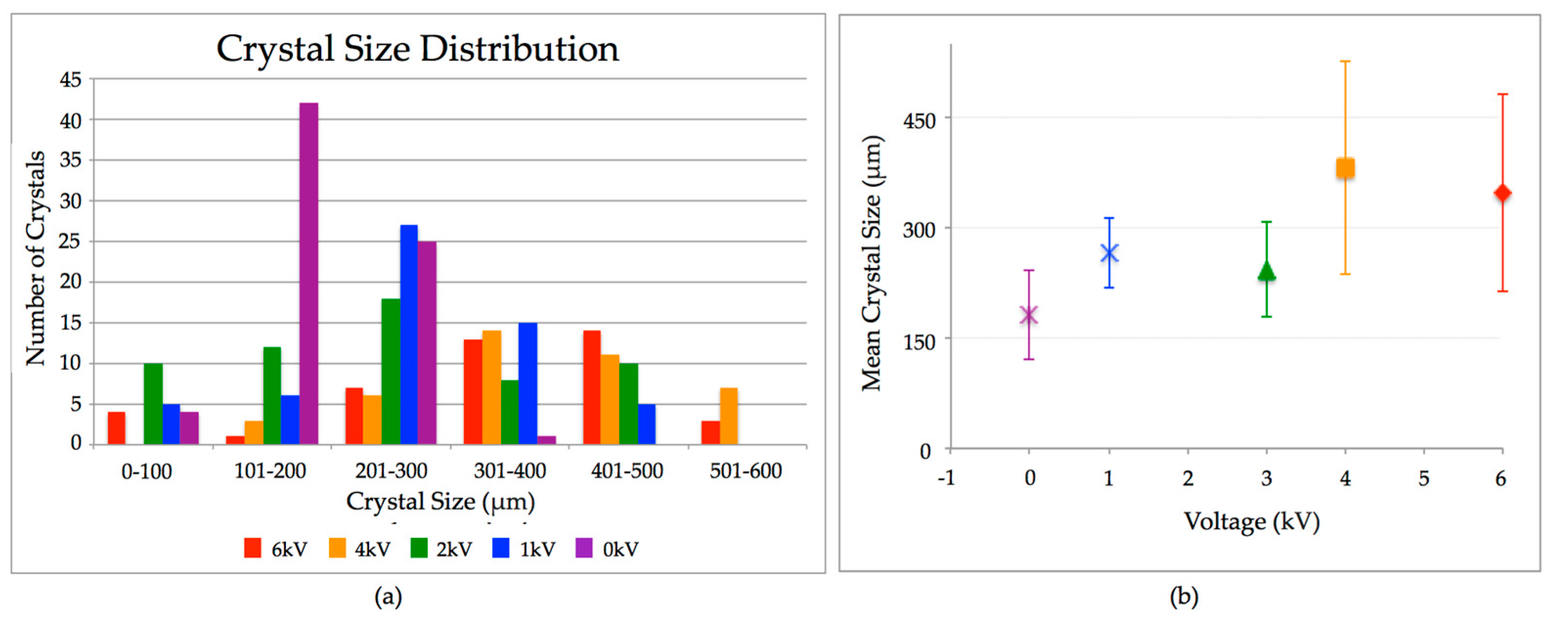
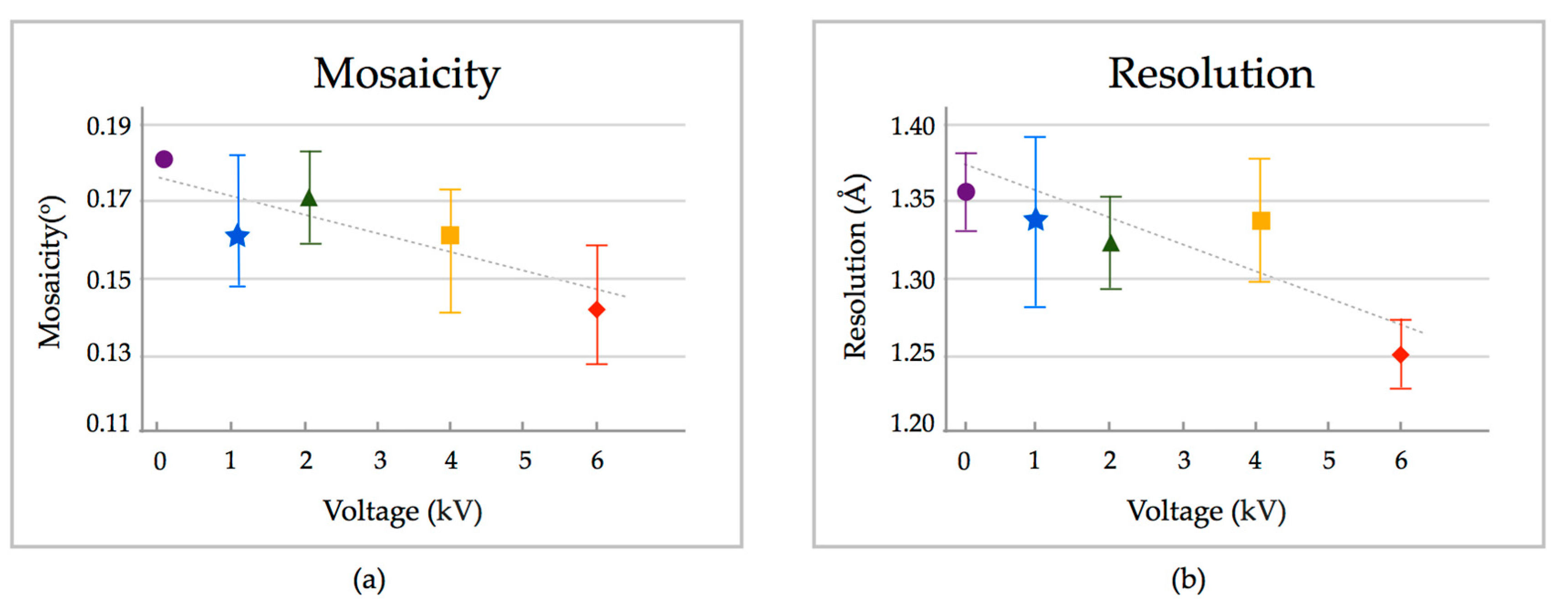
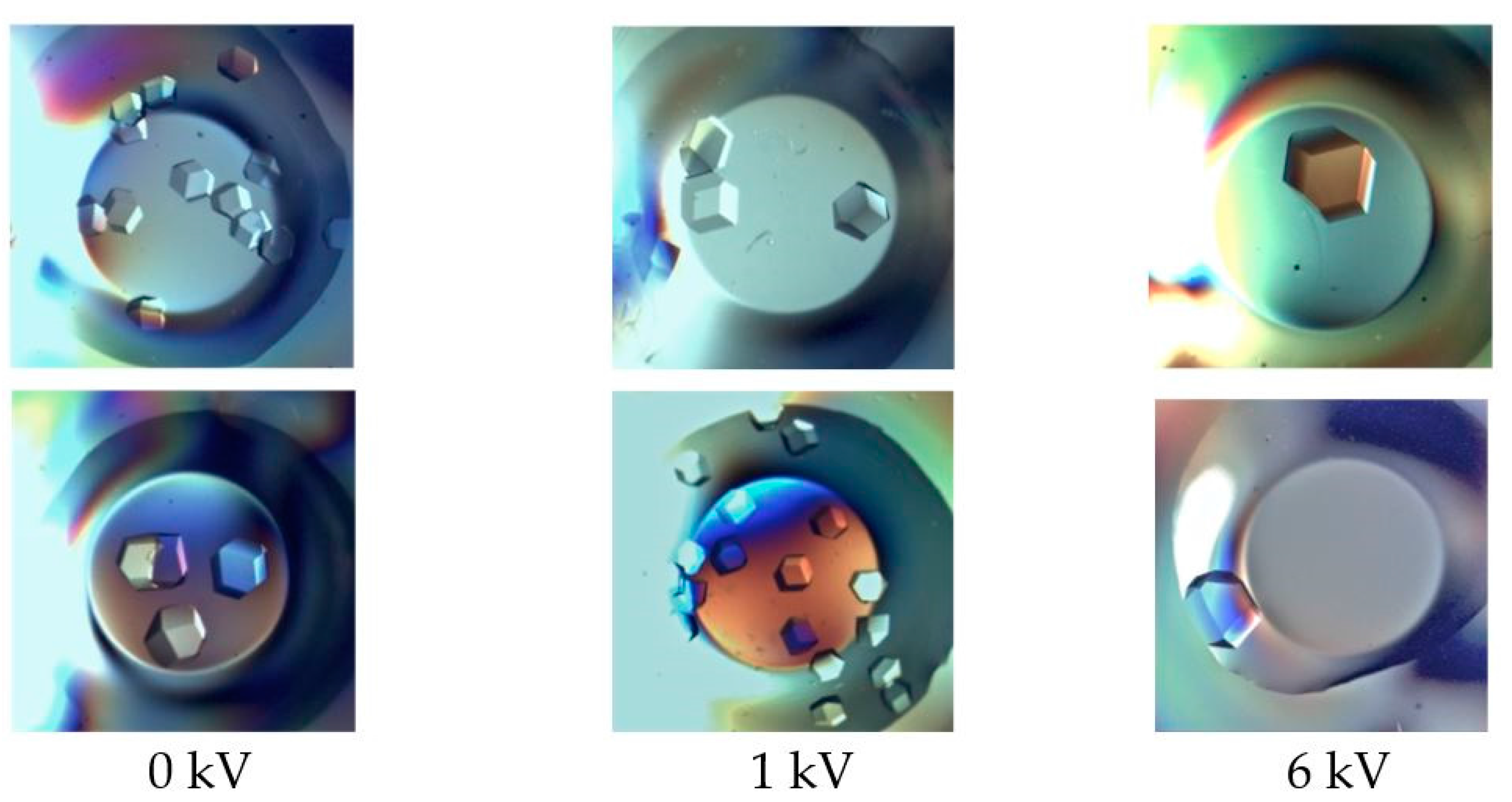
2. Results
3. Discussion
4. Materials and Methods
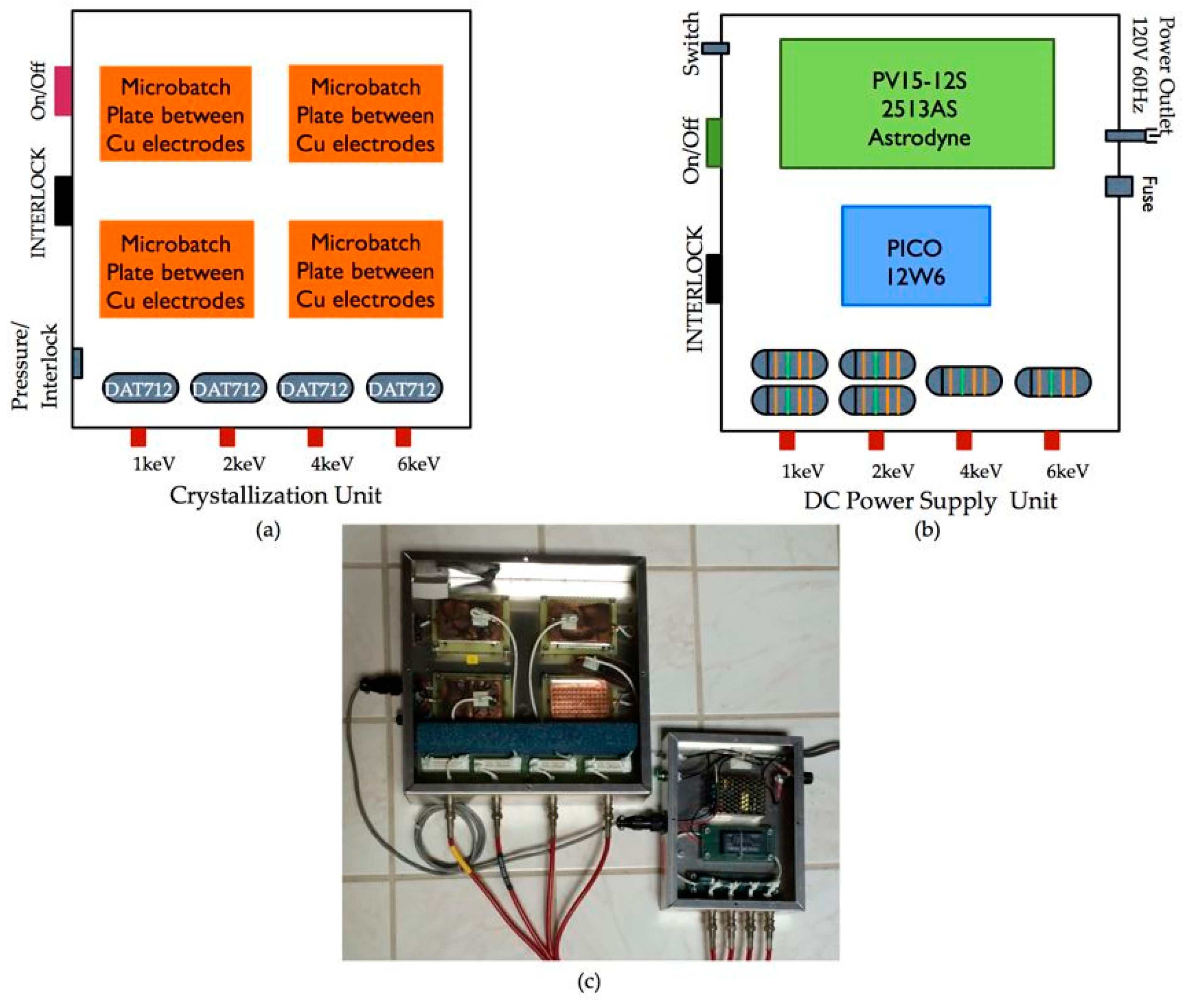
4.1. Electric Field Crystallization Device—Efield Microbatch
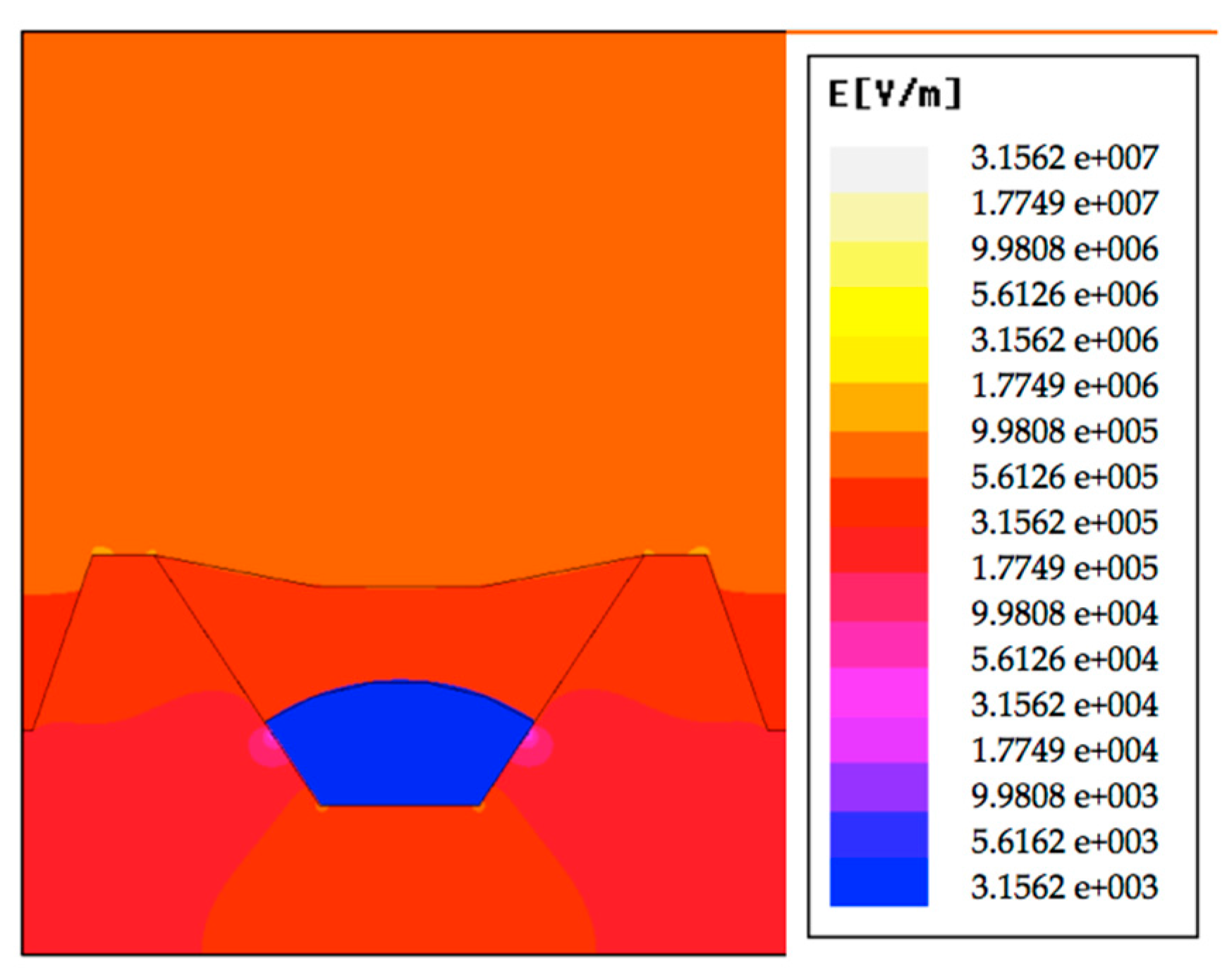
4.2. Electric Field Simulation
4.3. Crystallization Conditions in Electric Fields
4.4. Crystal Quality Assessment
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McPherson, A. Crystallization of Biological Macromolecules; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1999; 402p. [Google Scholar]
- Chayen, N.E.; Helliwell, J.R., Jr.; Snell, E.H. Macromolecular Crystallization and Crystal Perfection. In IUCr Monographs on Crystallography 24; Oxford University Press: Oxford, UK, 2010; 21p., ISBN 978-0-19-921325-2. [Google Scholar]
- Allegre, C.J.; Provost, A.; Jaupart, C. Oscillatory zoning: A pathological case of crystal growth. Nature 1981, 294, 223–228. [Google Scholar] [CrossRef]
- Vergara, A.; Lorber, B.; Zagari, A.; Giege, R. Physical aspects of protein crystal growth investigated with the Advanced Protein Crystallization Facility in reduced-gravity environments. Acta Crystallogr. D 2003, 59, 2–15. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.; DeLuca, L.J. Microgravity protein crystallization. NPJ Microgravity 2015, 1, 15010. [Google Scholar] [CrossRef]
- Petsev, D.N.; Thomas, B.R.; Yau, S.; Vekilov, P.G. Interactions and aggregation of apoferritin molecules in solution: Effects of added electrolytes. Biophys. J. 2000, 78, 2060–2069. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.M.; Moreno, A.; Viedma, C.; Coil, M. Crystal quality of lysozyme single crystas grown by the gel acupuncture method. Mater. Res. Bull. 1993, 28, 541–546. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.M. Counterdiffusion Methods for Macromolecular Crystallization. Methods Enzimol. 2003, 368, 130–154. [Google Scholar] [CrossRef]
- Rummel, G.; Hardmeyer, A.; Widmer, C.; Chiu, M.L.; Nollert, P.; Locher, K.P.; Pedruzzi, I.; Landau, E.M.; Rosenbusch, J.P. Lipidic Cubic Phases: New Matrices for the three-dimensional crystallization of membrane proteins. J. Struct. Biol. 1998, 121, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble porteins and complexes. Acta Crystallogr. F 2015, 71, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Chayen, N.E. A novel technique for containerless protein crystallization. Protein Eng. Des. Sel. 1996, 9, 927–929. [Google Scholar] [CrossRef]
- Govada, L.; Leese, H.S.; Saridakis, E.; Kassen, S.; Chain, B.; Khurshid, S.; Menzel, R.; Hu, S.; Shaffer, M.S.P.; Chayen, N.E. Exploring Carbon Nanomaterial Diversity for Nucleation of Protein Crystals. Sci. Rep. 2016, 6, 20053. [Google Scholar] [CrossRef] [PubMed]
- Bergfors, T. Screening and optimization methods for nonautomated crystallization laboratories. Methods Mol. Biol. 2007, 363, 131–151. [Google Scholar] [PubMed]
- Bergfords, T. Seeds to crystals. J. Struct. Biol. 2003, 142, 66–76. [Google Scholar] [CrossRef]
- Wakayama, N.I. Effects of a Strong Magnetic Field on Protein Crystal Growth. Cryst. Growth Des. 2003, 3, 17–24. [Google Scholar] [CrossRef]
- Surade, S.; Ochi, T.; Nietlispach, D.; Chirgadze, D.; Moreno, A. Investigations into Protein Crystallization in the Presence of a Strong Magnetic Field. Cryst. Growth Des. 2010, 10, 691–699. [Google Scholar] [CrossRef]
- Moreno, A.; Yokaichiya, F.; Dimasi, E.; Stojanoff, V. Growth and Characterization of high-quality protein crystals for X-ray crystallography. Ann. N. Y. Acad. Sci. 2009, 1161, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Hammadi, Z.; Veesler, S. New approaches on crystallization under electric fields. Prog. Biophys. Mol. Biol. 2009, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Rivera, C.; Cuéllar-Cruz, M.; Esturau-Escofet, N.; Demitri, N.; Polentarutti, M.; Stojanoff, V.; Moreno, A. Recent Advances in the Understanding of the Influence of Electric and Magnetic Fields on Protein Crystal Growth. Cryst. Growth Des. 2017, 17, 135–145. [Google Scholar] [CrossRef]
- Chin, C.C.; Dence, J.B.; Warren, J.C. Crystallization of human placental estradiol 17beta-dehydrogenase. A new method for crystallizing labile enzymes. J. Biol. Chem. 1976, 251, 3700–3705. [Google Scholar] [PubMed]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeot, J.P.; Capelle, B.; Aubry, A. Crystallization of proteins under an external electric field. J. Cryst. Growth 1999, 200, 575–582. [Google Scholar] [CrossRef]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeat, J.P.; Aubry, A. Equilibrium kinetics of lysozyme crystallization under an external electric field. J. Cryst. Growth 2001, 232, 250–255. [Google Scholar] [CrossRef]
- Ries-Kautt, M.; Ducruix, A. From solution to crystals with a physico-chemical aspect. In Crystallization of Nucleic Acids and Proteins: A Practical Approach, 2nd ed.; Ducruix, A., Giégé, R., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 269–312. [Google Scholar]
- Nanev, C.N.; Penkova, A. Nucleation of lysozyme crystals under external electric, ultrasonic fields. J. Cryst. Growth 2001, 232, 285–293. [Google Scholar] [CrossRef]
- Nanev, C.N.; Penkova, A. Nucleation and growth of lysozyme crystals under external electric field. Colloids Surf. A Physicochem. Eng. Asp. 2002, 209, 139–145. [Google Scholar] [CrossRef]
- Garetz, B.A.; Matic, J.; Myerson, A.S. Polarization Switching of Crystal Structure in the Nonphotochemical Light-Induced Nucleation of Supersaturated Aqueous Glycine Solutions. Phys. Rev. Lett. 2002, 89, 175501. [Google Scholar] [CrossRef] [PubMed]
- Penkova, A.; Gliko, O.; Dimitrov, I.L.; Hodjaoglu, F.V.; Nanev, C.; Vekilov, P.G. Enhancement and suppression of protein crystal nucleation due to electrically driven convection. J. Cryst. Growth 2005, 275, e1527–e1532. [Google Scholar] [CrossRef]
- Mirkin, N.; Frontana-Uribe, B.A.; Rodriguez-Romero, A.; Hernandez-Santoyo, A.; Moreno, A. The influence of an internal electric field upon protein crystallization using the gel-acupuncture method. Acta Crystallogr. 2003, D59, 1533–1538. [Google Scholar] [CrossRef]
- Moreno, A.; Sazaki, G. The use of a new ad hoc growth cell with parallel electrodes for the nucleation control of lysozyme. J. Cryst. Growth 2004, 264, 438–444. [Google Scholar] [CrossRef]
- Nieto-Mendoza, E.; Frontana-Uribe, B.A.; Sazaki, G.; Moreno, A. Investigations on electromigration phenomena for protein crystallization using crystal growth cells with multiple electrodes: Effect of the potential control. J. Cryst. Growth 2005, 275, e1437–e1446. [Google Scholar] [CrossRef]
- Gil-Alvaradejo, G.; Ruiz-Arellano, R.R.; Owen, C.; Rodriguez-Romero, A.; Rudino-Pinera, E.; Antwi, M.K.; Stojanoff, V.; Moreno, A. Novel Protein Crystal Growth Electrochemical Cell for Applications in X-ray Diffraction and Atomic Force Microscopy. Cryst. Growth Des. 2011, 11, 3917–3922. [Google Scholar] [CrossRef]
- Flores-Hernandez, E.; Stojanoff, V.; Arreguin-Espinosa, R.; Moreno, A.; Sanchez-Puig, N. An electrically assisted device for protein crystallization in a vapor-diffusion setup. J. Appl. Crystallogr. 2013, 46, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Caballero, S.; Cuellar-Cruz, M.; Demitri, N.; Polentarutti, M.; Rodriguez-Romero, A.; Moreno, A. Glucose Isomerase Polymorphs Obtained Using an Ad Hoc Protein Crystallization Temperature Device and a Growth Cell Applying an Electric Field. Cryst. Growth Des. 2016, 16, 1679–1686. [Google Scholar] [CrossRef]
- Koizumi, H.; Fujiwara, K.; Uda, S. Role of electric double layer in controlling the nucleation rate for tetragonal hen egg white lysozyme crystals by application of an external electric field. Cryst. Growth Des. 2010, 10, 2591–2595. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Improvement of crystal quality for tetragonal hen egg white lysozyme crystals under application of an external alternating current electric field. J. Appl. Crystallogr. 2013, 46, 25–29. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Crystallization of high-quality protein crystals using an external electric field. J. Appl. Crystallogr. 2015, 48, 1507–1513. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Technique for High-Quality Protein Crystal Growth by Control of Subgrain Formation under an External Electric Field. Crystals 2016, 6, 95. [Google Scholar] [CrossRef]
- Chayen, N.E.; Shaw Stewart, P.D.; Blow, D.M. Microbatch crystallization under oil—A new technique allowing many small-volume crystallization trials. J. Cryst. Growth 1992, 122, 176–180. [Google Scholar] [CrossRef]
- Chayen, N.E.; Shaw Stewart, P.D.; Maeder, D.L.; Blow, D.M. An automated system for micro-batch protein crystallization and screening. J. Appl. Cryst. 1990, 23, 297–302. [Google Scholar] [CrossRef]
- Stojanoff, V. Crystal quality a quest for structural proteomics. In Synchrotron Radiation and Structural Proteomics; Riekel, C., Ed.; Pan Stanford Publishing: Temasek Boulevard, Singapore, 2011; pp. 383–407. [Google Scholar]
- Felder, C.F.; Prilusky, J.; Silman, I.; Sussman, J.L. A server and database for dipole moments of proteins. Nucleic Acids Res. 2007, 35, W512–W521. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtand, K. Features and development of Coot. Acta Crystallogr. 2010, D66, 486–501. [Google Scholar] [CrossRef]
- Saban, K.; Thomas, J.; Varughese, P.A.; Varghese, G. Thermodynamics of Crystal Nucleation in an External Electric Field. Cryst. Res. Technol. 2002, 37, 1188–1199. [Google Scholar] [CrossRef]
- Warshavsky, V.B.; Bykov, T.V.; Zeng, X.C. Effects of external electric field on the interfacial properties of weakly dipolar fluid. J. Chem. Phys. 2001, 114, 504–512. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Method Enzymol. 1997, 276, 307–362. [Google Scholar]
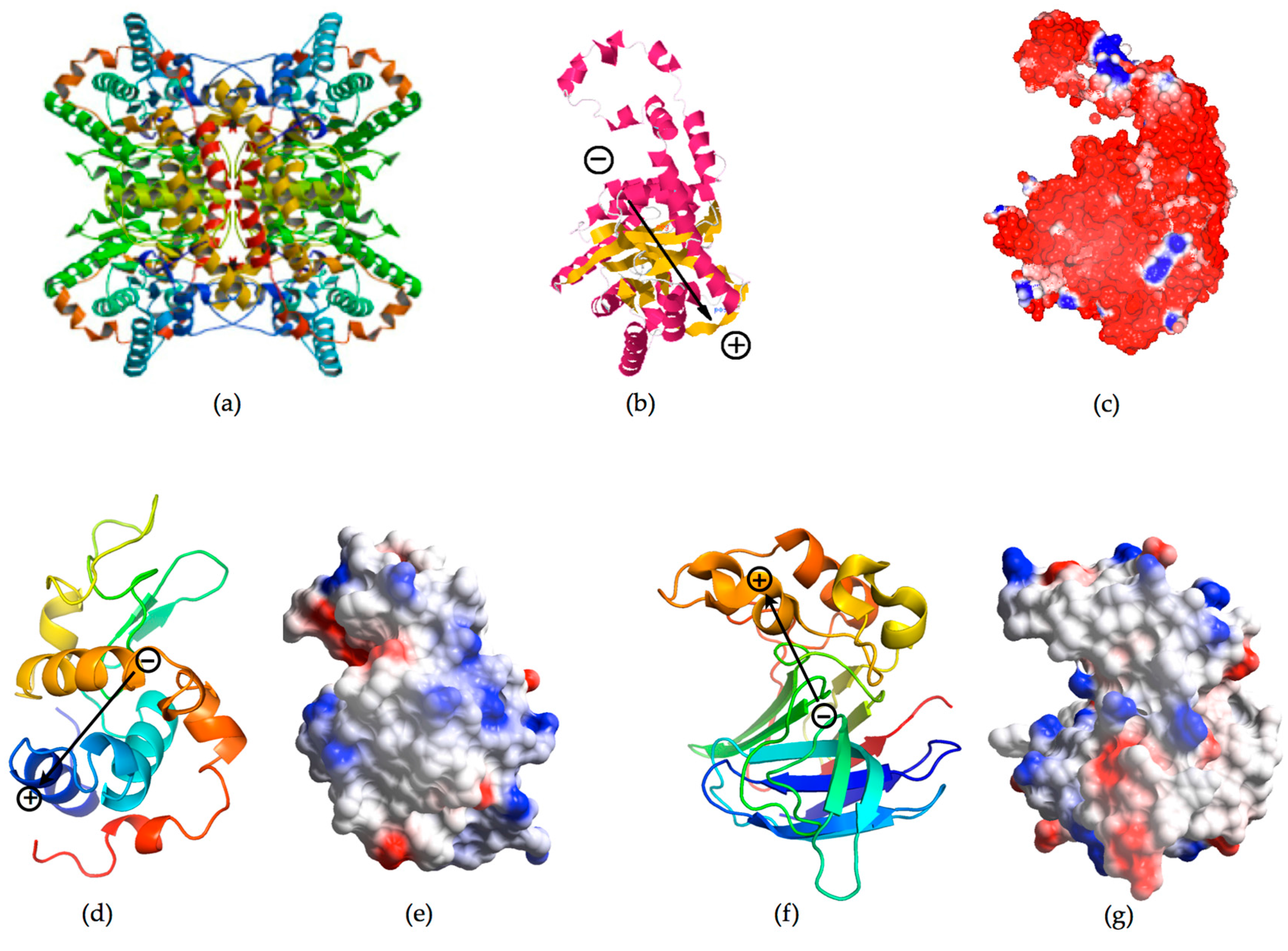
| GI | HEWL | Thaumatin | HEHH | Ferritin | |
|---|---|---|---|---|---|
| PDB ID | 4A8L | 193L | 1RQW | 1QYW | 3F32 |
| dipole moment (D) | 1045 | 198 | 478 | 770 | 529 |
| # of atoms | 3187 | 1012 | 1604 | 2123 | 1411 |
| # of residues | 386 | 129 | 207 | 276 | 168 |
| # of positive residues | 46 | 16 | 24 | 29 | 19 |
| # of negative residues | 65 | 9 | 19 | 30 | 26 |
| charge | −19 | 8 | 5 | −1 | −7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubin, E.; Owen, C.; Stojanoff, V. Crystallization under an External Electric Field: A Case Study of Glucose Isomerase. Crystals 2017, 7, 206. https://doi.org/10.3390/cryst7070206
Rubin E, Owen C, Stojanoff V. Crystallization under an External Electric Field: A Case Study of Glucose Isomerase. Crystals. 2017; 7(7):206. https://doi.org/10.3390/cryst7070206
Chicago/Turabian StyleRubin, Evgeniya, Christopher Owen, and Vivian Stojanoff. 2017. "Crystallization under an External Electric Field: A Case Study of Glucose Isomerase" Crystals 7, no. 7: 206. https://doi.org/10.3390/cryst7070206





