Synthesis and Characterization of a New Cobaloxime-Terpyridine Compound
Abstract
:1. Introduction
2. Results and Discussion
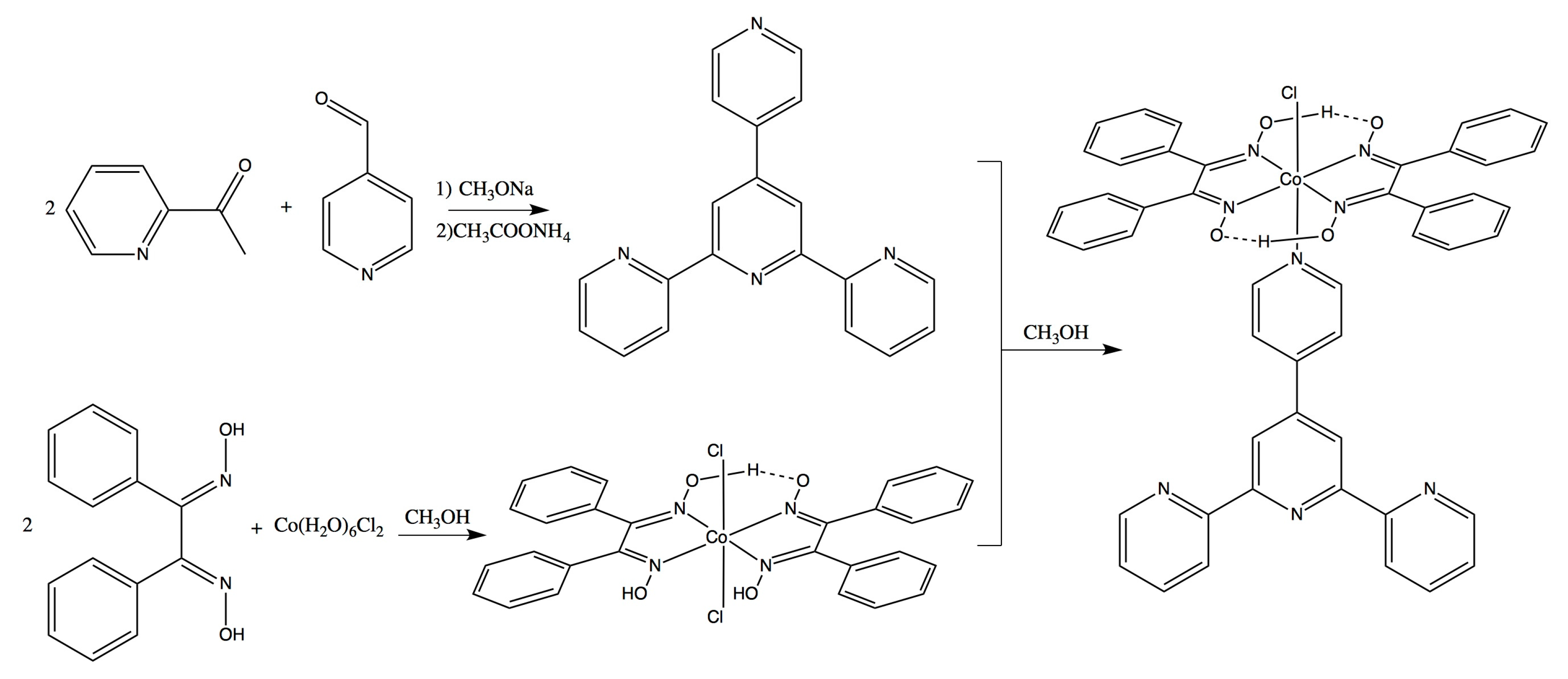
2.1. Synthesis
2.2. 1H-NMR
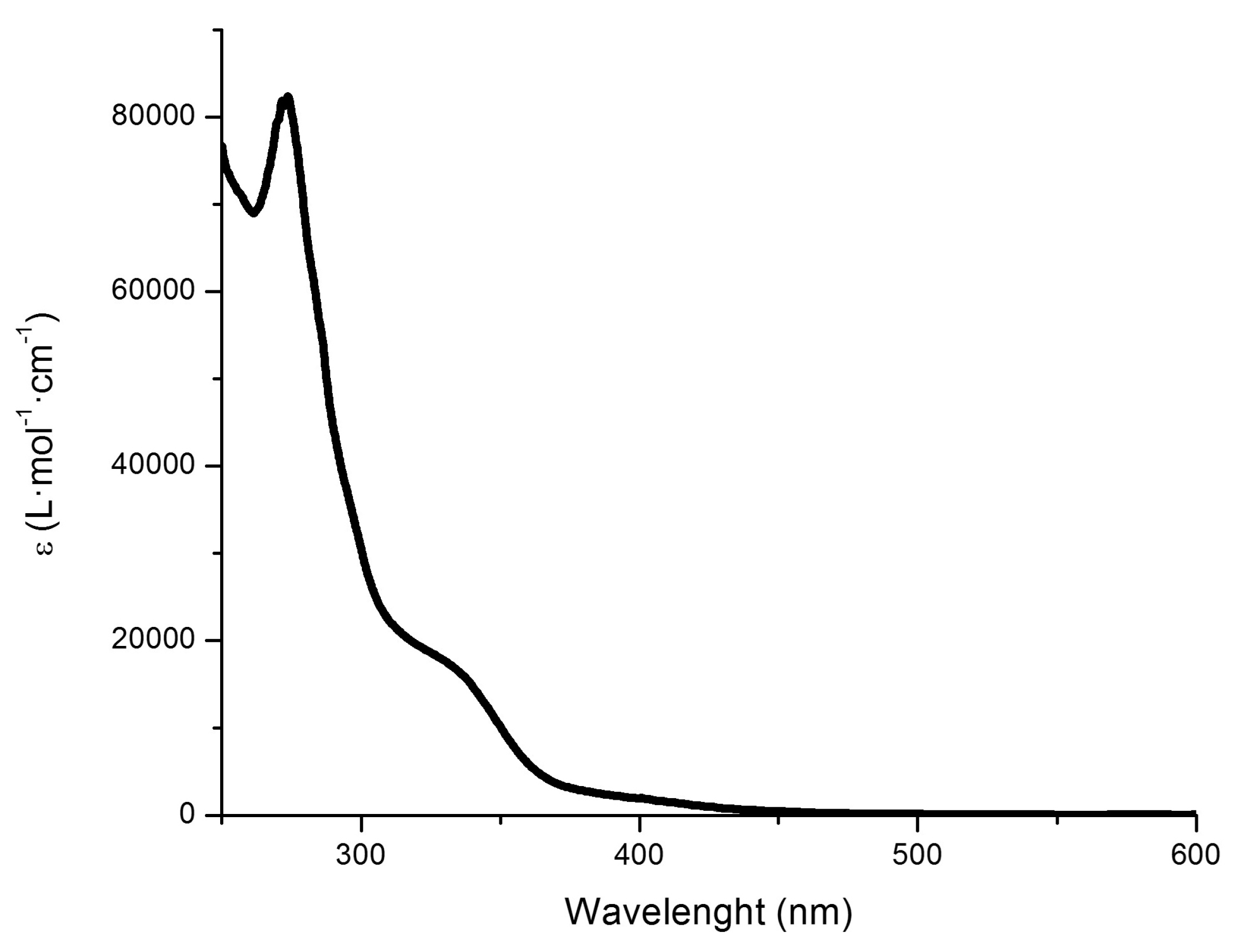
2.3. UV–Vis Spectroscopy
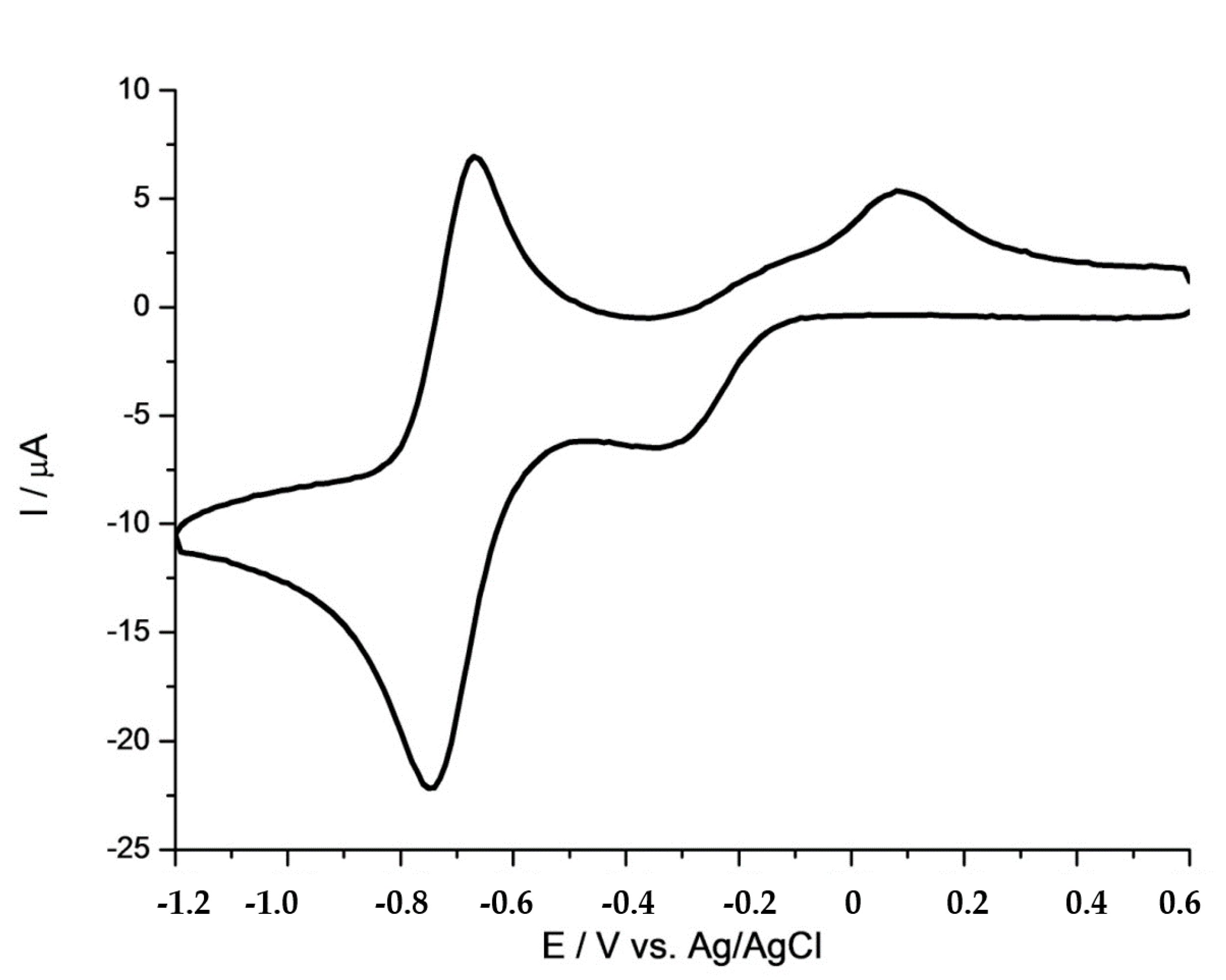
2.4. Electrochemistry
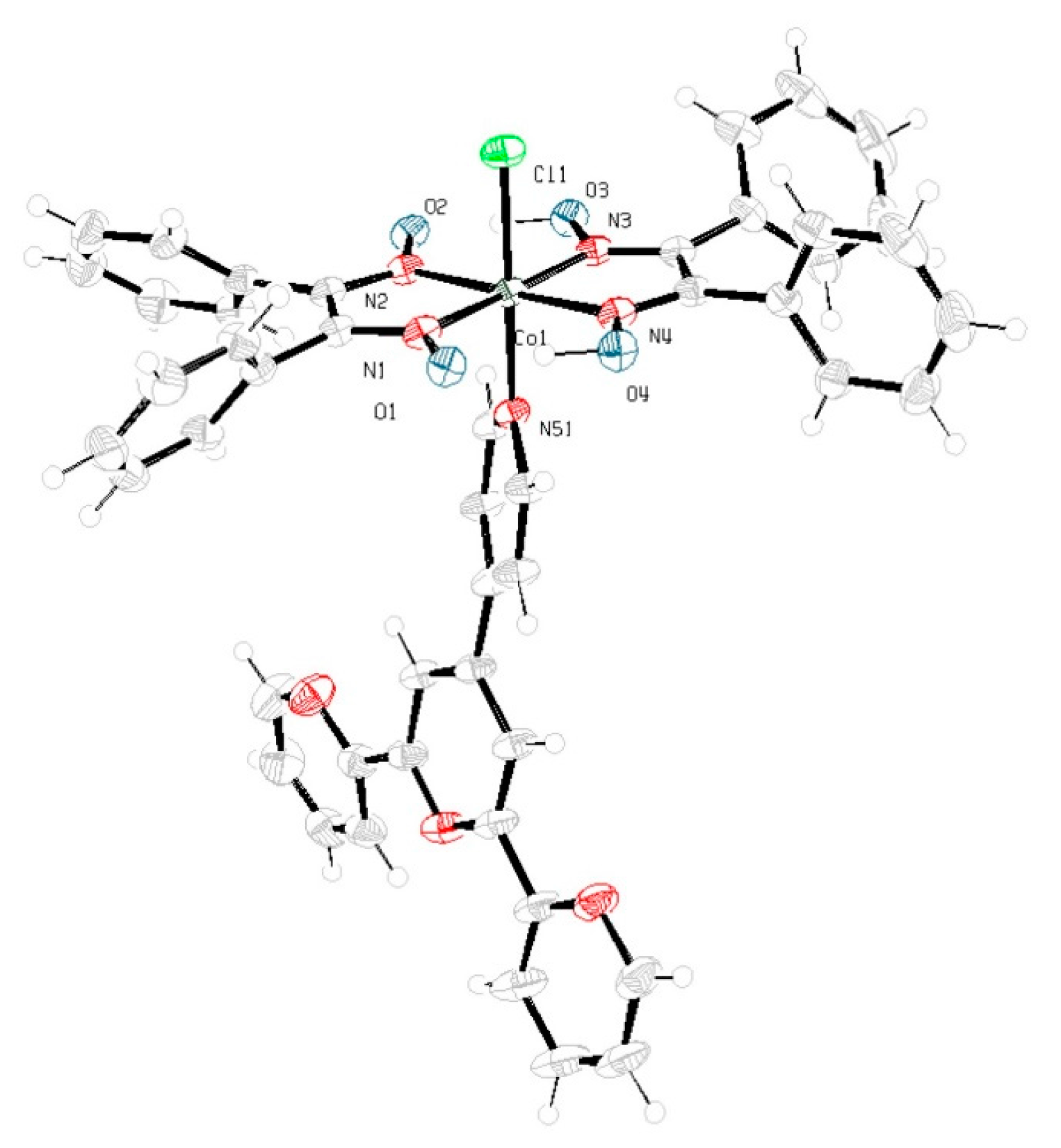
2.5. X-ray Crystallography
3. Materials and Methods
3.1. Apparatus and Reagents
3.2. 4′-(4′-Pyridyl)-2,2′:6′,2″-Terpyridine (Pytpy)
3.3. Synthesis of [CoCl(dpgH)2(Pytpy)]
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schrauzer, G. Organocobalt Chemistry of Vitamin B12 Model Compouns (Cobaloximes). Acc. Chem. Res. 1968, 1, 97–103. [Google Scholar] [CrossRef]
- Losse, S.; Vos, J.G.; Rau, S. Catalytic hydrogen production at cobalt centres. Coord. Chem. Rev. 2010, 254, 2492–2504. [Google Scholar] [CrossRef]
- Dempsey, J.L.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Hydrogen evolution catalyzed by cobaloximes. Acc. Chem. Res. 2009, 42, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.-A.V.; Artero, J.; Pécaut, M. Fontecave, Cobalt and nickel diimine-dioxime complexes as molecular electrocatalysts for hydrogen evolution with low overvoltages. Proc. Natl. Acad. Sci. USA 2009, 106, 20627–20632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-K.; Liu, J.-H. DFT studies of cobalt hydride intermediate on cobaloxime-catalyzed H2 evolution pathways. Int. J. Quantum Chem. 2012, 112, 2541–2546. [Google Scholar] [CrossRef]
- Solis, B.H.; Hammes-Schiffer, S. Substituent effects on cobalt diglyoxime catalysts for hydrogen evolution. J. Am. Chem. Soc. 2011, 133, 19036–19039. [Google Scholar] [CrossRef] [PubMed]
- Baffert, C.; Artero, V.; Fontecave, M. Cobaloximes as functional models for hydrogenases. 2. Proton electroreduction catalyzed by difluoroborylbis(dimethylglyoximato)cobalt(II) complexes in organic media. Inorg. Chem. 2007, 46, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mulfort, K.L.; Du, P.; Chen, L.X. Photodriven charge separation dynamics in CdSe/ZnS core/shell quantum dot/cobaloxime hybrid for efficient hydrogen production. J. Am. Chem. Soc. 2012, 134, 16472–16475. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Artero, V.; Razavet, M.; Baffert, C.; Leibl, W.; Fontecave, M. Cobaloxime-based photocatalytic devices for hydrogen production. Angew. Chem. Int. Ed. 2008, 47, 564–567. [Google Scholar] [CrossRef] [PubMed]
- McCormick, T.M.; Han, Z.; Weinberg, D.J.; Brennessel, W.W.; Holland, P.L.; Eisenberg, R. Impact of ligand exchange in hydrogen production from cobaloxime-containing photocatalytic systems. Inorg. Chem. 2011, 50, 10660–10666. [Google Scholar] [CrossRef] [PubMed]
- Razavet, M.; Artero, V.; Fontecave, M. Proton electroreduction catalyzed by cobaloximes: Functional models for hydrogenases. Inorg. Chem. 2005, 44, 4786–4795. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Thompson, A.M.W.C. Ligand reactivity in iron(II) complexes of 4′-(4‴-pyridyl)-2,2′: 6′,2″-terpyridine. J. Chem. Soc. Dalt. Trans. 1992, 2947–2950. [Google Scholar] [CrossRef]
- Toscano, P.J.; Lettko, L.; Schermerhorn, E.J.; Waechter, J.; Shufon, K.; Liu, S.; Dikarev, E.V.; Zubieta, J. Synthesis and characterization of diphenylglyoximato cobalt(III) complexes. The molecular structures of trans-bis(diphenylglyoximato)(alkyl)(pyridine)cobalt(III), with alkyl=CH2SiMe3, CH2CMe3 and CF3. Polyhedron 2003, 22, 2809–2820. [Google Scholar] [CrossRef]

| Compound | Epc CoIII/II (V) | E1/2 CoII/I (V) |
|---|---|---|
| [CoCl(dpgH)2(py)] # | −0.43 | −0.71 |
| [CoCl(dpgH)2(pytpy)] | −0.32 | −0.72 |
| Bond Length (Å) | Bond Angles (°) | ||
|---|---|---|---|
| Co1-N1 | 1.8930 | N1-Co1-N2 | 81.880 |
| Co1-N2 | 1.8866 | N2-Co1-N3 | 98.672 |
| Co1-N3 | 1.8995 | N3-O3-H1 | 101.476 |
| Co1-N4 | 1.8945 | N1-C1-C2 | 112.681 |
| Co1-N5 | 1.9640 | N2-C2-C1 | 112.563 |
| Co1-Cl1 | 2.2236 | N3-C3-C4 | 111.986 |
| N1-C1 | 1.3030 | N4-C4-C3 | 112.946 |
| N2-C2 | 1.3050 | Cl1-Co1-N1 | 89.776 |
| N3-C3 | 1.2980 | N1-Co1-N5 | 89.770 |
| N4-C4 | 1.2945 | N3-O3-H1 | 101.476 |
| N1-O1 | 1.3332 | N4-O4-H2 | 102.600 |
| N2-O2 | 1.3370 | O1-N1-C1 | 121.800 |
| N3-O3 | 1.3463 | O2-N2-C2 | 121.870 |
| N4-O4 | 1.3453 |
| Property | |
|---|---|
| Empirical formula | C49H38Cl3CoN8O4 |
| Temperature | 173(2) K |
| Crystal system | Triclinic |
| Space group | P-1 |
| a | 12.4698(6) Å |
| b | 14.1285(8) Å |
| c | 15.5801(8) Å |
| α | 109.681(4)° |
| β | 112.975(4)° |
| γ | 96.414(4)° |
| Volume | 2284.0(2) Å3 |
| Z | 2 |
| Density (calculated) | 1.408 Mg/m3 |
| Absorption coefficient | 0.606 mm−1 |
| F(000) | 996 |
| Crystal size | 0.370 × 0.350 × 0.340 mm3 |
| Theta range for data collection | 3.191° to 26.452° |
| Index ranges | −15 ≤ h ≤ 15, −17 ≤ k ≤ 17, −19 ≤ l ≤ 19 |
| Reflections collected | 41,194 |
| Independent reflections | 9334 [R(int) = 0.0560] |
| Completeness to theta = 25.000° | 99.7% |
| Absorption correction | Semi-empirical from equivalents |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 9334/0/604 |
| Goodness-of-fit on F2 | 0.987 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0564, wR2 = 0.1502 |
| R indices (all data) | R1 = 0.0630, wR2 = 0.1552 |
| Extinction coefficient | n/a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizarro, S.; Astudillo, P.; Gajardo, F.; Brito, I.; Delgadillo, A. Synthesis and Characterization of a New Cobaloxime-Terpyridine Compound. Crystals 2017, 7, 175. https://doi.org/10.3390/cryst7060175
Pizarro S, Astudillo P, Gajardo F, Brito I, Delgadillo A. Synthesis and Characterization of a New Cobaloxime-Terpyridine Compound. Crystals. 2017; 7(6):175. https://doi.org/10.3390/cryst7060175
Chicago/Turabian StylePizarro, Sebastián, Priscila Astudillo, Francisco Gajardo, Iván Brito, and Alvaro Delgadillo. 2017. "Synthesis and Characterization of a New Cobaloxime-Terpyridine Compound" Crystals 7, no. 6: 175. https://doi.org/10.3390/cryst7060175






