The Syntheses, Crystal Structure and Luminescence Properties of Cone-Like Octadentate Europium (III) Complexes with Four Short Alkoxy Substituents
Abstract
:1. Introduction
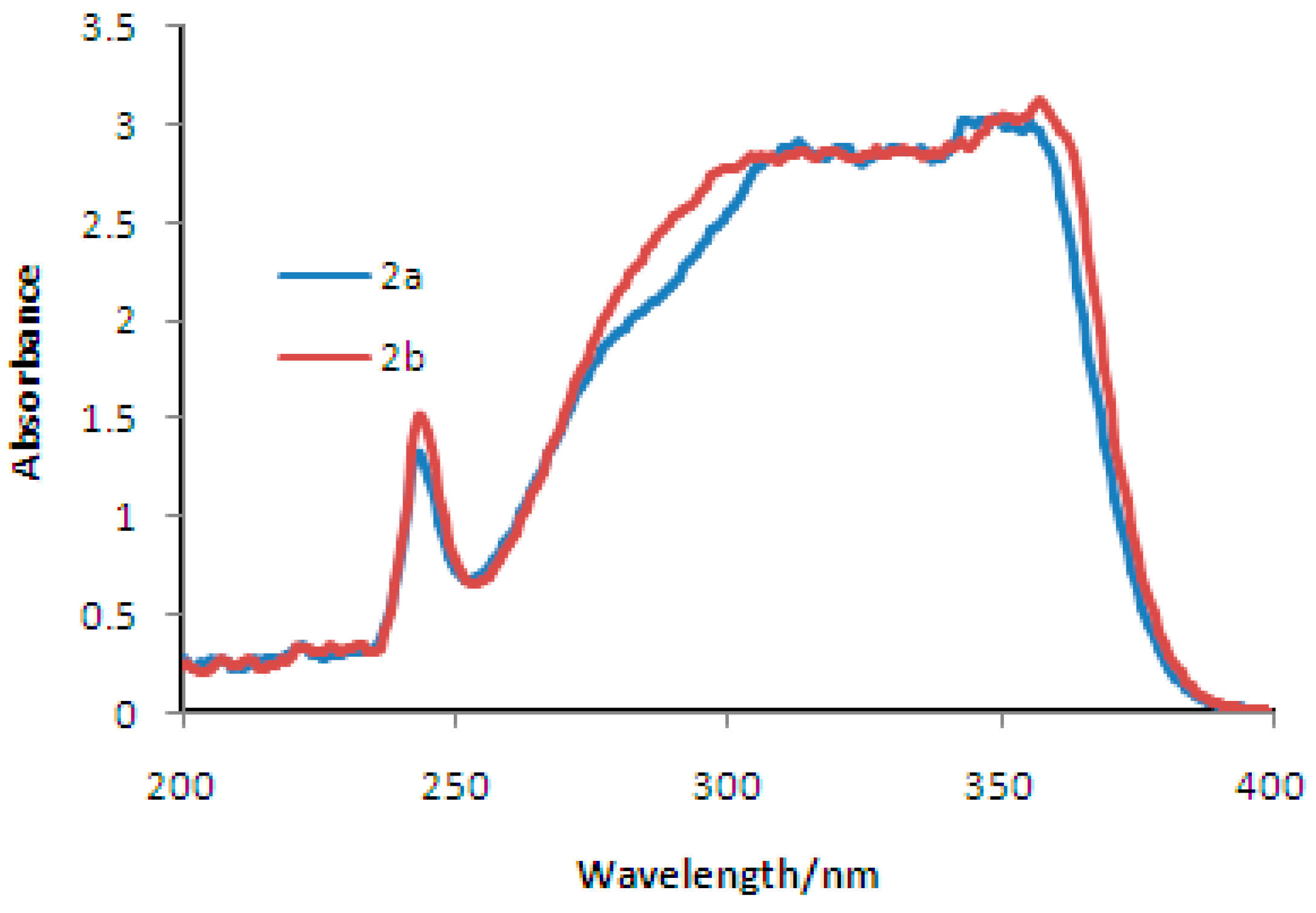
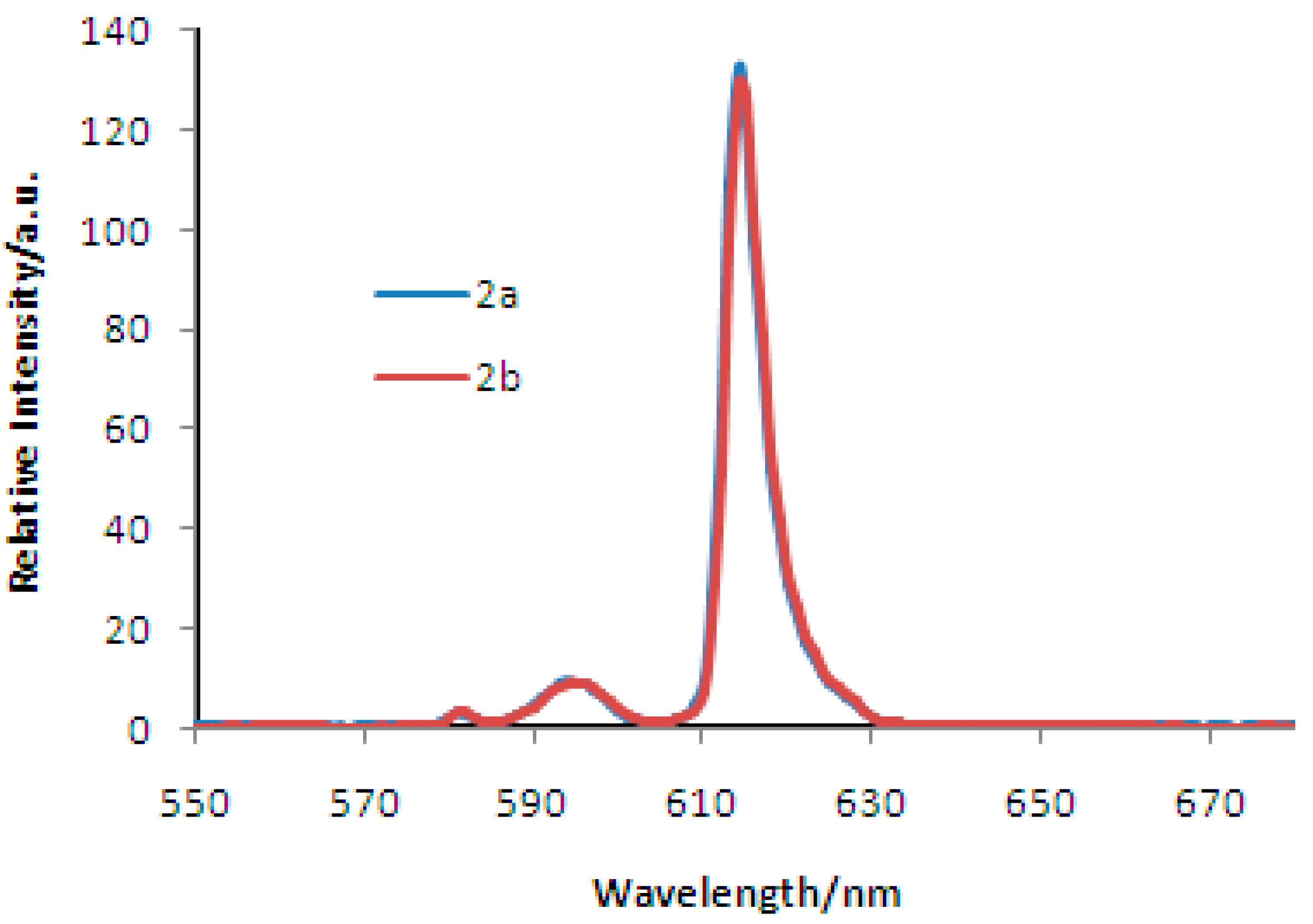
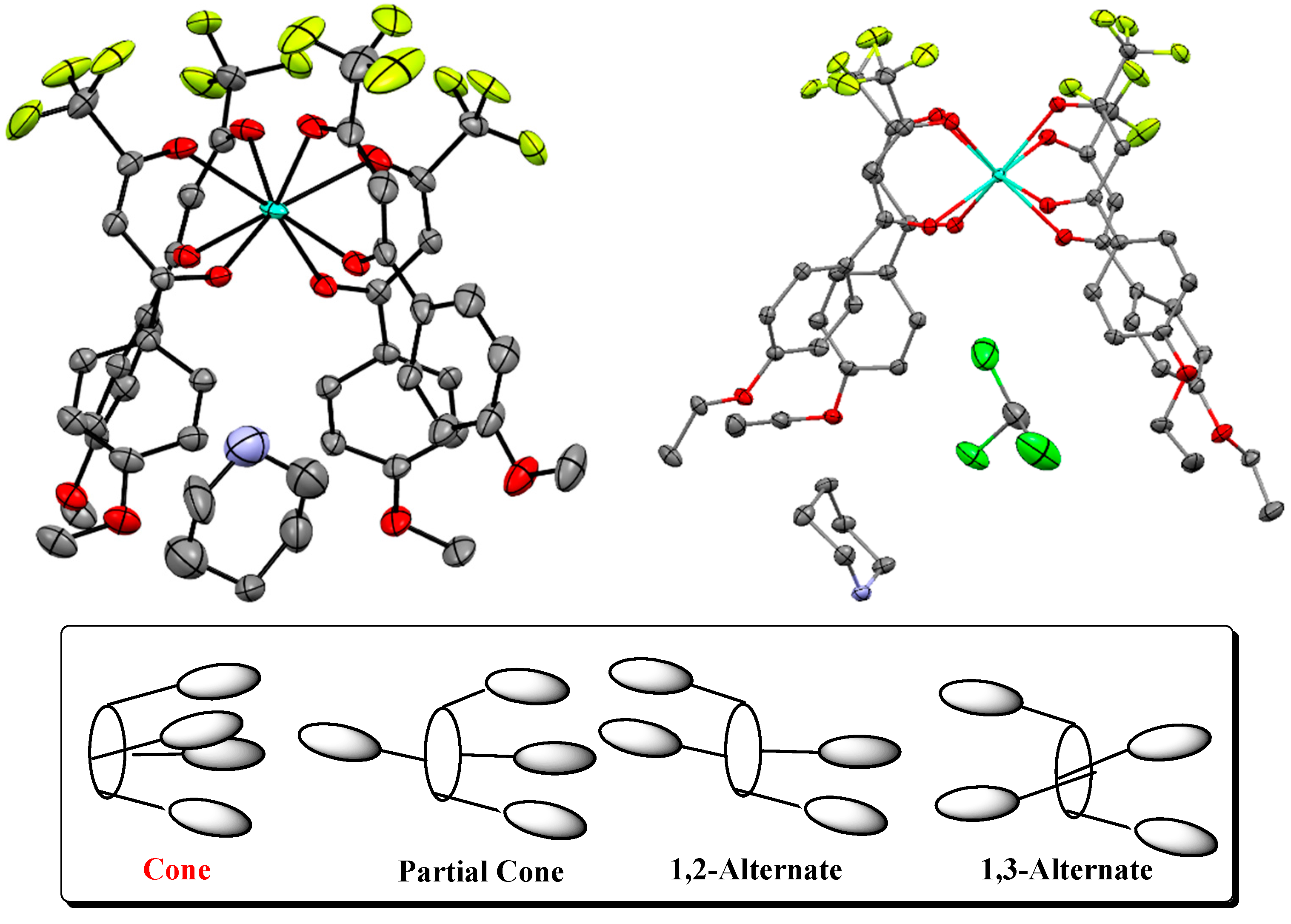
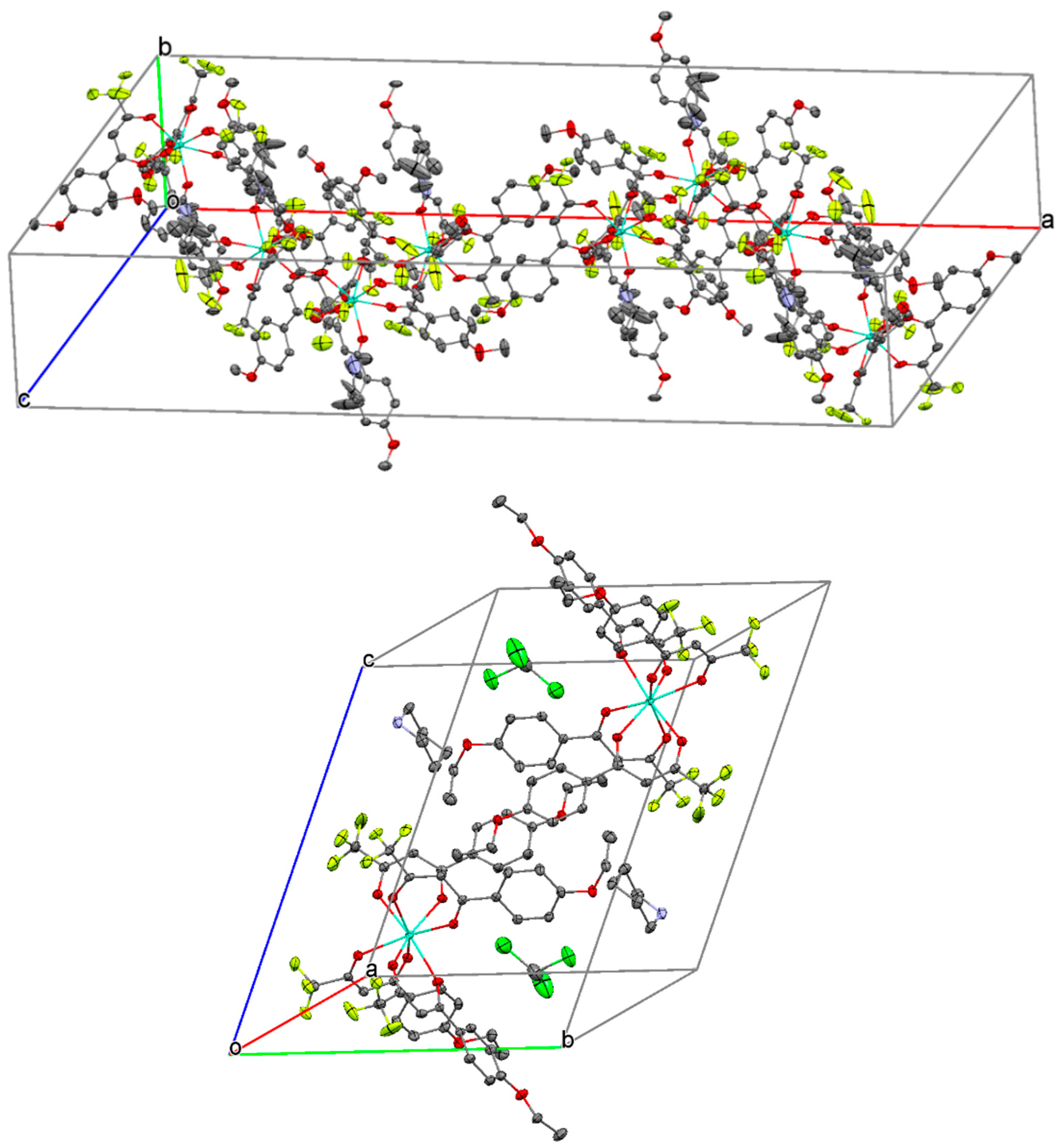
2. Results and Discussion
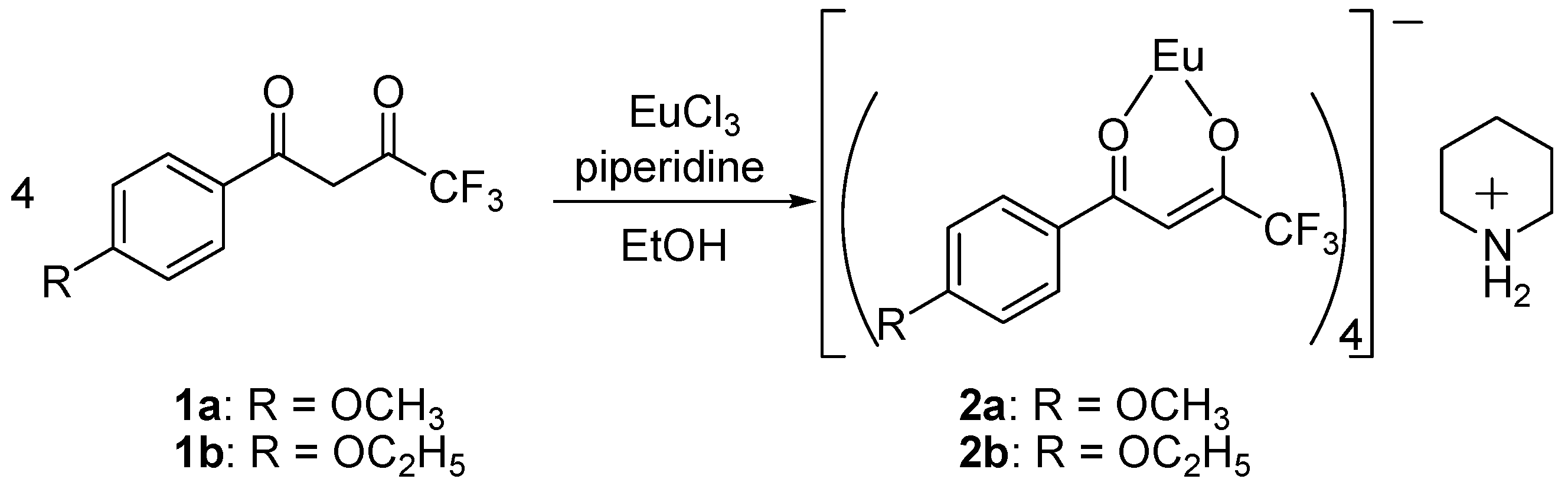
Synthesis and Spectra Analysis
3. Experiments
3.1. Materials and Instrumentation
3.2. Synthesis
3.2.1. Preparation of Piperidinium Tetrakis{1-(4′-substituted phenyl)-4,4,4-trifluoro-1,3-butanedionato}europate (III) Complexes 2a and 2b (Scheme 1)
Typical Procedure
3.2.2. Piperidinium Tetrakis{1-(4′-methoxy phenyl)-4,4,4-trifluoro-1,3-butanedionato}europate (III) 2a
3.2.3. Piperidinium Tetrakis{1-(4′-ethoxy phenyl)-4,4,4-trifluoro-1,3-butanedionato}europate (III) 2b
3.3. Data Collection, Refinement and Structural Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Binnemans, B. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Wada, Y.; Yanagida, S. Strategies for the design of luminescent lanthanide(III) complexes and their photonic applications. J. Photochem. Photobiol. C 2004, 5, 183–202. [Google Scholar] [CrossRef]
- Parker, D.; Senanayake, P.K.; Gareth Williams, J.A. Luminescent sensors for pH, pO2, halide and hydroxide ions using phenanthridine as a photosensitiser in macrocyclic europium and terbium complexes. J. Chem. Soc. Perkin Trans. 2 1998, 2129–2139. [Google Scholar] [CrossRef]
- Robinson, M.R.; O’Reganc, M.B.; Bazan, G.C. Synthesis, morphology and optoelectronic properties of tris [(N-ethylcarbazolyl)(3′,5′-hexyloxybenzoyl) methane](phenanthroline)-europium. Chem. Commun. 2000, 1645–1646. [Google Scholar] [CrossRef]
- Manju, B.; Satish, K.; Taxak, V.B.; Priti, B.; Khatkar, S.P. Synthesis, photoluminescent features and intramolecular energy transfer mechanism of europium (III) complexes with fluorinate β-diketone ligand and auxiliary ligands. J. Fluorine Chem. 2015, 178, 6–13. [Google Scholar]
- Wang, D.; Liu, H.; Fan, L.; Yin, G.; Hu, Y.; Zheng, J. Synthesis and photoluminescent behavior of Eu(III) complexes with 4,4,4-trifluoro-1-(6-methoxy-naphthalen-2-yl)-butane-1,3-dione. Synth. Met. 2015, 209, 267–272. [Google Scholar] [CrossRef]
- Kalinovskaya, I.V.; Mirochnik, A.G. Luminescent Properties of Compounds of Europium(III) with Quinaldic Acid and β-Diketones. Opt. Spectrosc. 2015, 119, 992–995. [Google Scholar] [CrossRef]
- Malba, C.M.; Enrichi, F.; Facchin, M.; Demitri, N.; Plaisier, J.R.; Natile, M.M.; Selva, M.; Riello, P.; Perosa, A.; Benedetti, A. Phosphonium-based tetrakis dibenzoylmethane Eu(III) and Sm(III) complexes: Synthesis, crystal structure and photoluminescence properties in a weakly coordinating phosphonium ionic liquid. RSC Adv. 2015, 5, 60898–60907. [Google Scholar] [CrossRef]
- Martins, J.P.; Martín-Ramos, P.; Coya, C.; Silva, M.R.; Eusebio, M.E.S.; deAndrés, A.; Álvarez, A.D.; Martín-Gil, J. Highly luminescent pure-red-emitting fluorinated β-diketonate europium(III) complex for full solution-processed OLEDs. J. Lumin. 2015, 159, 17–25. [Google Scholar] [CrossRef]
- Melby, L.R.; Rose, N.J.; Abramson, E.; Caris, J.C. Synthesis and Fluorescence of Some Trivalent Lanthanide Complexes. J. Am. Chem. Soc. 1964, 86, 5117–5125. [Google Scholar] [CrossRef]
- Richardson, F.S. Terbium(III) and europium(III) ions as luminescent probes and stains for biomolecular systems. Chem. Rev. 1982, 82, 541–552. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Zhang, L.; Chen, P.; Liu, S. Synthesis, Characterization, and Luminescent Properties of Europium Complexes with Fluorine Functionalized Phenanthroline. J. Electron. Soc. 2009, 156, H202–H207. [Google Scholar] [CrossRef]
- APEX2 Version 2009.9, Bruker AXS Inc.: Tokyo, Japan, 2009.
- SAINT Version 7.68A, Bruker AXS Inc.: Tokyo, Japan, 2009.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]


| Eu1-O1 | 2.383(5) | Eu1-O2 | 2.351(5) |
| Eu1-O4 | 2.373(4) | Eu1-O5 | 2.331(4) |
| Eu1-O7 | 2.381(5) | Eu1-O8 | 2.373(5) |
| Eu1-O10 | 2.350(6) | Eu1-O11 | 2.316(4) |
| O1-Eu1-O2 | 73.60(16) | O4-Eu1-O5 | 72.67(14) |
| O7-Eu1-O8 | 71.83(15) | O10-Eu1-O11 | 72.26(14) |
| Eu1-O1 | 2.404(3) | Eu1-O2 | 2.371(3) |
| Eu1-O4 | 2.439(2) | Eu1-O5 | 2.339(3) |
| Eu1-O7 | 2.411(2) | Eu1-O8 | 2.424(3) |
| Eu1-O10 | 2.420(2) | Eu1-O11 | 2.331(2) |
| O1-Eu1-O2 | 70.41(8) | O4-Eu1-O5 | 70.63(8) |
| O7-Eu1-O8 | 71.19(8) | O10-Eu1-O11 | 71.81(8) |
| Crystal Information | 2a | 2b |
|---|---|---|
| Empirical formula | C49H44EuF12NO12 | C53H52EuF12NO12, CHCl3 |
| Formula weight | 1218.82 | 1394.30 |
| Temperature | 90 K | 90 K |
| Wavelength | 0.71073 Å | 0.71073 Å |
| Crystal system | Monoclinic | Triclinic |
| Space group | C2/c (no. 15) | P − 1 (no. 2) |
| Unit cell dimensions | a = 49.553(5) Å b = 11.2988(12) Å c = 18.8552(19) Å β = 111.599(2)° | a = 12.9414(12) Å b = 15.5698(13) Å c = 17.5075(16) Å α = 69.6130(10)° β = 70.0410(10)° γ = 76.1220(10)° |
| Cell volume | 9815.6(18) Å3 | 2880.9(5) Å3 |
| Z | 8 | 2 |
| Density (calculated) | 1.650 g/cm3 | 1.607 g/cm3 |
| Absorption coefficient | 1.387 mm−1 | 1.327 mm−1 |
| F(000) | 4896 | 1404 |
| Crystal size(mm) | 0.45 × 0.30 × 0.30 | 0.30 × 0.20 × 0.10 |
| θ range for data collection | 1.77° to 25.03° | 1.29° to 25.03° |
| Index ranges | −58 ≤ h ≤ 46, −13 ≤ k ≤13 | −15 ≤ h ≤ 10, −17 ≤ k ≤ 12 |
| Reflections collected | −22 ≤ l ≤ 21 | −20 ≤ l ≤ 20 |
| Independent reflections | 8642 [R(int) = 0.0308] | 9950 [R(int) = 0.0223] |
| Reflections | 7138(25.03°) | 9356(25.03°) |
| [I > 2sigma(I)] | 99.4% | 97.6% |
| Completeness to theta° | Empirical | Empirical |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Absorption correction | Empirical | Empirical |
| Data/restraints/ parameters | 8642/0/681 | 9950/0/752 |
| Goodness-of-fit on F2 | 1.130 | 1.168 |
| Final R1 indices [I >2sigma(I)] | R1 = 0.0570 | R1 = 0.0361 |
| wR2 indices (all data) | wR2 = 0.1716 | wR2 = 0.1090 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriguchi, T.; Hirosaki, S.; Jalli, V.; Tsuge, A.; Yoza, K. The Syntheses, Crystal Structure and Luminescence Properties of Cone-Like Octadentate Europium (III) Complexes with Four Short Alkoxy Substituents. Crystals 2017, 7, 85. https://doi.org/10.3390/cryst7030085
Moriguchi T, Hirosaki S, Jalli V, Tsuge A, Yoza K. The Syntheses, Crystal Structure and Luminescence Properties of Cone-Like Octadentate Europium (III) Complexes with Four Short Alkoxy Substituents. Crystals. 2017; 7(3):85. https://doi.org/10.3390/cryst7030085
Chicago/Turabian StyleMoriguchi, Tetsuji, Satoshi Hirosaki, Venkataprasad Jalli, Akihiko Tsuge, and Kenji Yoza. 2017. "The Syntheses, Crystal Structure and Luminescence Properties of Cone-Like Octadentate Europium (III) Complexes with Four Short Alkoxy Substituents" Crystals 7, no. 3: 85. https://doi.org/10.3390/cryst7030085






