Synthesis, Crystal Structure and Water Vapor Adsorption Properties of a Porous Supramolecular Architecture
Abstract
:1. Introduction
2. Results and Discussion
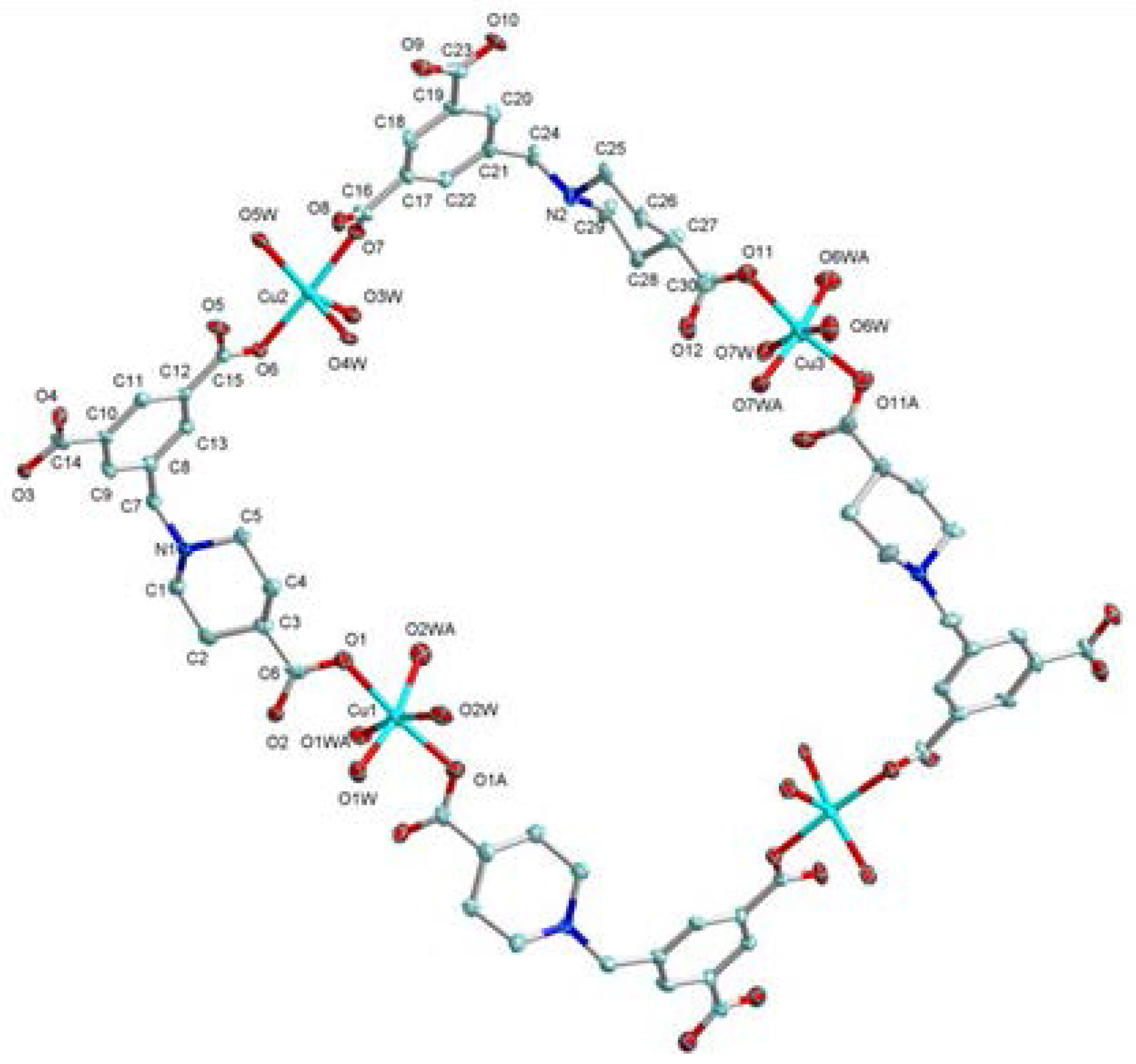
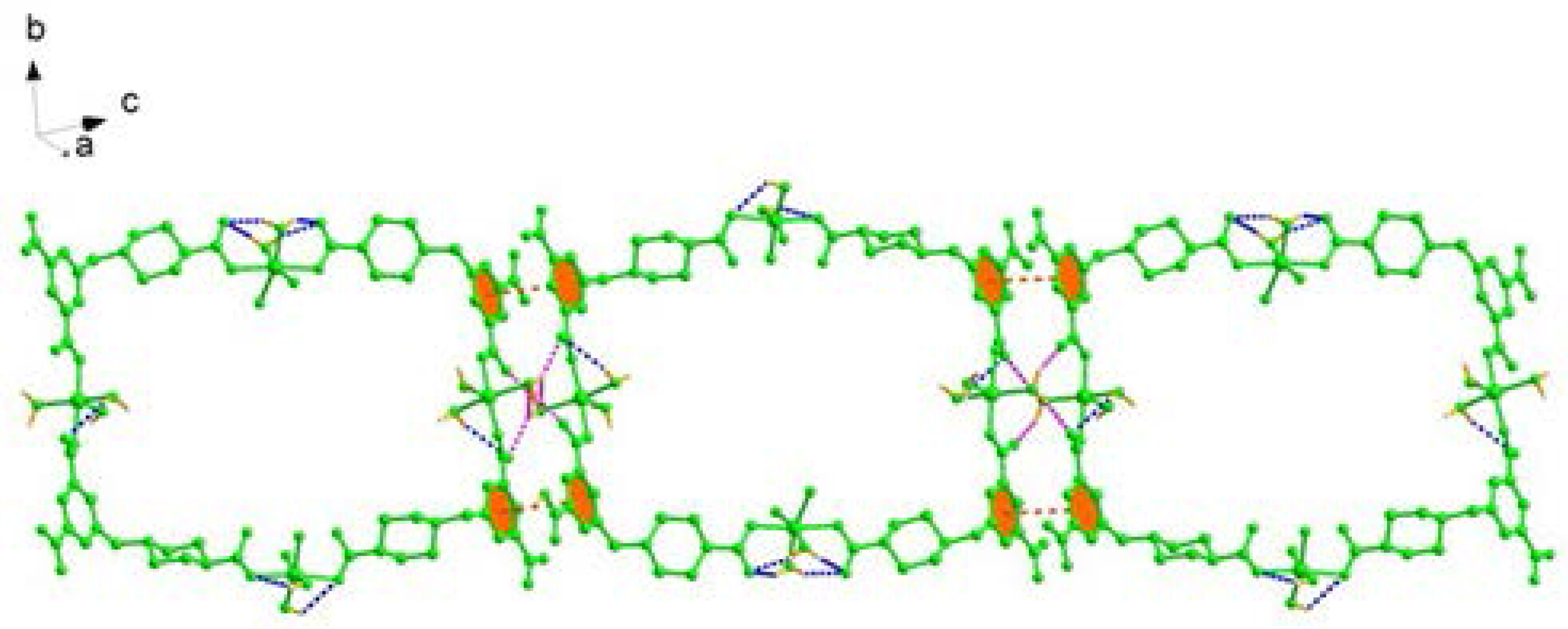
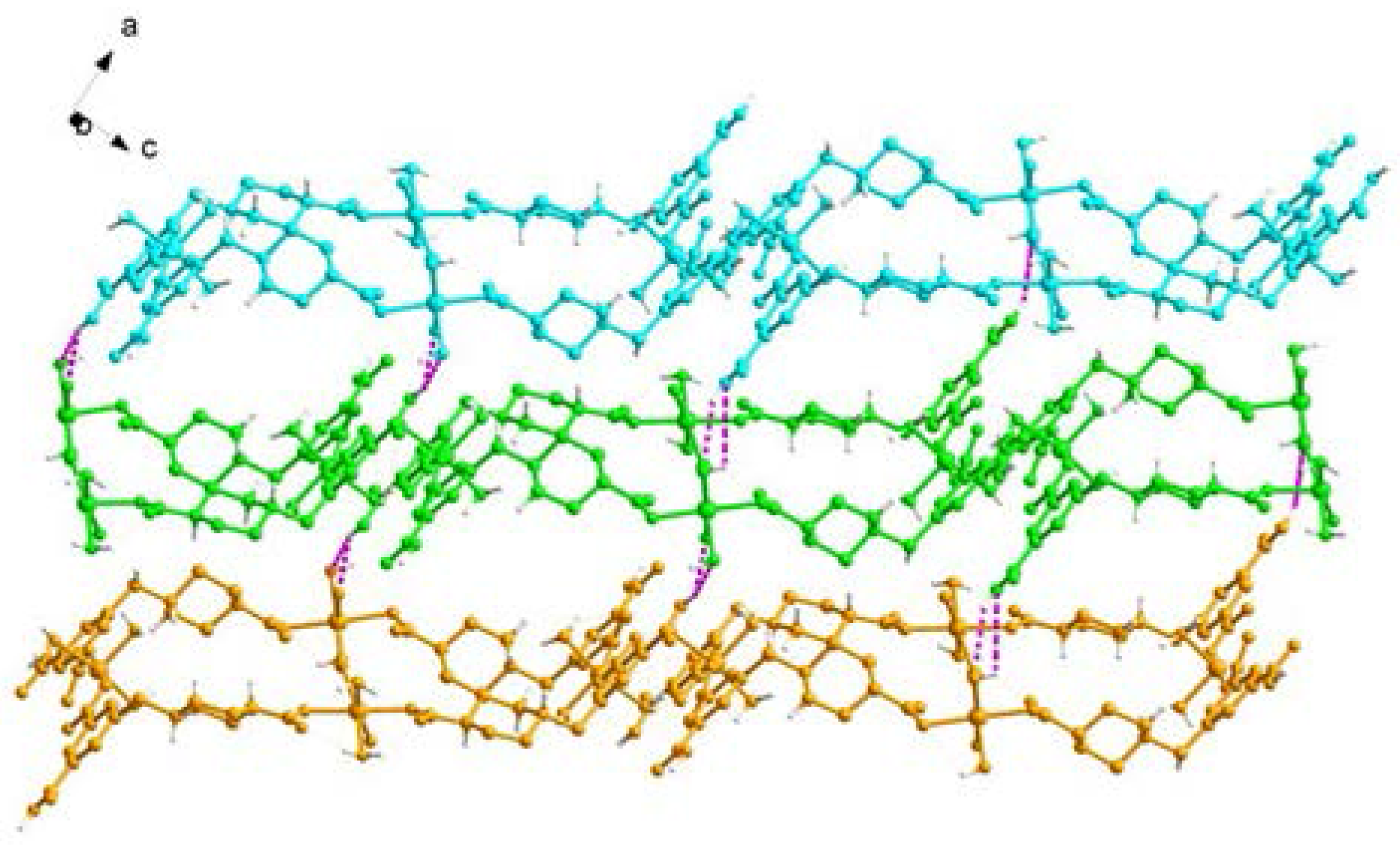
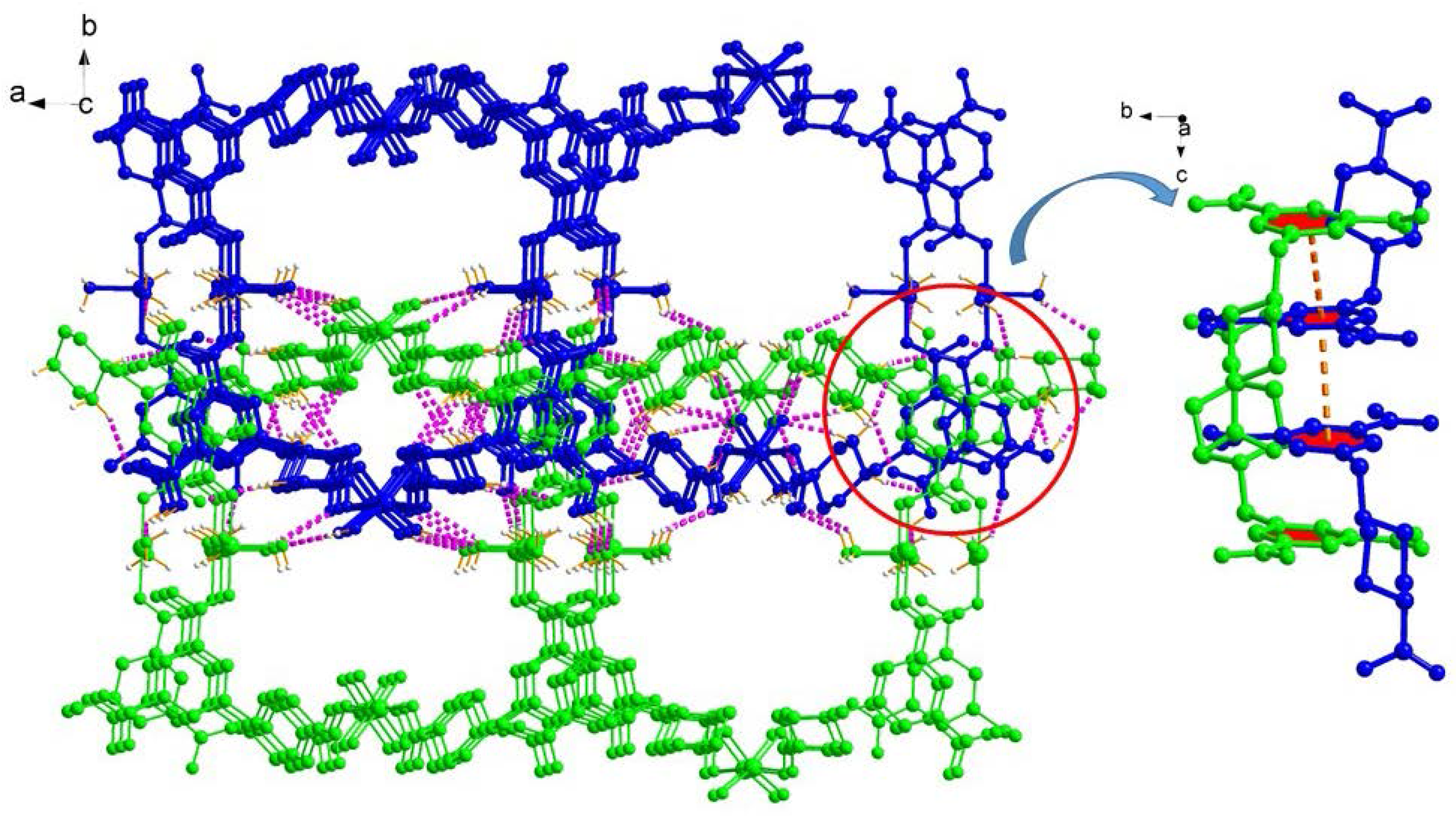
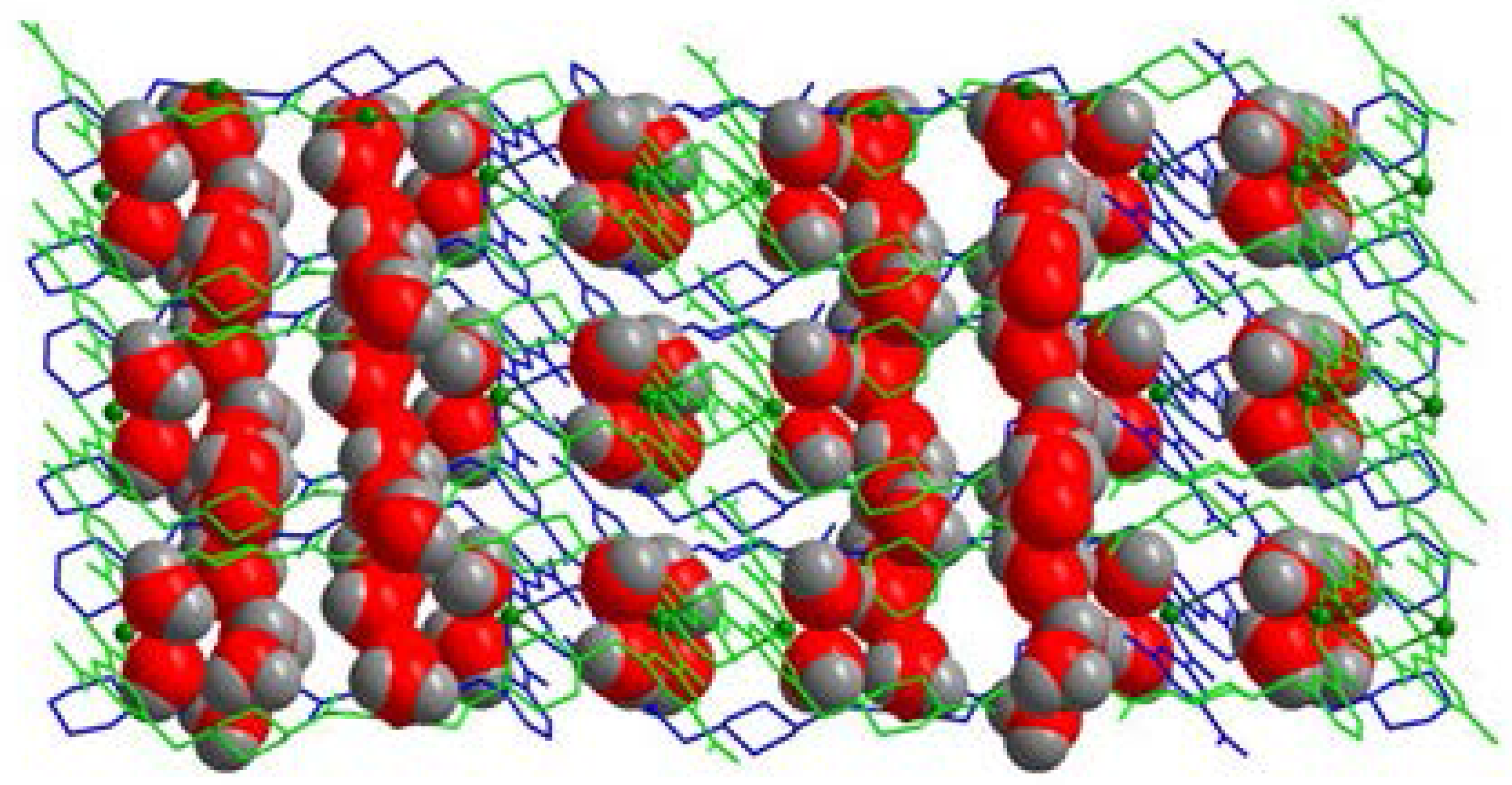
2.1. Structural Description of [Cu4(HL)4(H2O)14] (1)
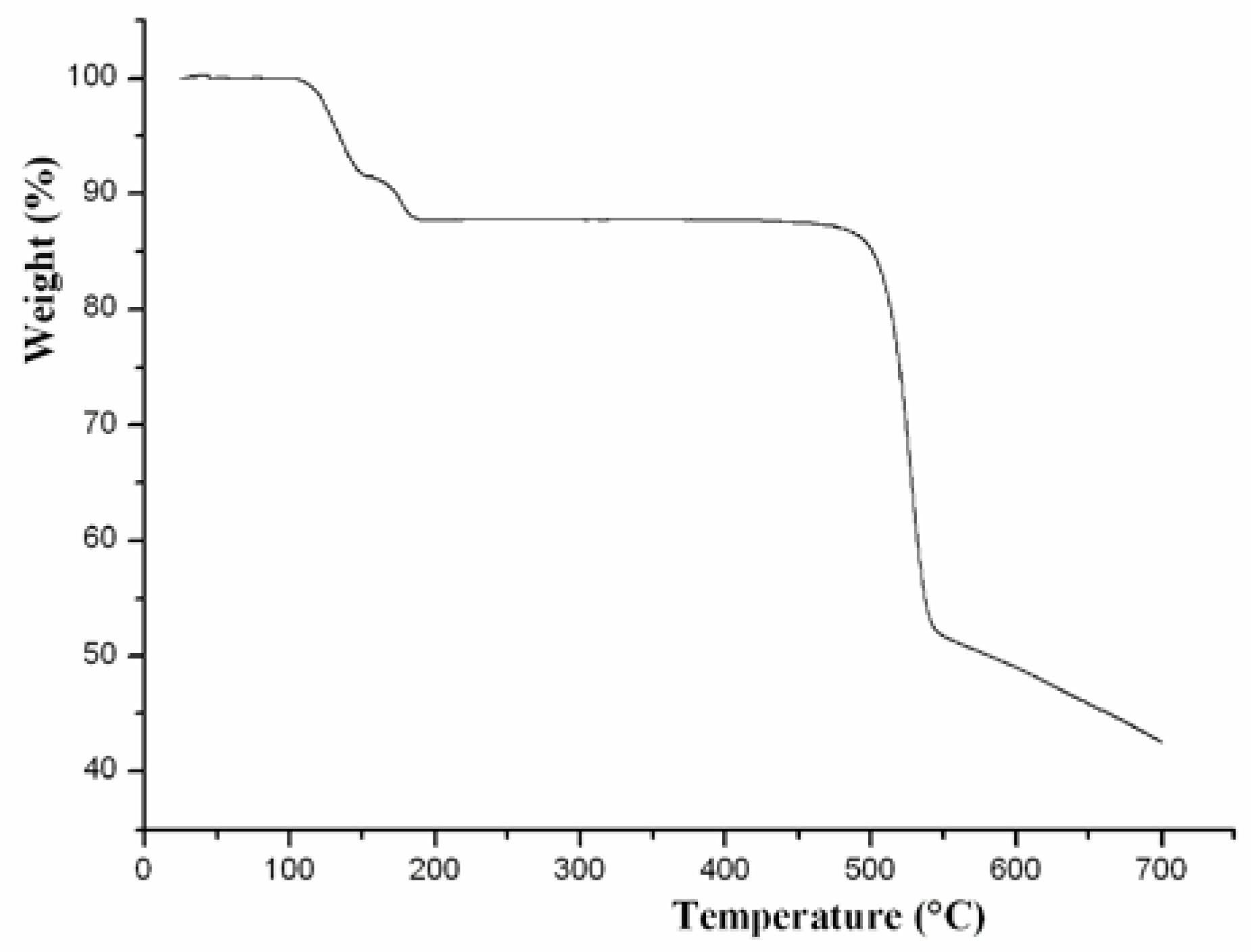
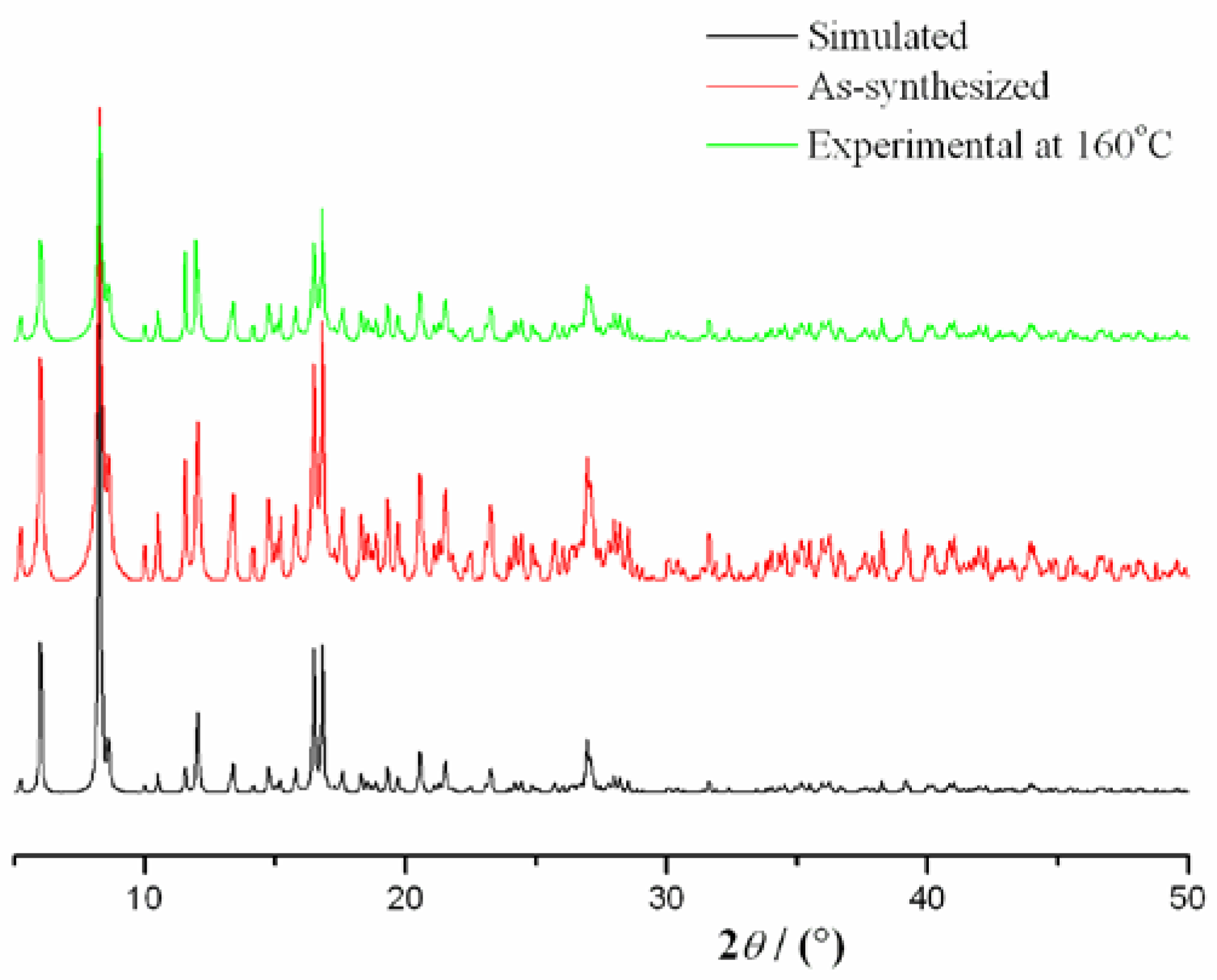
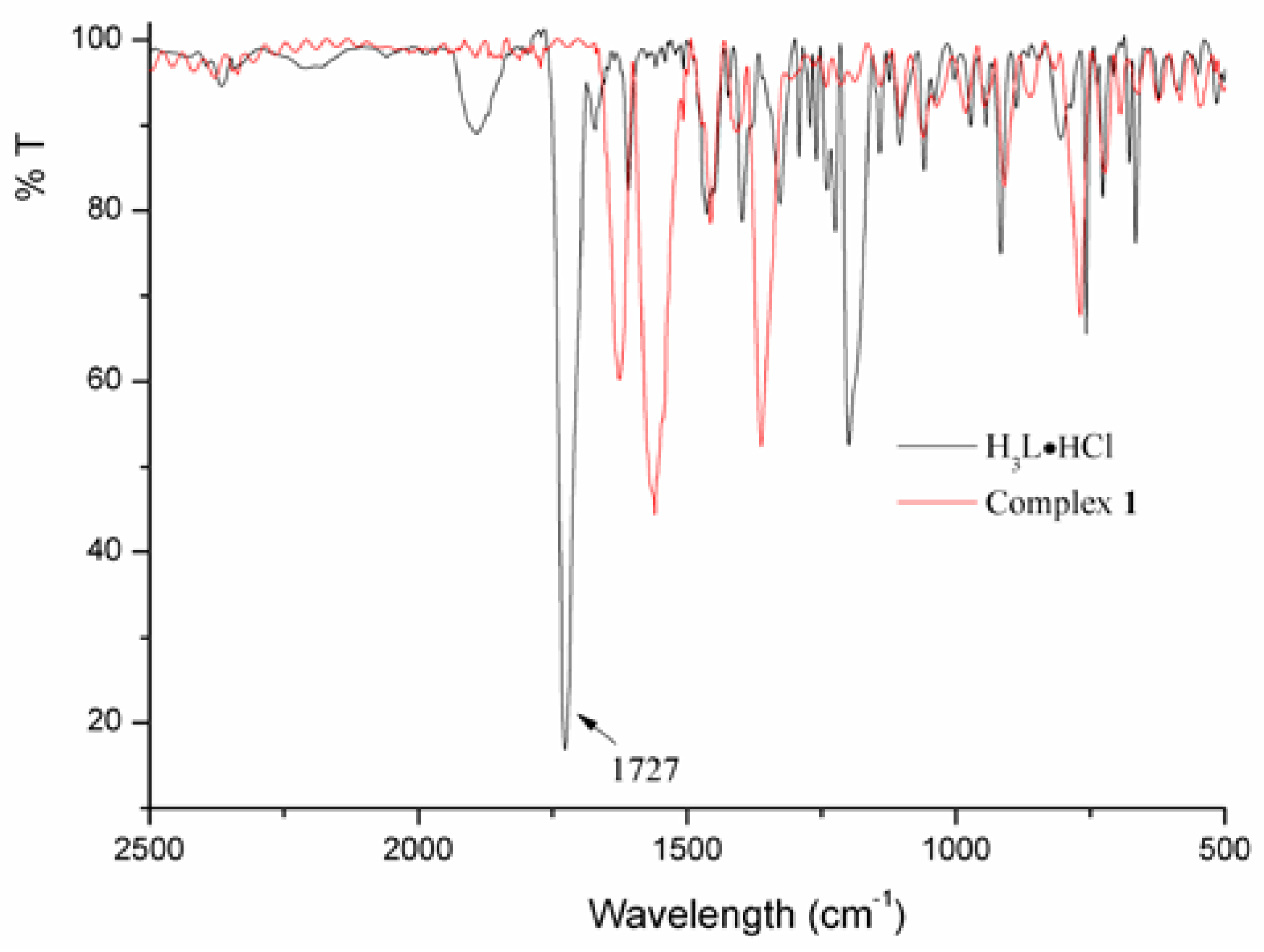
2.2. Thermal Analysis and Powder X-ray Diffraction Analysis
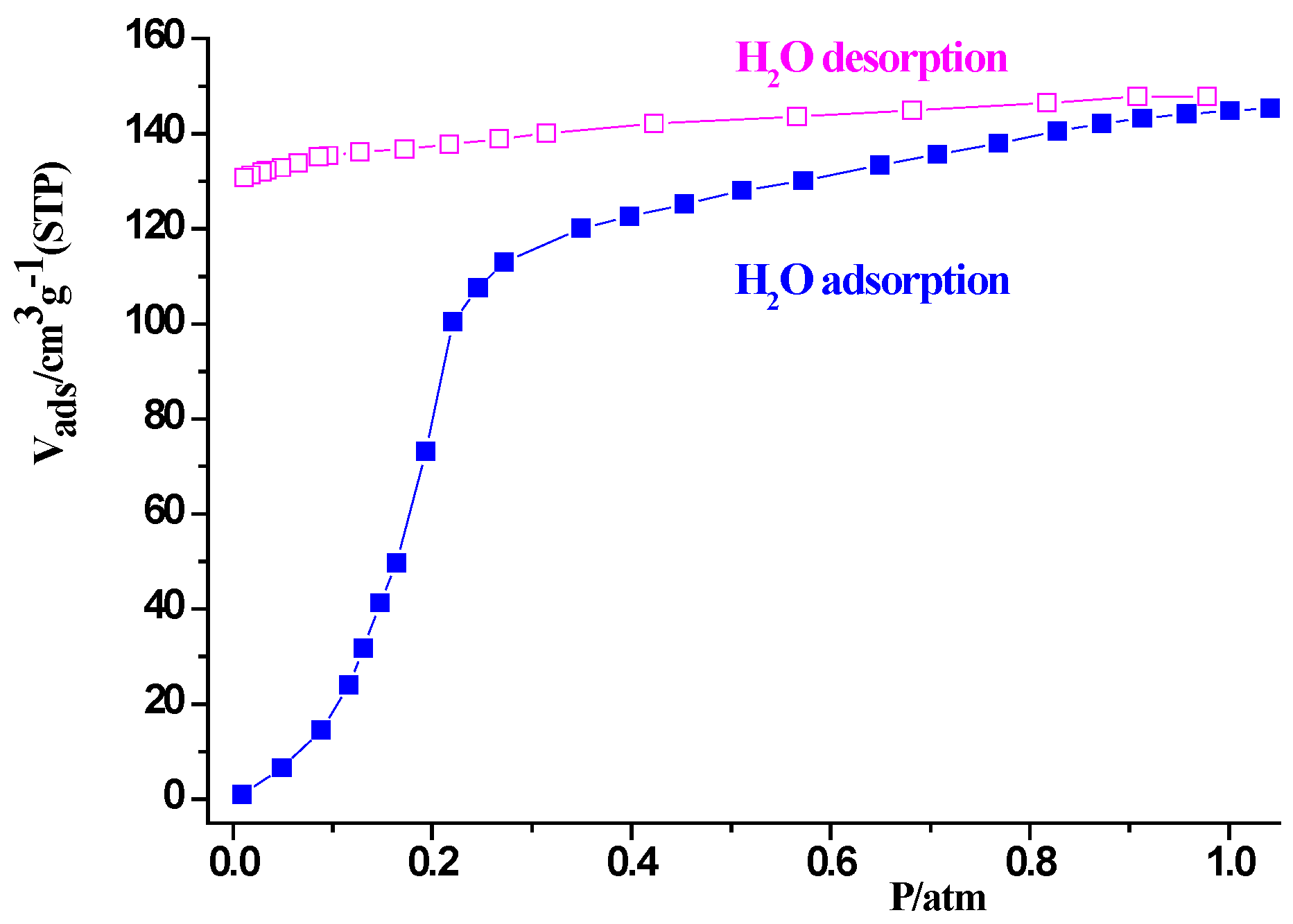
2.3. Sorption Property of Complex 1
3. Experimental Section
3.1. Materials and Instrumentation
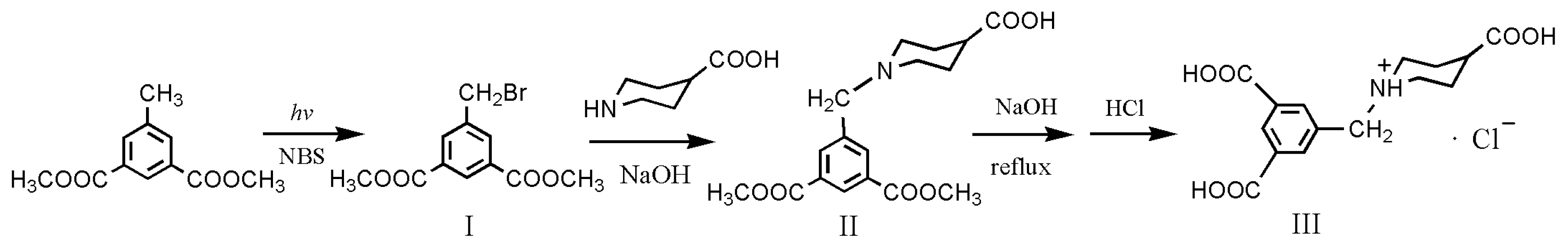
3.2. Synthesis of Dimethyl 5-(Bromomethyl)isophthalate (I)
3.3. Synthesis of 1-(3,5-bis(Methoxycarbonyl)benzyl)piperidine-4-carboxylic Acid (II)
3.4. Synthesis of 5-((4-Carboxypiperidin-1-yl)methyl)isophthalic Acid Hydrochloride (III, H3L·HCl)
3.5. Synthesis of Complex [Cu4(HL)4(H2O)14] (1)
3.6. Crystal Structure Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Almeida Paz, F.A.; Klinowski, J.; Vilela, S.M.F.; Tomé, J.P.C.; Cavaleiro, J.A.S.C.; Rocha, J. Ligand design for functional metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Wang, P.; Lv, G.C.; Sun, W.Y. Porous cobalt(II)-imidazolate supramolecular isomeric frameworks with selective gas sorption property. Chem. Commun. 2011, 47, 4902–4904. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Luan, Y.; Qi, C.; Ramella, D. The synthesis of a bifunctional copper metal organic framework and its application in the aerobic oxidation/Knoevenagel condensation sequential reaction. Dalton Trans. 2016, 45, 13917–13924. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S. The roles of imidazole ligands in coordination supramolecular systems. CrystEngComm 2016, 18, 6543–6565. [Google Scholar] [CrossRef]
- Fang, W.H.; Zhang, L.; Zhang, J.; Yang, G.Y. Halogen dependent symmetry change in two series of wheel cluster organic frameworks built from La18 tertiary building units. Chem. Commun. 2016, 52, 1455–1457. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.J.; Chang, Z.; Xu, J.; Hu, Z.; Liu, Y.Q.; Xu, Y.L.; Bu, X.H. Construction of a polyhedron decorated MOF with a unique network through the combination of two classic secondary building units. Chem. Commun. 2016, 52, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Sheng, L.Q.; Zhao, Y.; Liu, Z.D.; Qiao, R.; Yang, S. Syntheses, structures, and properties of a series of polyazaheteroaromatic core-based Zn(II) coordination polymers together with carboxylate auxiliary ligands. Cryst. Growth Des. 2016, 16, 229–241. [Google Scholar] [CrossRef]
- Mihaly, J.J.; Zeller, M.; Genna, D.T. Ion-directed synthesis of indium-derived 2,5-thiophenedicarboxylate metal-organic frameworks: Tuning framework dimensionality. Cryst. Growth Des. 2016, 16, 1550–1558. [Google Scholar] [CrossRef]
- Hua, J.A.; Zhao, Y.; Kang, Y.S.; Lu, Y.; Sun, W.Y. Solvent-dependent zinc(II) coordination polymers with mixed ligands: Selective sorption and fluorescence sensing. Dalton Trans. 2015, 44, 11524–11532. [Google Scholar] [CrossRef] [PubMed]
- Kamari, A.A.; Haque, R.A.; Razali, M.R. A heterobimetallic 2-D coordination polymer [Na2(Cu2I2(2pyCOO)4)(H2O)4]n (2pyCOO−=picolinate) within a 3-D supramolecular architecture. Crystals 2016, 6, 96. [Google Scholar] [CrossRef]
- Zhu, M.A.; Guo, X.Z.; Chen, S.S. Synthesis, Crystal Structure and Luminescent Property of a Zn(II) Complex Based on 4-Imidazole-carboxylate Ligand. Chin. J. Struct. Chem. 2017, 36, 1348–1354. [Google Scholar]
- Suckert, S.; Germann, L.S.; Dinnebier, R.E.; Werner, J.; Näther, C. Synthesis, structures and properties of cobalt thiocyanate coordination compounds with 4-(hydroxymethyl)pyridine as Co-ligand. Crystals 2016, 6, 38. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Furukawa, H.; Ko, N.; Nie, W.; Park, H.J.; Okajima, S.; Cordova, K.E.; Deng, H.; Kim, J.; Yaghi, O.M. Introduction of functionality, selection of topology, and enhancement of gas adsorption in multivariate metal-organic framework-177. J. Am. Chem. Soc. 2015, 137, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Liu, Q.; Zhao, Y.; Qiao, R.; Sheng, L.Q.; Liu, Z.D.; Yang, S.; Song, C.F. New metal-organic frameworks constructed from the 4-imidazole-carboxylate ligand: Structural diversities, luminescence, and gas adsorption properties. Cryst. Growth Des. 2014, 14, 3727–3741. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xiao, L.; Chen, S.S.; Qiao, R.; Yang, S. A novel Zn(II) complex with 4-connected umc topology: Synthesis, crystal structure and luminescent property. Chin. J. Struct. Chem. 2017, 36, 819–824. [Google Scholar]
- Chen, S.S.; Qiao, R.; Sheng, L.Q.; Zhao, Y.; Yang, S.; Chen, M.M.; Liu, Z.D.; Wang, D.H. Cadmium(II) and zinc(II) complexes with rigid 1-(1H-imidazol-4-yl)-3-(4H-tetrazol-5-yl)benzene and varied carboxylate ligands. CrystEngComm 2013, 15, 5713–5725. [Google Scholar] [CrossRef]
- Xiao, L.; Li, W.D.; Fang, X.; Jiang, L.Y.; Chen, S.S. Two three-dimensional supramolecular polymer built from mixed N-donor and carboxylate ligands. Chin. J. Struct. Chem. 2016, 35, 781–788. [Google Scholar]
- Qiao, R.; Chen, S.S.; Sheng, L.Q.; Yang, S.; Li, W.D. Syntheses, crystal structures, and properties of four complexes based on polycarboxylate and imidazole ligands. J Solid State Chem. 2015, 228, 199–207. [Google Scholar] [CrossRef]
- Li, W.D.; Fang, X.; Qiao, R.; Chen, S.S. Non-covalent bonded 3D supramolecular architectures based on acid-base adducts. Chin. J. Struct. Chem. 2016, 35, 46–54. [Google Scholar]
- Spek, A.L. Single-Crystal Structure Validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Li, Y.L.; Hua, J.A.; Zhao, Y.; Kang, Y.S.; Sun, W.Y. Metal-organic frameworks with 1,3,5-tris(1-imidazolyl)benzene and dicarboxylate ligands: Synthesis, anion exchange and gas adsorption. Microporous Mesoporous Mater. 2015, 214, 188–194. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
| Bond | d | Bond | d |
| Cu(1)-O(1W) | 2.003(3) | Cu(1)-O(1) | 2.053(3) |
| Cu(1)-O(2W) | 2.048(3) | Cu(2)-O(3W) | 2.066(3) |
| Cu(2)-O(4W) | 2.003(3) | Cu(2)-O(5W) | 2.119(3) |
| Cu(2)-O(6) | 1.979(3) | Cu(2)-O(7) | 2.013(3) |
| Cu(3)-O(6W) | 2.046(3) | Cu(3)-O(7W) | 2.009(3) |
| Cu(3)-O(11) | 2.131(3) | - | - |
| >Angle | >ω | >Angle | >ω |
| O(1)-Cu(1)-O(1W) | 94.90(13) | O(1)-Cu(1)-O(2W) | 103.39(13) |
| O(1)-Cu(1)-O(1) i | 174.45(17) | O(1)-Cu(1)-O(1W) i | 88.99(13) |
| O(1)-Cu(1)-O(2W) i | 73.05(12) | O(1W)-Cu(1)-O(2W) i | 73.00(11) |
| O(1W)-Cu(1)-O(2W) | 83.85(12) | O(1W)-Cu(1)-O(1W) i | 91.16(18) |
| O(1W)-Cu(1)-O(2W) i | 166.98(12) | O(2W)-Cu(1)-O(2W) i | 103.45(17) |
| O(3W)-Cu(2)-O(5W) | 97.37(12) | O(3W)-Cu(2)-O(6) | 90.65(11) |
| O(3W)-Cu(2)-O(7) | 88.08(11) | O(4W)-Cu(2)-O(7) | 94.05(11) |
| O(4W)-Cu(2)-O(5W) | 169.00(11) | O(4W)-Cu(2)-O(6) | 91.45(11) |
| O(6W)-Cu(3)-O(7W) | 74.90(12) | O(6W)-Cu(3)-O(11) | 88.51(14) |
| O(6W)-Cu(3)-O(7W) i | 165.99(12) | O(6W)-Cu(3)-O(6W) i | 119.1(2) |
| O(6W)-Cu(3)-O(11) i | 87.35(11) | O(7W)-Cu(3)-O(11) | 96.51(13) |
| O(7W)-Cu(3)-O(7W) i | 91.36(19) | O(7W)-Cu(3)-O(11) i | 89.06(13) |
| O(6W)i-Cu(3)-O(11) | 87.46(14) | O(11)-Cu(3)-O(11) i | 172.04(19) |
| D-H···A | d(D-H) | d(H···A) | d(D···A) | ∠DHA |
|---|---|---|---|---|
| N(1)-H(1)···O(8) a | 0.91 | 1.90 | 2.787(4) | 166 |
| O(1W)-H(1X)···O(2) | 0.85 | 2.05 | 2.838(4) | 154 |
| O(1W)-H(1Y)···O(2) b | 0.85 | 2.08 | 2.790(4) | 140 |
| N(2)-H(2)···O(4) c | 0.91 | 1.87 | 2.772(4) | 170 |
| O(3W)-H(3X)···O(11) d | 0.80 | 2.36 | 2.722(4) | 114 |
| O(3W)-H(3Y)···O(2) e | 0.88 | 2.36 | 2.959(4) | 125 |
| O(4)-H(4C)···O(7W) f | 0.96 | 2.49 | 3.298(4) | 142 |
| O(4W)-H(4X)···O(8) | 0.96 | 2.51 | 3.261(4) | 135 |
| O(4W)-H(4Y)···O(3) c | 0.96 | 1.85 | 2.667(4) | 141 |
| O(5W)-H(5X)···O(5) g | 0.96 | 1.63 | 2.525(4) | 153 |
| O(5W)-H(5Y)···O(5W) g | 0.96 | 2.53 | 3.047(4) | 114 |
| O(5W)-H(5Y)···O(8) g | 0.96 | 2.10 | 2.768(4) | 125 |
| O(6W)-H(6Y)···O(3W) h | 0.87 | 2.47 | 3.336(4) | 174 |
| C(3)-H(3)···O(2W) e | 0.98 | 2.29 | 3.142(5) | 145 |
| C(5)-H(5A)···O(9) a | 0.97 | 2.48 | 3.309(5) | 143 |
| C(5)-H(5B)···O(2W) e | 0.97 | 2.25 | 3.063(5) | 141 |
| C(24)-H(24A)···O(12) d | 0.97 | 2.44 | 3.383(5) | 163 |
| C(25)-H(25A)···O(5) c | 0.97 | 2.49 | 3.318(5) | 142 |
| Empirical Formula | C60H88N4O38Cu4 |
|---|---|
| Formula weight | 1727.50 |
| Temperature/K | 291(2) |
| Crystal system | Monoclinic |
| Space group | C 2/c |
| a/Å | 29.539(12) |
| b/Å | 20.530(9) |
| c/Å | 13.280(8) |
| α/° | 90 |
| β/° | 95.365(9) |
| γ/° | 90 |
| Volume/Å3 | 8018(7) |
| Z | 4 |
| ρcalcmg/mm3 | 1.434 |
| μ/mm−1 | 1.136 |
| S | 1.089 |
| F(000) | 3600 |
| Index ranges | −38 ≤ h ≤ 38, −26 ≤ k ≤ 22, −17 ≤ l ≤ 16 |
| Reflections collected | 36887 |
| Independent reflections | 9546 |
| Data/restraints/parameters | 9546/4/479 |
| Goodness-of-fit on F2 | 1.021 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.061, wR2 = 0.1617 |
| Final R indexes [all data] | R1 = 0.0985, wR2 = 0.1694 |
| Largest diff. peak/hole/e Å−3 | 0.092/−1.194 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, R.; Zhang, Z.-Y.; Zhu, M.-A. Synthesis, Crystal Structure and Water Vapor Adsorption Properties of a Porous Supramolecular Architecture. Crystals 2017, 7, 297. https://doi.org/10.3390/cryst7100297
Qiao R, Zhang Z-Y, Zhu M-A. Synthesis, Crystal Structure and Water Vapor Adsorption Properties of a Porous Supramolecular Architecture. Crystals. 2017; 7(10):297. https://doi.org/10.3390/cryst7100297
Chicago/Turabian StyleQiao, Rui, Zi-You Zhang, and Mei-An Zhu. 2017. "Synthesis, Crystal Structure and Water Vapor Adsorption Properties of a Porous Supramolecular Architecture" Crystals 7, no. 10: 297. https://doi.org/10.3390/cryst7100297





