The New Method of XRD Measurement of the Degree of Disorder for Anode Coke Material
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization
2.2.1. XRD Technique
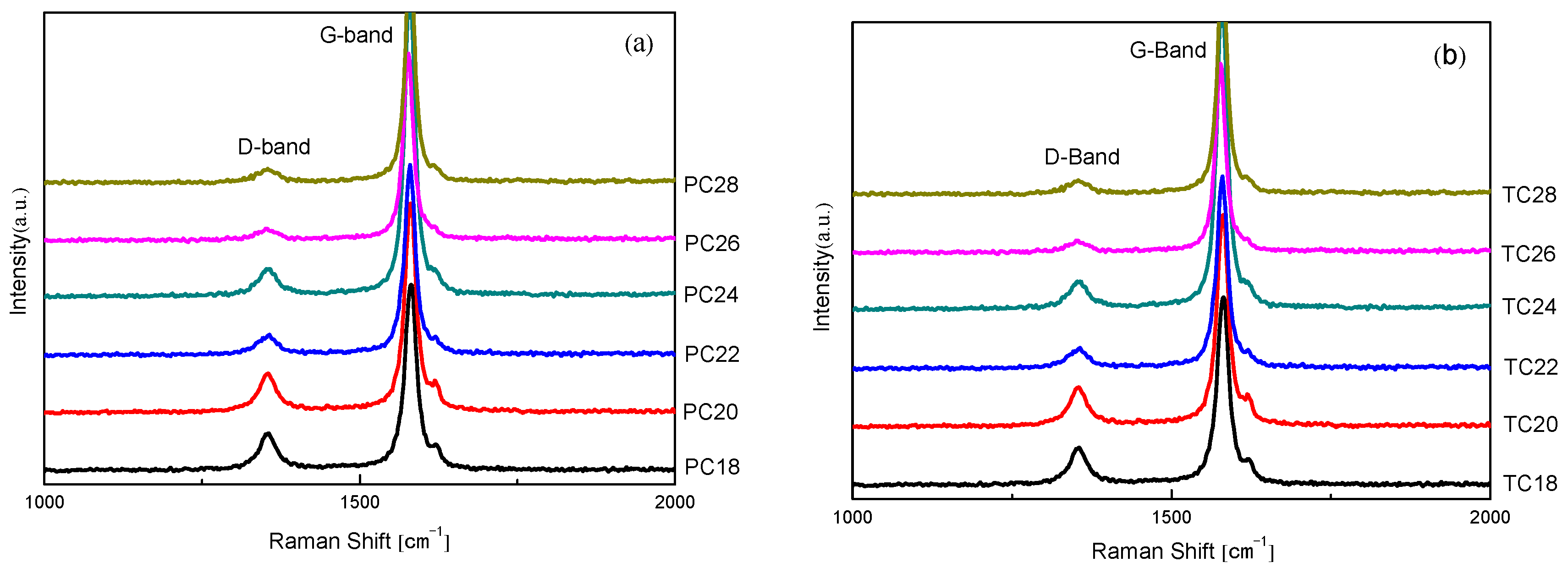
2.2.2. Raman Technique
2.2.3. Electrochemical Measurement
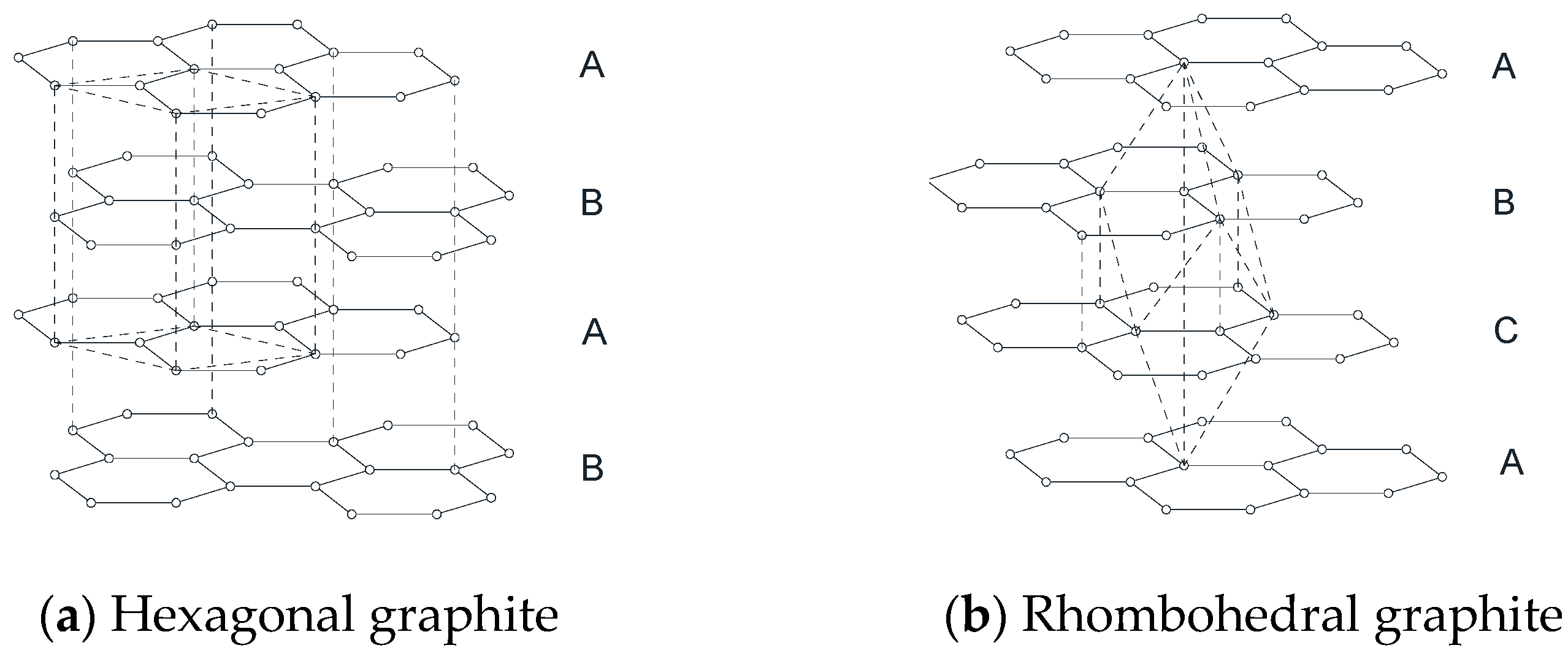
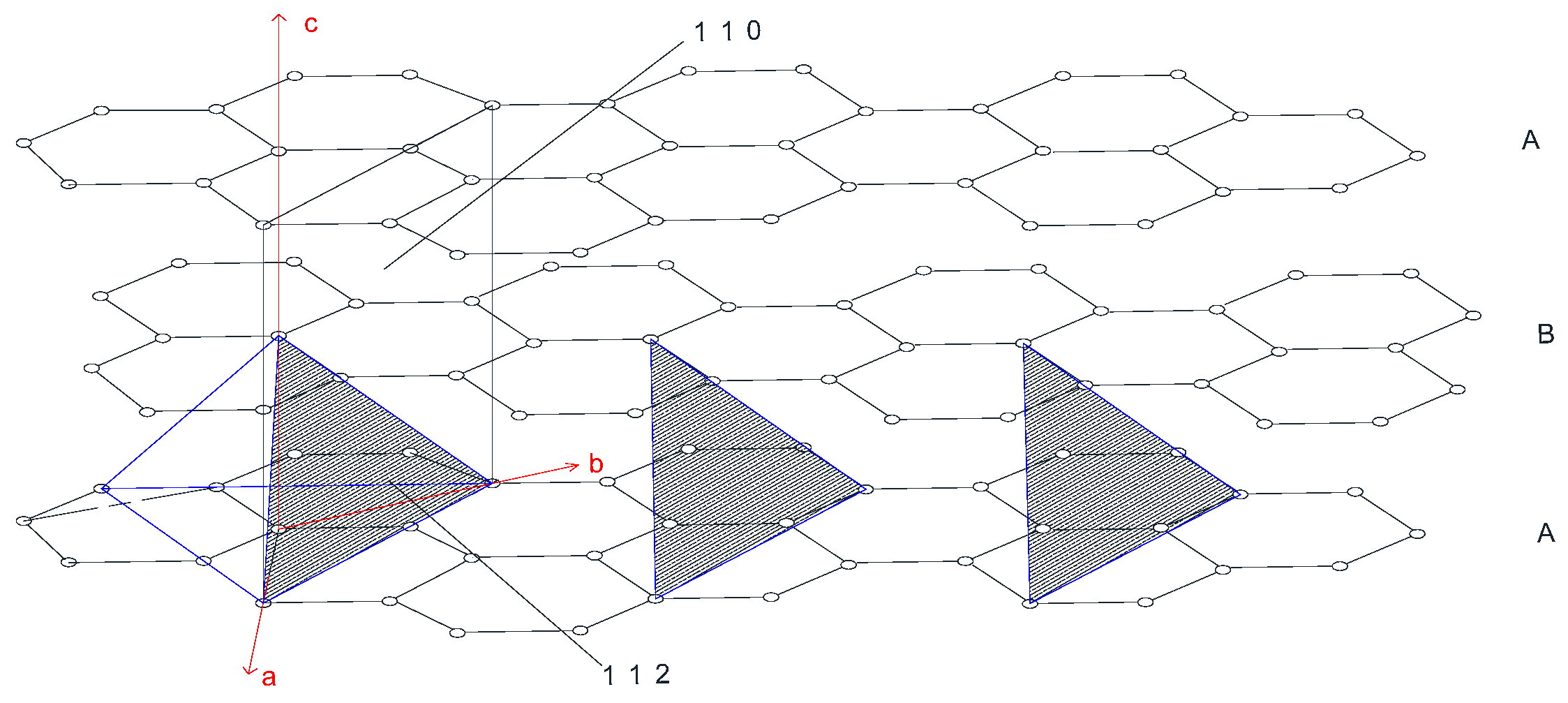
2.3. Review of the Stacking Degree Disorder of 2H-Graphite Method Proposed by Li et al.
h – k = 3n ± 1, l even M = 3P/(2c)
h – k = 3n ± 1, l odd M = P/(2c)
3. Results and Discussion
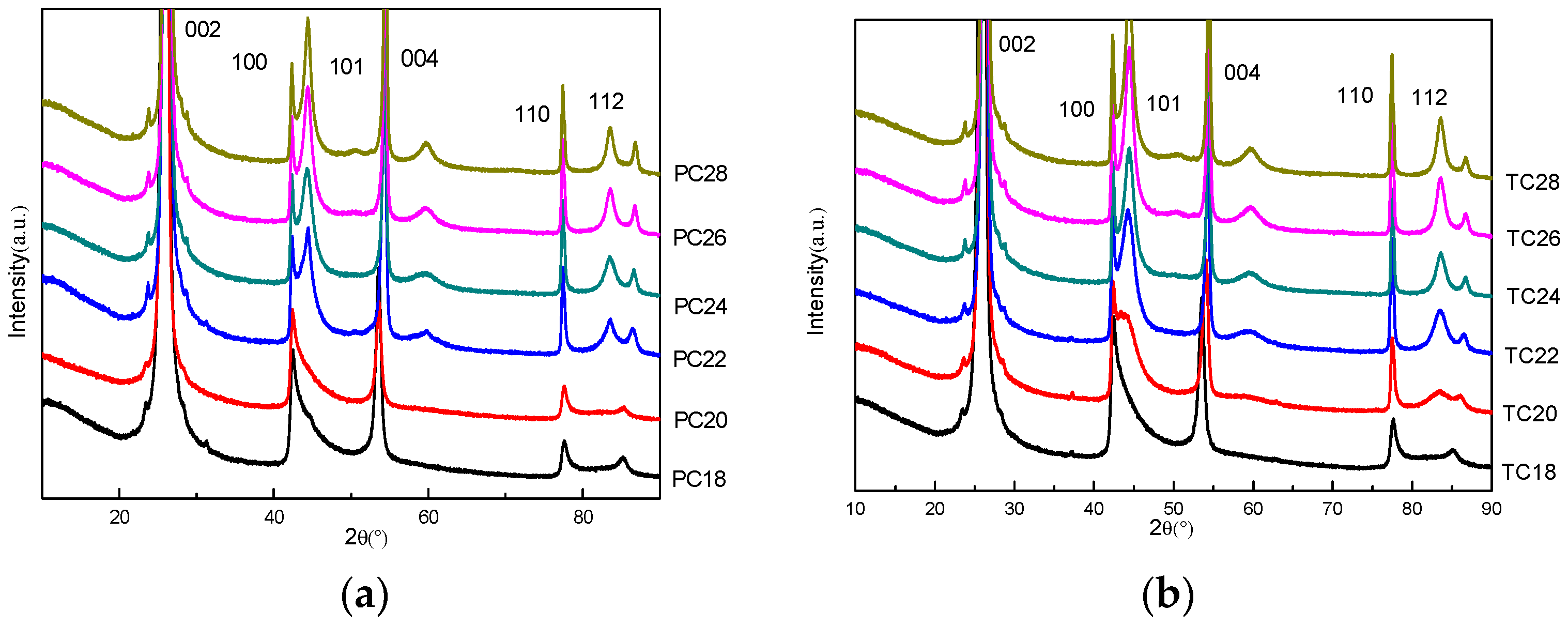
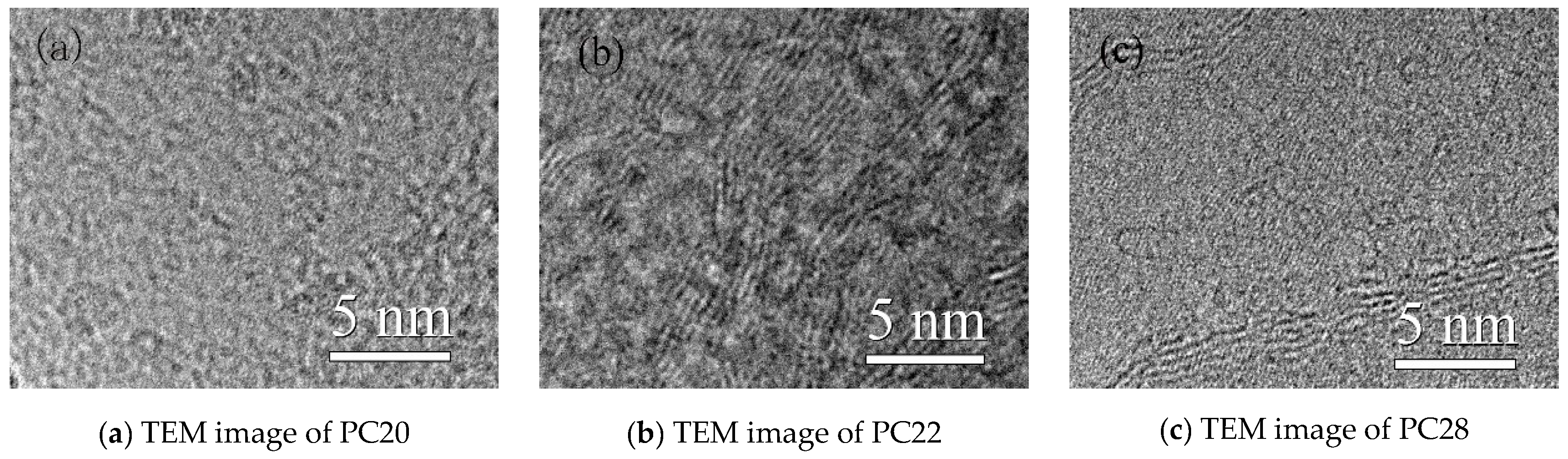
3.1. XRD Spectra and Data Analysis of Two Kinds of Graphitized Coke
3.2. Improved Calculation Method
3.3. Raman Spectral Data Analysis in Two Kinds of Cokes after Graphitization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franklin, E.R. The structure of graphitic carbons. Acta Crystallogr. 1951, 4, 253–261. [Google Scholar] [CrossRef]
- Shi, H.; Reimers, J.N.; Dahn, J.R. Structure-refinement program for disorderd carbon. J. Appl. Cryst. 1993, 26, 827–836. [Google Scholar] [CrossRef]
- Inagaki, M.; Shiraishi, M. The evaluation of graphitization degree. Carbon Tech. 1951, 5, 165–175. [Google Scholar]
- Yang, C.Z.; Jiang, C.H. Line profile analysis and microstructure characterization of diffraction line broaden. PTCA Part A Phys. Test 2014, 50, 658–667. [Google Scholar]
- Li, H.; Yang, C.Z.; Liu, F. Novel method for determining stacking disorder degree in hexagonal graphite by X-ray diffraction. Sci. China Chem. 2009, 52, 174–180. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.Z.; Liu, F. Determining graphitization and disordered degrees in 2H-Graphite by X-Ray diffraction methods. J. Test Meas. Technol. 2009, 23, 161–167. [Google Scholar]
- Langford, J.I.; Bouitif, A. The use of pattern decomposition to study the combined X-ray diffraction effects of crystallite size and stacking faults in ex-oxalate zinc oxide. J. Appl. Crystallogr. 1993, 26, 22–33. [Google Scholar] [CrossRef]
- Inagaki, M.; Feiyu, K. Carbon Materials Science and Engineering: From Fundamentals to Applications; Tsinghua University Press: Beijing, China, 2006. [Google Scholar]
- Wang, H.J.; Wang, H.F. The effect of graphitization temperature on the microstructure and mechanical properties of carbon fibers. New Carbon Mater. 2005, 20, 158–163. [Google Scholar]
- Zerda, W.T.; Xu, W. High pressure Raman and neutron scattering study on structure of carbon black particles. Carbon 2000, 38, 355–361. [Google Scholar] [CrossRef]
- Yang, S. Studies on the Microstructure and Properties of Carbon Fibers by Raman Spectroscopy. Master’s Thesis, Donghua University, Shanghai, China, 2010; pp. 29–36. [Google Scholar]
- Niu, P.X.; Wang, Y.L.; Zhan, L. Electrochemical Performance of Needle Coke and Pitch Coke Used as Anode Material for Li-ion Battery. J. Mater. Sci. Eng. 2011, 29, 204–209. [Google Scholar]
- Zhang, B.; Guo, H.; Li, X.; Wang, Z.; Peng, W. Mechanism for effects of structure and properties of carbon on its electrochemical characteristics as anode of lithium ion battery. J. Cent. South Univ. (Sci. Technol.) 2007, 38, 454–460. [Google Scholar]

| Raw Material | 1800 °C | 2000 °C | 2200 °C | 2400 °C | 2600 °C | 2800 °C | |
|---|---|---|---|---|---|---|---|
| Sample Numbers | Petroleum coke | PC18 | PC20 | PC22 | PC24 | PC26 | PC28 |
| pitch coke | TC18 | TC20 | TC22 | TC24 | TC26 | TC28 |
| Categories | 2θ002 (°) | 2θ101 (°) | d002 (Å) | D002 (nm) | D100 (nm) | D004 (nm) | D112 (nm) |
|---|---|---|---|---|---|---|---|
| PC18 | 25.947 | 3.433 | 24.598 | 10.391 | 13.775 | ||
| PC20 | 25.934 | 3.432 | 25.371 | 10.944 | 14.108 | ||
| PC22 | 26.282 | 44.276 | 3.388 | 33.923 | 20.909 | 18.910 | 8.647 |
| PC24 | 26.328 | 44.212 | 3.382 | 37.209 | 21.386 | 25.026 | 8.788 |
| PC26 | 26.357 | 44.301 | 3.378 | 36.538 | 25.846 | 28.599 | 10.325 |
| PC28 | 26.378 | 44.337 | 3.376 | 39.391 | 26.748 | 29.364 | 12.479 |
| TC18 | 25.928 | 3.434 | 21.697 | 3.864 | 13.721 | ||
| TC20 | 26.171 | 44.031 | 3.402 | 24.772 | 2.838 | 18.130 | 2.254 |
| TC22 | 26.298 | 44.212 | 3.386 | 28.441 | 15.789 | 20.799 | 6.151 |
| TC24 | 26.354 | 44.287 | 3.379 | 29.373 | 22.305 | 23.025 | 8.339 |
| TC26 | 26.370 | 44.288 | 3.377 | 28.851 | 21.731 | 24.363 | 9.802 |
| TC28 | 26.366 | 44.306 | 3.377 | 30.716 | 28.482 | 26.322 | 10.812 |
| Categories | P1 (%) | P2 (%) | Pd002 (%) | Categories | P1 (%) | P2 (%) | Pd002 (%) |
|---|---|---|---|---|---|---|---|
| PC18 | - | - | 71.27 | TC18 | - | - | 73.23 |
| PC20 | - | - | 61.74 | TC20 | 309.85 | 44.88 | 38.22 |
| PC22 | 102.22 | 31.61 | 22.99 | TC22 | 157.74 | 27.28 | 20.92 |
| PC24 | 115.22 | 27.76 | 15.86 | TC24 | 118.02 | 21.17 | 15.92 |
| PC26 | 76.58 | 22.70 | 10.95 | TC26 | 80.21 | 19.32 | 13.49 |
| PC28 | 60.41 | 19.59 | 10.50 | TC28 | 67.50 | 18.26 | 11.81 |
| Categories | ID | IG | R (%) | PR (%) | Categories | ID | IG | R (%) | PR (%) |
|---|---|---|---|---|---|---|---|---|---|
| PC18 | 36996.633 | 132947.533 | 27.78 | 21.76 | TC18 | 14633.458 | 54986.276 | 26.61 | 21.01 |
| PC20 | 11402.538 | 42307.602 | 26.95 | 21.22 | TC20 | 11162.159 | 49591.129 | 22.50 | 18.37 |
| PC22 | 6191.185 | 36309.367 | 17.05 | 14.56 | TC22 | 5100.839 | 29241.519 | 17.43 | 14.85 |
| PC24 | 8125.216 | 57023.172 | 14.24 | 12.47 | TC24 | 6090.779 | 36191.928 | 16.82 | 14.40 |
| PC26 | 4499.386 | 37766.283 | 11.91 | 10.64 | TC26 | 2294.976 | 17445.023 | 13.15 | 11.62 |
| PC28 | 2483.245 | 24031.675 | 10.33 | 9.36 | TC28 | 4954.759 | 49667.213 | 9.97 | 9.07 |
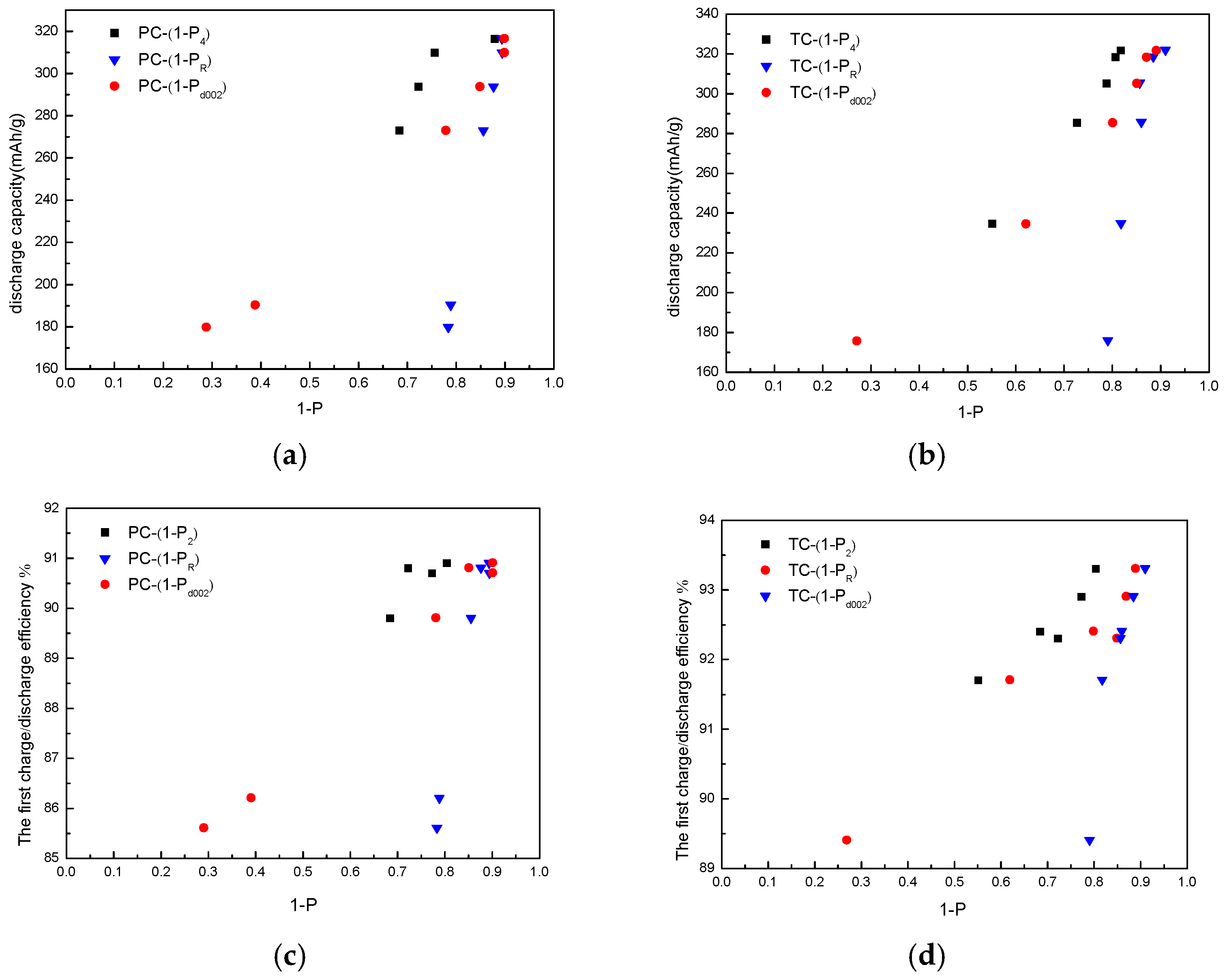
| Categories | PR (%) | Pd002 (%) | P1 (%) | P2 (%) | Charge/Discharge Capacity (mAh/g) | The First Charge-Discharge Efficiency (%) |
|---|---|---|---|---|---|---|
| PC18 | 21.76 | 71.27 | - | - | 179.8/210.0 | 85.6 |
| PC20 | 21.22 | 61.74 | - | - | 190.3/220.8 | 86.2 |
| PC22 | 14.56 | 22.99 | 102.22 | 31.61 | 272.9/303.9 | 89.8 |
| PC24 | 12.47 | 15.86 | 115.22 | 27.76 | 293.6/323.3 | 90.8 |
| PC26 | 10.64 | 10.95 | 76.58 | 22.70 | 309.8/341.6 | 90.7 |
| PC28 | 9.36 | 10.5 | 60.41 | 19.59 | 316.4/348.1 | 90.9 |
| TC18 | 21.01 | 73.23 | - | - | 175.8/196.6 | 89.4 |
| TC20 | 18.37 | 38.22 | - | 44.88 | 234.6/255.8 | 91.7 |
| TC22 | 14.85 | 20.92 | 157.74 | 27.28 | 285.4/308.9 | 92.4 |
| TC24 | 14.4 | 15.92 | 118.02 | 21.17 | 305.1/330.6 | 92.3 |
| TC26 | 11.62 | 13.49 | 80.21 | 19.32 | 318.3/342.6 | 92.9 |
| TC28 | 9.07 | 11.81 | 67.5 | 18.26 | 321.7/344.8 | 93.3 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, Q. The New Method of XRD Measurement of the Degree of Disorder for Anode Coke Material. Crystals 2017, 7, 5. https://doi.org/10.3390/cryst7010005
Zhang Z, Wang Q. The New Method of XRD Measurement of the Degree of Disorder for Anode Coke Material. Crystals. 2017; 7(1):5. https://doi.org/10.3390/cryst7010005
Chicago/Turabian StyleZhang, Zhuo, and Qi Wang. 2017. "The New Method of XRD Measurement of the Degree of Disorder for Anode Coke Material" Crystals 7, no. 1: 5. https://doi.org/10.3390/cryst7010005






