Ionic Liquid Crystals Modifier for Selective Determination of Terazosin Antihypertensive Drug in Presence of Common Interference Compounds
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Electrochemical Cell and Equipment
2.3. Preparation of Carbon Paste Electrode (CPE)
2.4. Preparation of Working Electrode (ILCCPE)
2.5. Analysis of Urine
3. Results and Discussion
3.1. Morphology Study
3.2. Comparison between Different Modified Electrodes
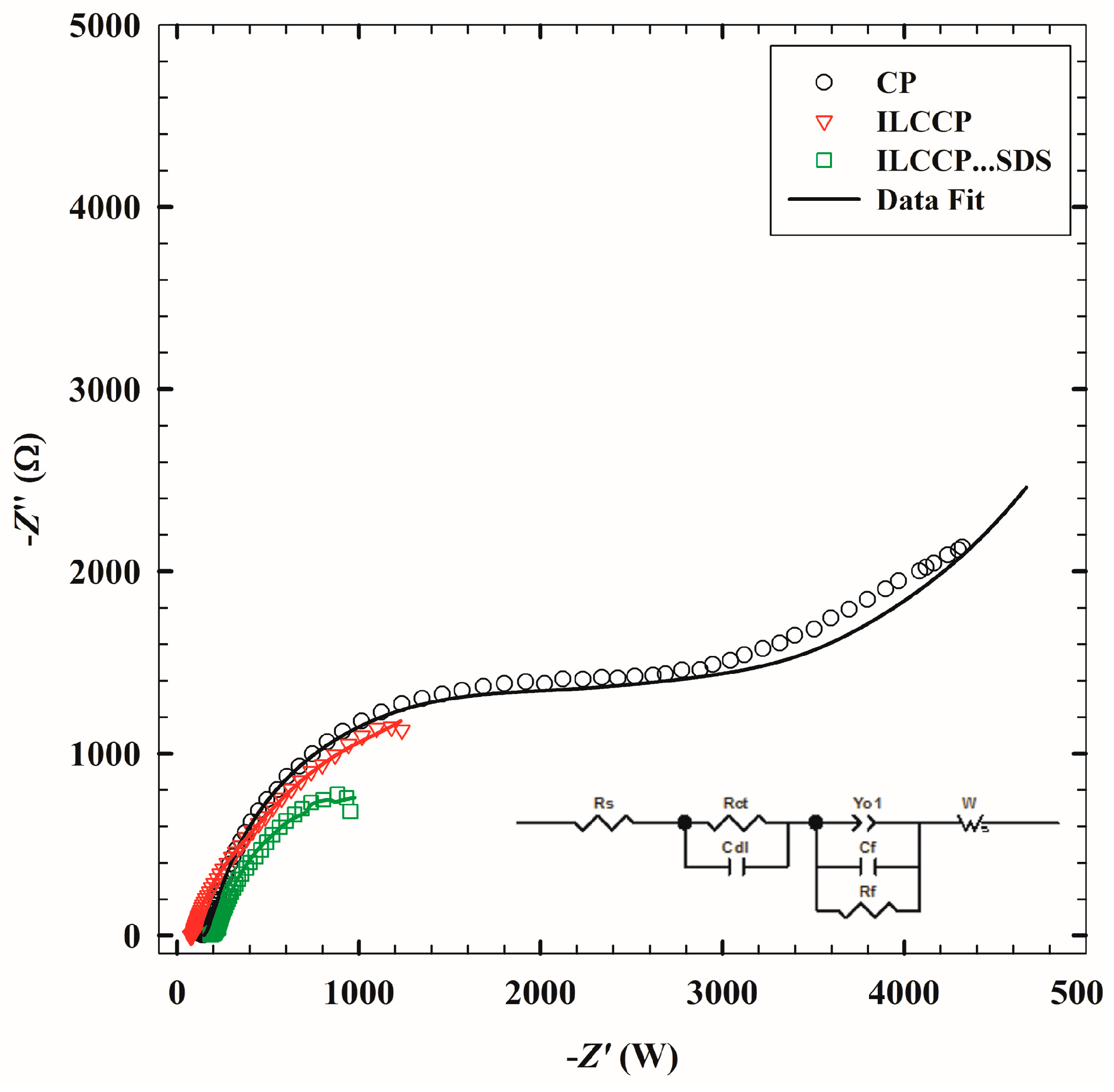
3.3. Electrochemical Impedance Spectroscopy
3.4. Effect of Scan Rate
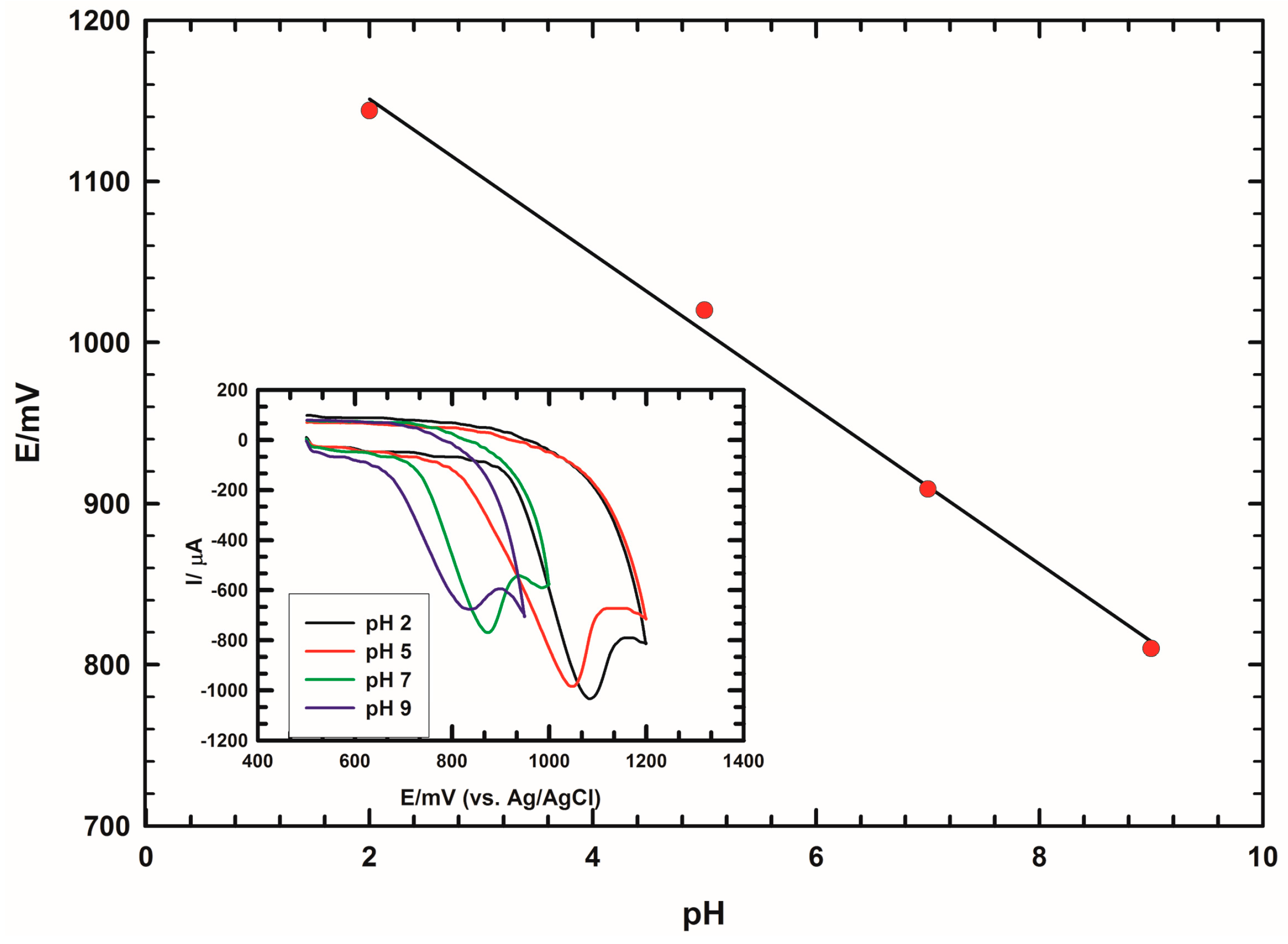
3.5. pH Effect
3.6. Stability, Repeatability and Reproducibility of the Proposed Sensor
4. Determination of TZ in Real Samples
4.1. Determination of TZ in Urine
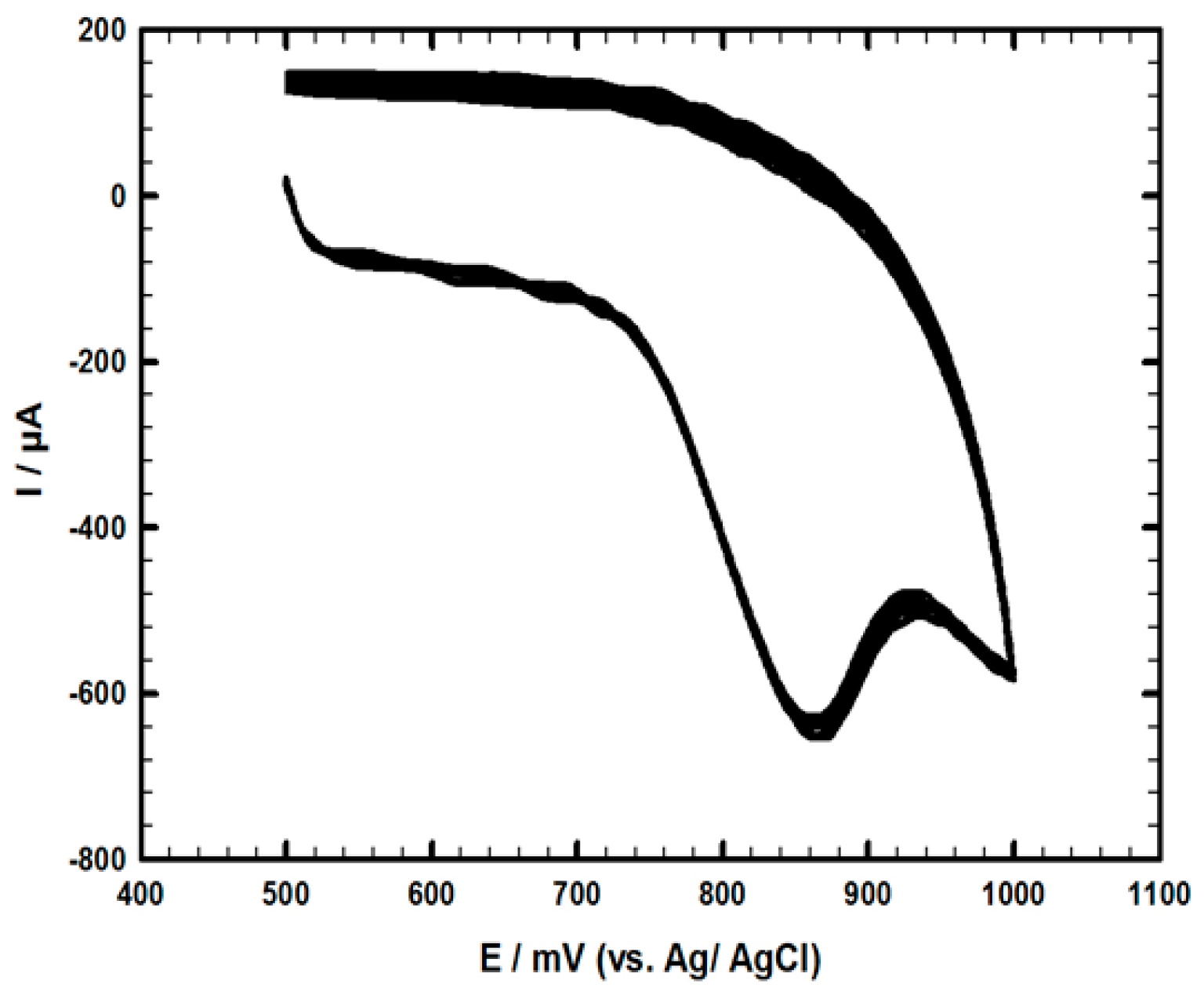
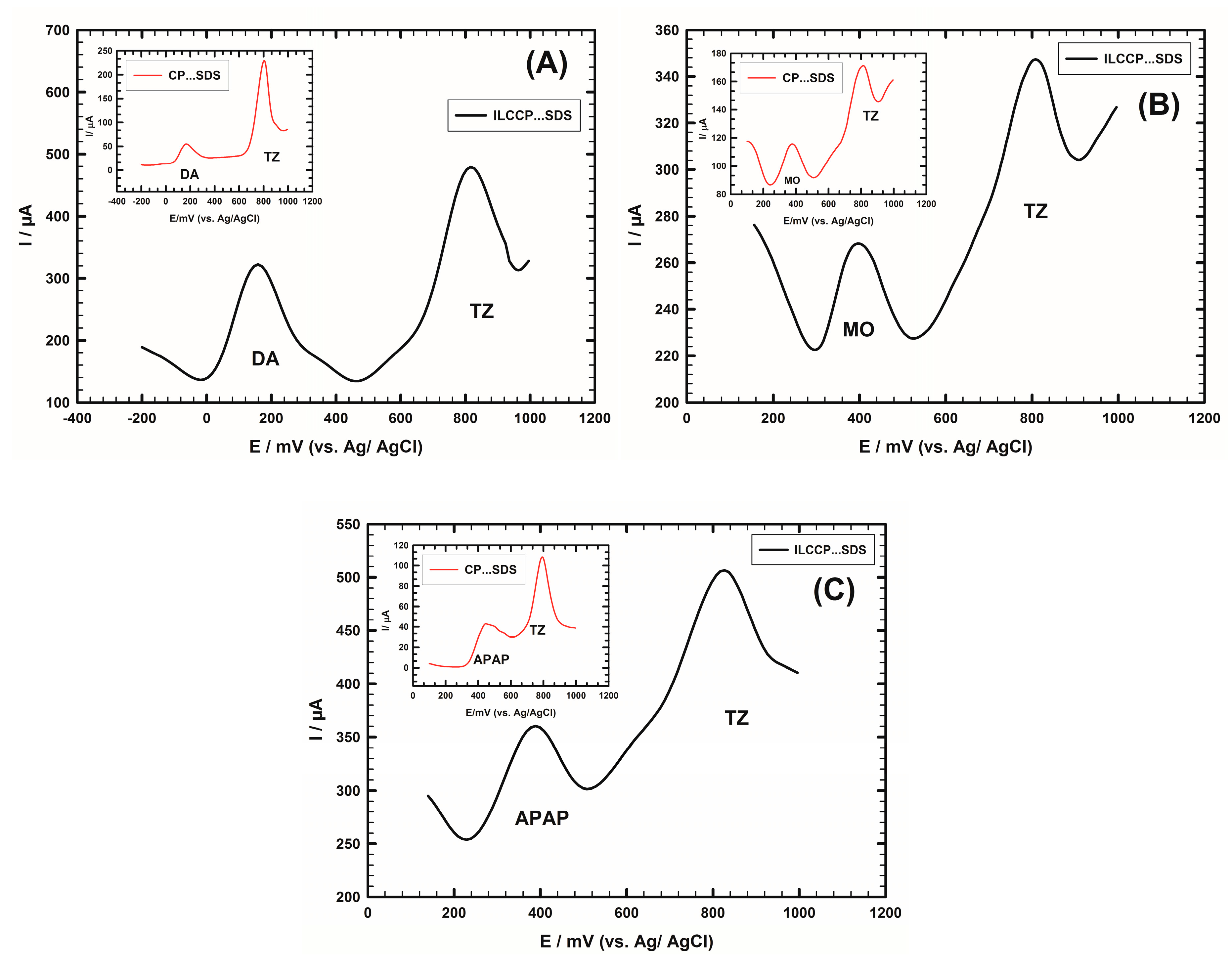
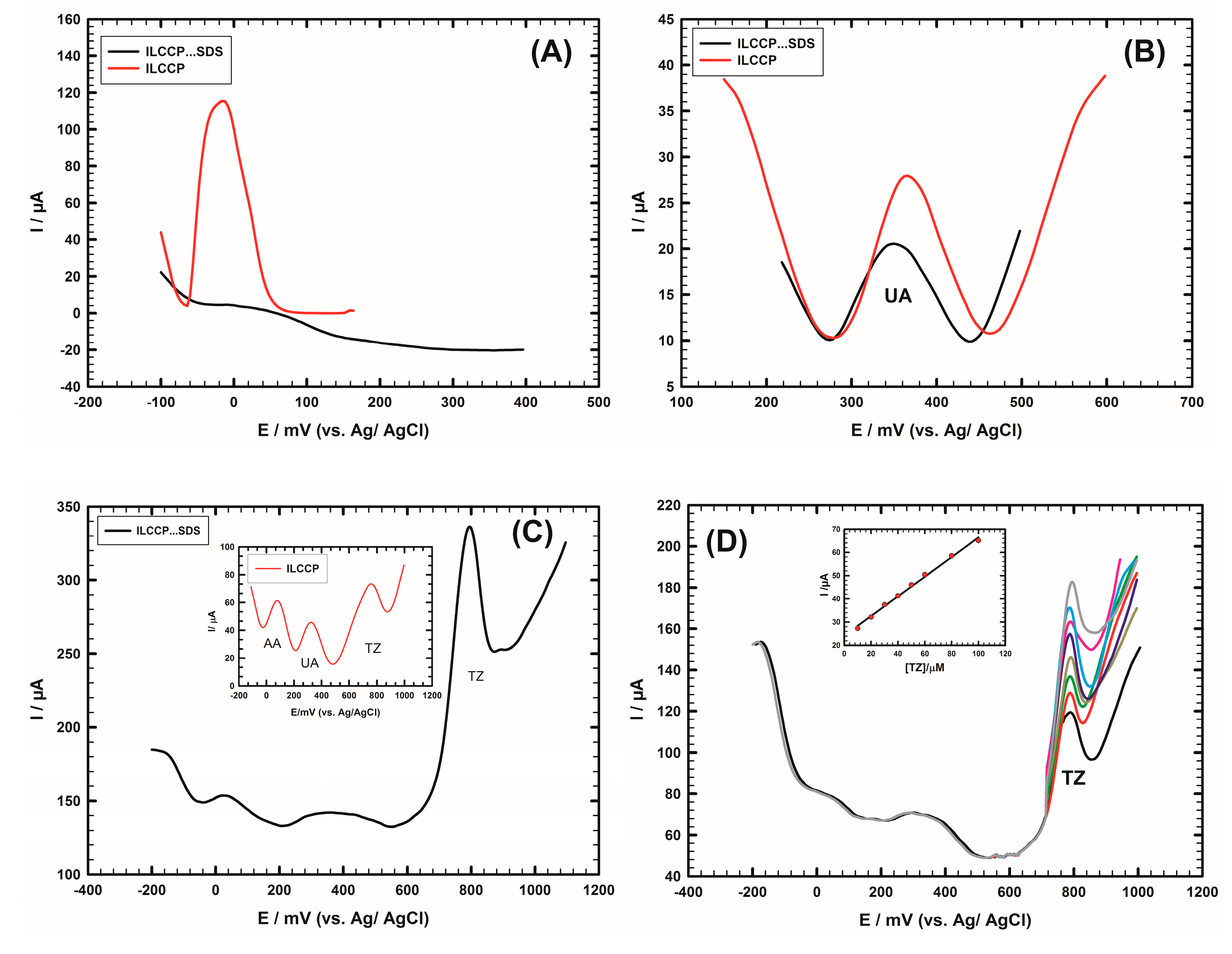
4.2. Interference Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abraham, P.A.; Halstenson, C.E.; Matzke, G.R.; Napier, J.L.; Keane, W.F. Antihypertensive therapy with once-daily administration of terazosin, a new alpha1-adrenergic-receptor blocker. Pharmacotherapy 1985, 5, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Miller, K. Pharmacological management of hypertension in paediatric patients. Drugs 1994, 48, 868–887. [Google Scholar] [CrossRef] [PubMed]
- Deger, G. Effect of terazosin on serum lipids. Am. J. Med. 1986, 80, 82–85. [Google Scholar] [CrossRef]
- Fabricius, P.G.; Weizert, P.; Dunzendorfer, U.; Hannaford, J.M.; Maurath, C. Efficacy of once-a-day terazosin in benign prostatic hyperplasia: A randomized, double-blind placebo-controlled clinical trial. Prostate 1990, 17, 85–93. [Google Scholar] [CrossRef]
- Patterson, S.E. Determination of terazosin in human plasma using high performance liquid chromatography with fluorescence detection. J. Chromatogr. B 1984, 311, 206–212. [Google Scholar] [CrossRef]
- Bhamra, R.K.; Flanagan, R.J.; Holt, D.W. High performance liquid chromatographic measurement of prazosin and terazosin in biological fluids. J. Chromatogr. B 1986, 380, 216–221. [Google Scholar] [CrossRef]
- Bauer, J.F.; Krogh, S.K.; Chang, Z.L.; Wong, C.F. Determination of minor impurities in terazosin hydrochloride by high-performance liquid chromatography. J. Chromatogr. A 1993, 648, 175–181. [Google Scholar] [CrossRef]
- Ferretti, R.; Gallinella, B.; La Torre, F.; Zanitti, L.; Turchetto, L.; Mosca, A.; Cirilli, R. Direct high-performance liquid chromatography enantioseparation of terazosin on an immobilised polysaccharide-based chiral stationary phase under polar organic and reversed-phase conditions. J. Chromatogr. A 2009, 1216, 5385–5390. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.-Y.; Wu, H.-L.; Yang, L.-Q.O.; Zhang, Y.; Nie, J.-F.; Fu, H.-Y.; Yu, R.-Q. Fluorescent quantification of terazosin hydrochloride content in human plasma and tablets using second-order calibration based on both parallel factor analysis and alternating penalty trilinear decomposition. Anal. Chim. Acta 2009, 650, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zavitsanos, A.P.; Alebic-Kolbah, T. Enantioselective determination of terazosin in human plasma by normal phase high-performance liquid chromatography–electrospray mass spectrometry. J. Chromatogr. A 1998, 794, 45–56. [Google Scholar] [CrossRef]
- Srinivas, J.S.; Avadhavulu, A.B.; Anjaneyulu, Y. HPLC determination of terazosin hydrochloride in its pharmaceutical dosage forms. Indian Drugs 1998, 35, 269–273. [Google Scholar]
- Sekhar, E.C.; Rao, T.R.K.; Sekhar, K.R.; Naidu, M.U.R.; Shobha, J.C.; Rani, P.U.; Kumar, T.V.; Kumar, V.P. Determination of terazosin in human plasma, using high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 1998, 710, 137–142. [Google Scholar] [CrossRef]
- Cheah, P.Y.; Yuena, K.H.; Liong, M.L. Improved high performance liquid chromatographic analysis of terazosin in human plasma. J. Chromatogr. B 2000, 745, 439–443. [Google Scholar] [CrossRef]
- Niu, C.Q.; Ren, L.M. Chiral separation and preparation of three new antagonists of alpha 1-adrenoceptors by chiral mobile phase HPLC. Yao Xue Xue Bao 2002, 37, 450–453. [Google Scholar] [PubMed]
- Bakshi, M.; Ojha, T.; Singh, S. Validated specific HPLC methods for determination of prazosin, terazosin and doxazosin in the presence of degradation products formed under ICH-recommended stress conditions. J. Pharm. Biomed. Anal. 2004, 34, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, C. Determination of terazosin in human plasma by high performance liquid chromatography with ultraviolet detection. Asian J. Tradit. Med. 2006, 52, 1–3. [Google Scholar]
- Ren, X.; Dong, Y.; Huang, A.; Sun, Y.; Sun, Z. Separation of the enantiomers of four chiral drugs by neutral cyclodextrin-mediated capillary zone electrophoresis. Chromatographia 1999, 49, 411–414. [Google Scholar] [CrossRef]
- Abdine, H.H.; El-Yazbi, F.A.; Blaih, S.M.; Shaalan, R.A. Spectrophotometric and spectrofluorimetric methods for the determination of terazosin in dosage forms. Spectrosc. Lett. 1998, 31, 969–980. [Google Scholar] [CrossRef]
- Jiang, C.-Q.; Gao, M.-X.; He, J.-X. Study of the interaction between terazosin and serum albumin: synchronous fluorescence determination of terazosin. Anal. Chim. Acta 2002, 452, 185–189. [Google Scholar] [CrossRef]
- Atta, N.F.; Darwish, S.A.; Khalil, S.E. Effect of surfactants on the voltammetric response and determination of an antihypertensive drug. Talanta 2007, 72, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Pournaghi-Azar, M.H.; Saadatirad, A. Simultaneous voltammetric and amperometric determination of morphine and codeine using a chemically modified-palladized aluminum electrode. J. Electroanal. Chem. 2008, 624, 293–298. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, R.A. Direct and simple electrochemical determination of morphine at PEDOT modified Pt electrode. Electroanalysis 2011, 23, 737–746. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El Ries, M.A.; Hammam, E.; Beltagi, A.M. A validated stripping voltammetric procedure for quantification of the anti-hypertensive and benign prostatic hyperplasia drug terazosin in tablets and human serum. Talanta 2004, 64, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Molaakbari, E.; Mostafavi, A.; Beitollahi, H.; Alizadeh, R. Synthesis of ZnO nanorods and their application in the construction of a nanostructure-based electrochemical sensor for determination of levodopa in the presence of carbidopa. Analyst 2014, 139, 4356–4364. [Google Scholar] [CrossRef] [PubMed]
- Shabani-Nooshabadi, M.; Tahernejad-Javazmi, F. Electrocatalytic determination of hydroxylamine in the presence of thiosulfate in water and wastewater samples using a nanostructure modified carbon paste electrode. Electroanalysis 2015, 27, 1733–1741. [Google Scholar] [CrossRef]
- Mahmoudi Moghaddam, H.; Beitollahi, H.; Tajik, S.; Soltani, H. Fabrication of a Nanostructure Based Electrochemical Sensor for Voltammetric Determination of Epinephrine, Uric Acid and Folic Acid. Electroanalysis 2015, 27, 2620–2628. [Google Scholar] [CrossRef]
- Novoselov, K.S.A.; Geim, A.K.; Morozov, S.; Jiang, D.; Katsnelson, M.; Grigorieva, I.; Dubonos, S.; Firsov, A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon Paste Electrodes in Facts, Numbers, and Notes: A Review on the Occasion of the 50-Years Jubilee of Carbon Paste in Electrochemistry and Electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Binnemans, K. Ionic liquid crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, M.; Jiao, K. Electrocatalytic oxidation of dopamine at an ionic liquid modified carbon paste electrode and its analytical application. Anal. Bioanal. Chem. 2007, 389, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, J.; Wang, X.; Li, T.; Li, G.; Wu, J.; Zhang, L. Electrochemical oxidation of adenosine-5′-triphosphate on a chitosan–graphene composite modified carbon ionic liquid electrode and its determination. Mater. Sci. Eng. C 2012, 32, 2129–2134. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Qin, X.; Zhan, G.; Ma, M.; Li, C. Electrochemical fabrication of TiO2 nanoparticles/[BMIM] BF4 ionic liquid hybrid film electrode and its application in determination of p-acetaminophen. Mater. Sci. Eng. C 2012, 32, 2280–2285. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M. Coupling of NiO Nanoparticles and Room Temperature Ionic Liquid for Fabrication of Highly Sensitive Voltammetric Sensor in Tryptophan Analysis. Anal. Bioanal. Electrochem. 2012, 8, 578–588. [Google Scholar]
- Beitollahi, H.; Tajik, S.; Jahani, S. Electrocatalytic Determination of Hydrazine and Phenol Using a Carbon Paste Electrode Modified with Ionic Liquids and Magnetic Core-shell Fe3O4@SiO2/MWCNT Nanocomposite. Electroanalysis 2016, 28, 1093–1099. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M.; Tahernejad-Javazmi, F. Graphene oxide/NiO nanoparticle composite-ionic liquid modified carbon paste electrode for selective sensing of 4-chlorophenol in the presence of nitrite. J. Mol. Liq. 2016, 219, 142–148. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M. Modification of carbon paste electrode with NiO/graphene oxide nanocomposite and ionic liquids for fabrication of high sensitive voltammetric sensor on sulfamethoxazole analysis. J. Mol. Liq. 2016, 220, 329–333. [Google Scholar] [CrossRef]
- Sun, W.; Dong, L.; Deng, Y.; Yu, J.; Wang, W.; Zhu, Q. Application of N-doped graphene modified carbon ionic liquid electrode for direct electrochemistry of hemoglobin. Mater. Sci. Eng. C 2014, 39, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Analytical applications of room-temperature ionic liquids: A review of recent efforts. Anal. Chim. Acta 2006, 556, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, S.F.Y. An ionic liquid coating for determination of sildenafil and UK-103,320 in human serum by capillary zone electrophoresis-ion trap mass spectrometry. Electrophoresis 2002, 23, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Silvester, D.S. Recent advances in the use of ionic liquids for electrochemical sensing. Analyst 2011, 136, 4871–4882. [Google Scholar] [CrossRef] [PubMed]
- Opallo, M.; Lesniewski, A. A review on electrodes modified with ionic liquids. J. Electroanal. Chem. 2011, 656, 2–16. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Rezaei, B.; Krimi-Maleh, H. An ionic liquid-type multiwall carbon nanotubes paste electrode for electrochemical investigation and determination of morphine. Ionics (Kiel) 2011, 17, 659–668. [Google Scholar] [CrossRef]
- Liu, X.; Ding, Z.; He, Y.; Xue, Z.; Zhao, X.; Lu, X. Electrochemical behavior of hydroquinone at multi-walled carbon nanotubes and ionic liquid composite film modified electrode. Colloids Surf. B Biointerfaces 2010, 79, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.N.; Swamy, B.E.K.; Ashoka, N.B.; Bannanakere, M.P. Simultaneous electrochemical determination of epinephrine and uric acid at 1-butyl-4-methyl-pyridinium tetrafluroborate ionic liquid modified carbon paste electrode: A voltammetric study. J. Mol. Liq. 2012, 165, 168–172. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Bahrami, H.; Rezaei, B.; Karimi-Maleh, H. Application of ionic liquid-TiO2 nanoparticle modified carbon paste electrode for the voltammetric determination of benserazide in biological samples. Mater. Sci. Eng. C 2013, 33, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiang, Q.; Yang, M.; Jiao, K. Electrochemical behaviors of hydroquinone on a carbon paste electrode with ionic liquid as binder. Bull. Korean Chem. Soc. 2008, 29, 915–921. [Google Scholar]
- Liu, L.-H.; Duan, C.-Q.; Gao, Z.-N. Electrochemical behavior and electrochemical determination of carbamazepine at an ionic liquid modified carbon paste electrode in the presence of sodium dodecyl sulfate. J. Serbian Chem. Soc. 2012, 77, 483–496. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 2399–2407. [Google Scholar] [CrossRef]
- Huang, K.-J.; Niu, D.-J.; Sun, J.-Y.; Zhu, X.-L.; Zhu, J.-J. Label-free amperometric immunobiosensor based on a gold colloid and Prussian blue nanocomposite film modified carbon ionic liquid electrode. Anal. Bioanal. Chem. 2010, 397, 3553–3561. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, X.; Jiao, K. Direct Electrochemistry of Myoglobin in a Nafion-Ionic Liquid Composite Film Modified Carbon Ionic Liquid Electrode. Electroanalysis 2009, 21, 959–964. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, F.; Li, J.; Liu, L.; Zeng, B. Characterization of hydrophobic ionic liquid-carbon nanotubes—Gold nanoparticles composite film coated electrode and the simultaneous voltammetric determination of guanine and adenine. Electrochim. Acta 2008, 53, 7781–7788. [Google Scholar] [CrossRef]
- Arvand, M.; Shiraz, M.G. Voltammetric Determination of Clozapine in Pharmaceutical Formulations and Biological Fluids Using an In Situ Surfactant-Modified Carbon Ionic Liquid Electrode. Electroanalysis 2012, 24, 683–690. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Musameh, M.M.; Abu-Yousef, I.; Yousef, J.M.; Kanan, S.M.; Xiao, L.; Davies, S.G.; Russell, A.; Compton, R.G. Carbon nanotube- ionic liquid composite sensors and biosensors. Anal. Chem. 2008, 81, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shan, C.; Wang, X.; Chen, L.; Niu, L.; Chen, B. New ionic liquid crystals based on azobenzene moiety with two symmetric imidazolium ion group substituents. Liq. Cryst. 2008, 35, 1299–1305. [Google Scholar] [CrossRef]
- Cheng, X.; Bai, X.; Jing, S.; Ebert, H.; Prehm, M.; Tschierske, C. Self-Assembly of Imidazolium-Based Rodlike Ionic Liquid Crystals: Transition from Lamellar to Micellar Organization. Chem. Eur. J. 2010, 16, 4588–4601. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Kumar, S. Microwave-assisted synthesis of novel imidazolium-based ionic liquid crystalline dimers. Tetrahedron Lett. 2006, 47, 8993–8997. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Azab, S.M.; Ibrahim, A.H. Electrochemical Sensor Based on Ionic Liquid Crystal Modified Carbon Paste Electrode in Presence of Surface Active Agents for Enoxacin Antibacterial Drug. J. Electrochem. Soc. 2015, 162, B9–B15. [Google Scholar] [CrossRef]
- Galal, A.; Atta, N.F.; Azab, S.M.; Ibrahim, A.H. Electroanalysis of Benazepril Hydrochloride Antihypertensive Drug Using an Ionic Liquid Crystal Modified Carbon Paste Electrode. Electroanalysis 2015, 27, 1282–1292. [Google Scholar] [CrossRef]
- Zanardi, C.; Terzi, F.; Seeber, R. Composite electrode coatings in amperometric sensors. Effects of differently encapsulated gold nanoparticles in poly(3,4-ethylendioxythiophene) system. Sens. Actuators B Chem. 2010, 148, 277–282. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Abu-Attia, F.M.; Azab, S.M. Characterization and electrochemical investigations of micellar/drug interactions. Electrochim. Acta 2011, 56, 2510–2517. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ekram, H. A novel sensor of cysteine self-assembled monolayers over gold nanoparticles for the selective determination of epinephrine in presence of sodium dodecyl sulfate. Analyst 2012, 137, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Galal, A.; Atta, N.F.; Ekram, H. Probing cysteine self-assembled monolayers over gold nanoparticles—Towards selective electrochemical sensors. Talanta 2012, 93, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.S.; Swamy, B.E.K. Detection of epinephrine in presence of serotonin and ascorbic acid by TTAB modified carbon paste electrode: A voltammetric study. Int. J. Electrochem. Sci. 2014, 9, 1321–1339. [Google Scholar]
- Shrivastav, R.; Satsangee, S.P.; Jain, R. Effect of Surfactants on the Voltammetric Response and Determination of an Antihypertensive Drug Phentolamine at Boron Doped Diamond Electrode. ECS Trans. 2013, 50, 23–26. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Kurbanoglu, S.; Gumustas, M.; Uslu, B.; Ozkan, S.A. Electrochemical approach for the sensitive determination of anticancer drug epirubicin in pharmaceuticals in the presence of anionic surfactant. Rev. Roum. Chim. 2013, 58, 647–658. [Google Scholar]
- Teradal, N.L.; Kalanur, S.S.; Prashanth, S.N.; Seetharamappa, J. Electrochemical investigations of an anticancer drug in the presence of sodium dodecyl sulfate as an enhancing agent at carbon paste electrode. J. Appl. Electrochem. 2012, 42, 917–923. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, S. Glassy carbon electrode modified with multi-walled carbon nanotubes sensor for the quantification of antihistamine drug pheniramine in solubilized systems. J. Pharm. Anal. 2012, 2, 56–61. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ali, S.M.; Hassan, S.H. Electrochemistry and detection of dopamine at a poly(3,4-ethylenedioxythiophene) electrode modified with ferrocene and cobaltocene. Ionics (Kiel) 2015, 21, 2371–2382. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ekram, H.E. Gold nanoparticles-coated poly(3,4-ethylene-dioxythiophene) for the selective determination of sub-nano concentrations of dopamine in presence of sodium dodecyl sulfate. Electrochim. Acta 2012, 69, 102–111. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Ads, E.H. Smart electrochemical morphine sensor using poly(3,4-ethylene-dioxythiophene)/gold-nanoparticles composite in presence of surfactant. Int. J. Electrochem. Sci 2014, 9, 2113–2131. [Google Scholar]
- Giang, H.T.; Duy, H.T.; Ngan, P.Q.; Thai, G.H.; Toan, N.N. Hydrocarbon gas sensing of nano-crystalline perovskite oxides LnFeO3 (Ln = La, Nd and Sm). Sens. Actuators B Chem. 2011, 158, 246–251. [Google Scholar] [CrossRef]
- Axenov, K.V.; Laschat, S. Thermotropic ionic liquid crystals. Materials (Basel) 2011, 4, 206–259. [Google Scholar] [CrossRef]
- Wang, X.; Hu, S.; Li, Q.; Li, F.; Yao, K.; Shi, M. Inducting effects of ionic liquid crystal modified-PEDOT: PSS on the performance of bulk heterojunction polymer solar cells. RSC Adv. 2015, 5, 52874–52881. [Google Scholar] [CrossRef]
- Maleki, N.; Safavi, A.; Tajabadi, F. High-performance carbon composite electrode based on an ionic liquid as a binder. Anal. Chem. 2006, 78, 3820–3826. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Madrakian, T.; Ghasemi, H.; Afkhami, A.; Haghshenas, E. ZnO/rGO nanocomposite/carbon paste electrode for determination of terazosin in human serum samples. RSC Adv. 2016, 6, 2552–2558. [Google Scholar] [CrossRef]
- Afkhami, A.; Ghaedi, H.; Madrakian, T.; Ahmadi, M.; Mahmood-Kashani, H. Fabrication of a new electrochemical sensor based on a new nano-molecularly imprinted polymer for highly selective and sensitive determination of tramadol in human urine samples. Biosens. Bioelectron. 2013, 44, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shawish, H.M.; Ghalwa, N.A.; Zaggout, F.R.; Saadeh, S.M.; Al-Dalou, A.R.; Assi, A.A.A. Improved determination of tramadol hydrochloride in biological fluids and pharmaceutical preparations utilizing a modified carbon paste electrode. Biochem. Eng. J. 2010, 48, 237–245. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Srivastava, A.K. Simultaneous voltammetric determination of acetaminophen and tramadol using Dowex50wx2 and gold nanoparticles modified glassy carbon paste electrode. Anal. Chim. Acta 2011, 706, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Galal, A.; Ahmed, R.A. Poly(3,4-ethylene-dioxythiophene) electrode for the selective determination of dopamine in presence of sodium dodecyl sulfate. Bioelectrochemistry 2011, 80, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Galal, A.; Azab, S.M. Gold Nanoparticles Modified Electrode for the Determination of an Antihypertensive Drug. Electroanalysis 2012, 24, 1431–1440. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Stability-Indicating RP-HPLC Method for the Simultaneous Determination of Prazosin, Terazosin, and Doxazosin in Pharmaceutical Formulations. Sci. Pharm. 2012, 80, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.S.; Mohamed, T.A. Potentiometric and Fluorimetric Methods for the Determination of Terazosin HCl in Drug Substance and Dosage Forms. Int. J. Electrochem. 2014, 9, 7394–7413. [Google Scholar]
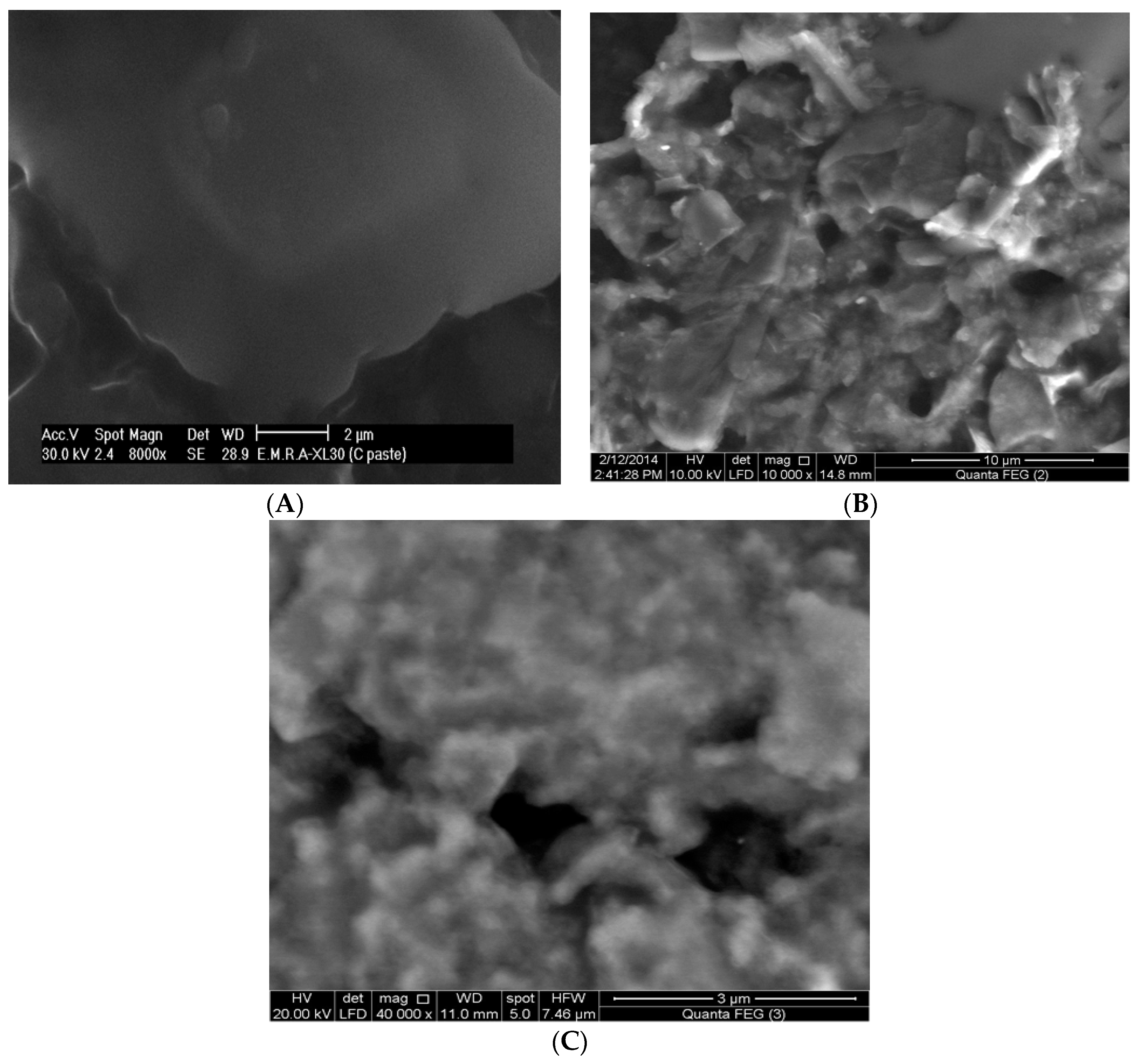



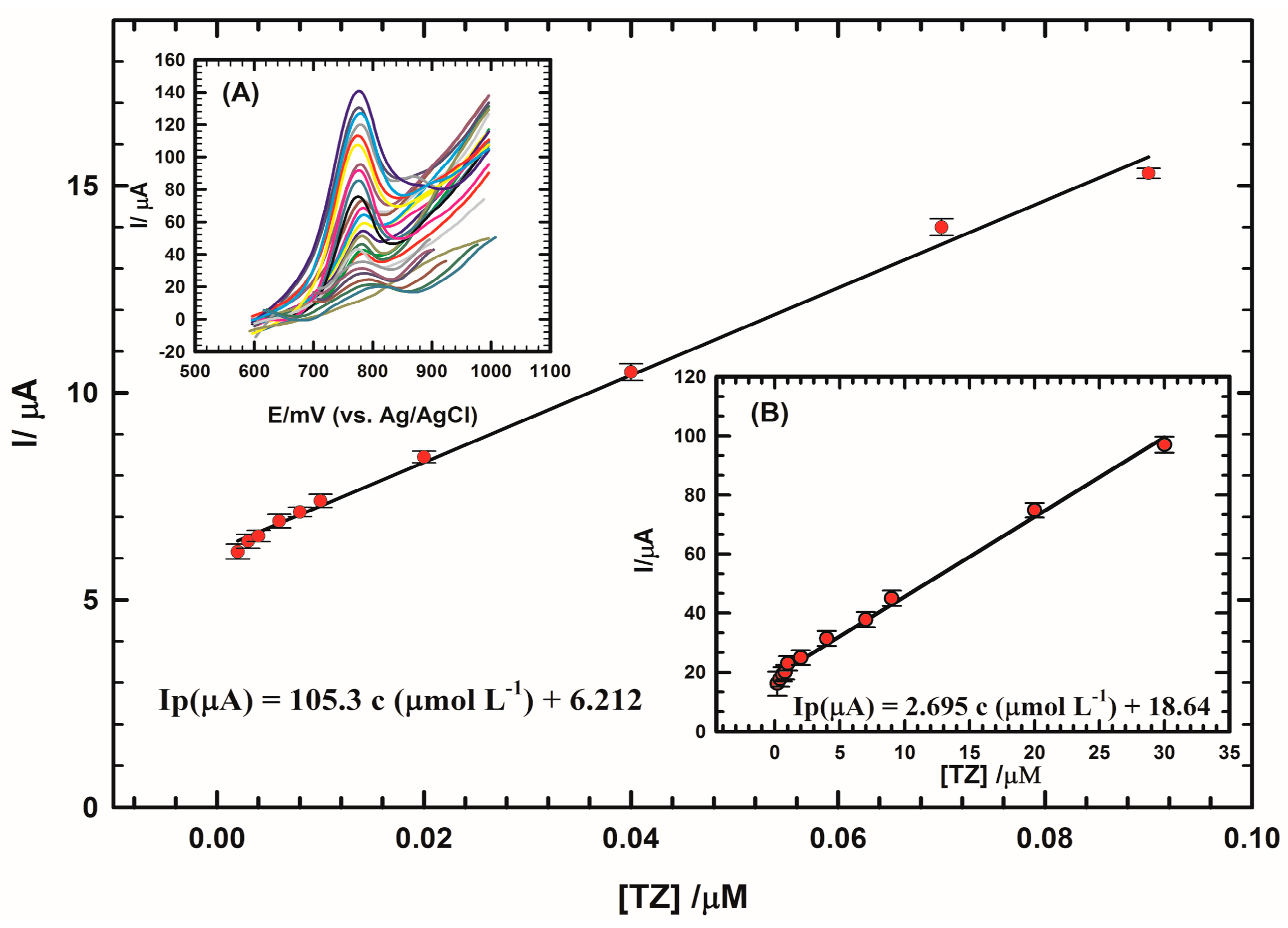
| Electrode | Epa (mV) | Ipa (µA) | Dapp × 10−5 (cm2/s) |
|---|---|---|---|
| CPE | 850 | 86 | 0.424 |
| CPE…SDS | 863 | 120 | 0.827 |
| ILCCPE | 828 | 285 | 4.64 |
| ILCCPE…SDS | 874 | 707 | 28.7 |
| IL1CPE…SDS | 842 | 319 | 5.85 |
| IL2CPE…SDS | 839 | 290 | 4.83 |
| Electrode | Rs (Ω·cm2) | Rct (×10−3 Ω·cm2) | Cdl (×103 F·cm−2) | Yo1 (×10−4 F·cm−2) | n | Cf (×104 F·cm−2) | Rf (×10−3 Ω·cm2) | W (×10−3 Ω·s−1/2) |
|---|---|---|---|---|---|---|---|---|
| CPE | 183 | 3.23 | 0.0199 | 1.09 | 0.980 | 2.94 | 1.11 | 1.13 |
| ILCCPE | 148 | 1.59 | 1.21 | 2.04 | 0.910 | 7.32 | 0.848 | 1.10 |
| ILCCPE…SDS | 80.9 | 0.739 | 1.48 | 53.0 | 0.933 | 4.49 | 0.366 | 0.552 |
| Sample | [TZ] Added (µmol·L−1) | [TZ] Found (µmol·L−1) a | Recovery (%) | Standard Deviation | Standard Error b |
|---|---|---|---|---|---|
| /10−8 | /10−8 | ||||
| 1 | 0.002 | 0.0019 | 99.94 | 0.57735 | 0.3333 |
| 2 | 0.01 | 0.0099 | 99.86 | 1 | 0.5774 |
| 3 | 2 | 1.968 | 98.4 | 57.735 | 33.33 |
| 4 | 20 | 20.03 | 100.13 | 10 | 5.774 |
| Electrode | LDR | Sensitivity (µA/µM) | LOD | Reference |
|---|---|---|---|---|
| GNMCPE (urine) | 0.2 µM–30 µM | - | 0.28 (nM) | [82] |
| GC/SDS (buffer) | 0.04 µM–2.4 µM | - | 4.58 (nM) | [20] |
| HPLC (buffer) | 2 μg/mL–500 μg/mL | - | 0.065 μg/mL | [83] |
| (TRZ.PMA) cp | 1 µM–10 mM | - | 800 (nM) | [84] |
| ILCCPE…SDS (urine) | 0.002 µM–0.09 µM | 105 | 0.0169 (nM) | This work |
| 0.2 µM–30 µM | 2.695 | 6.43 (nM) |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, N.F.; BinSabt, M.H.; Hassan, S.H.; Galal, A. Ionic Liquid Crystals Modifier for Selective Determination of Terazosin Antihypertensive Drug in Presence of Common Interference Compounds. Crystals 2017, 7, 27. https://doi.org/10.3390/cryst7010027
Atta NF, BinSabt MH, Hassan SH, Galal A. Ionic Liquid Crystals Modifier for Selective Determination of Terazosin Antihypertensive Drug in Presence of Common Interference Compounds. Crystals. 2017; 7(1):27. https://doi.org/10.3390/cryst7010027
Chicago/Turabian StyleAtta, Nada F., Mohammad H. BinSabt, Samar H. Hassan, and Ahmed Galal. 2017. "Ionic Liquid Crystals Modifier for Selective Determination of Terazosin Antihypertensive Drug in Presence of Common Interference Compounds" Crystals 7, no. 1: 27. https://doi.org/10.3390/cryst7010027





