Potassium Disorder in the Defect Pyrochlore KSbTeO6: A Neutron Diffraction Study
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alonso, J.A.; Turrillas, X. Location of H+ sites in the fast proton-conductor (H3O)SbTeO6 pyrochlore. Dalton Trans. 2005, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.; Lemus, J.; Pina, M.P.; Sanz, J.; Aguadero, A.; Alonso, J.A. Evaluation of the pyrochlore (H3O)SbTeO6 as a candidate for electrolytic membranes in PEM fuel cells. J. New Mater. Electrochem. Syst. 2009, 12, 77–80. [Google Scholar]
- England, W.A.; Cross, M.G.; Hamnett, A.; Wiseman, P.J.; Goodenough, J.B. Fast proton conduction in inorganic ion-exchange compounds. Solid State Ion. 1980, 1, 231–249. [Google Scholar] [CrossRef]
- Ozawa, K.; Wang, J.; Ye, J.; Sakka, Y.; Amano, M. Preparation and Some Electrical Properties of Yttrium-Doped Antimonic Acids. Chem Mater. 2003, 15, 928–934. [Google Scholar] [CrossRef]
- Turrillas, X.; Delabouglise, G.; Joubert, J.G.; Fournier, T.; Muller, J. Un nouveau conducteur protonique HSbTeO6·xH2O. Conductivite en fonction de la temperature et de la pression partielle de vapeur d’eau. Solid State Ion. 1985, 17, 169–174. [Google Scholar]
- Subramanian, M.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Alonso, J.A.; Castro, A.; Rasines, I. Study of the defect pyrochlores A(SbTe)O6 (A = K, Rb, Cs, Tl). J. Mater. Sci. 1988, 23, 4103–4107. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Cryst. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Babel, D.; Pausegang, G.; Werner, V. Die Struktur einiger Fluoride, Oxide und Oxidfluoride AMe2X6: Der RbNiCrF6-Typ. Zeitschrift für Naturforschung B 1967, 22, 1219–1220. [Google Scholar] [CrossRef]
- Darriet, B.; Rat, M.; Galy, J.; Hagenmuller, R. Sur quelques nouveaux pyrochlores des systemes MTO3 − WO3 et MTO3 − TeO3 (M = K, Rb, Cs, Tl; T = Nb, Ta). Mater. Res. Bull. 1971, 6, 1305–1315. [Google Scholar] [CrossRef]
- El Haimouti, A.; Zambon, D.; El-Ghozzi, M.; Avignant, D.; Leroux, F.; Daoud, M.; El Aatmani, M. Synthesis, structural and physico-chemical characterization of new defect pyrochlore-type antimonates K0.42Lny′Sb2O6+z′ and Na0.36LnySb2O6+z (0 < y, y′;' z, z′ < 1; Ln = Y, Eu and Gd) prepared by soft chemistry route. J. Alloy. Compd. 2004, 363, 130–137. [Google Scholar] [CrossRef]
- Fourquet, J.L.; Javobini, C.; de Pape, R. Les pyrochlores AIB2X6: Mise en evidence de l’occupation par le cation AI de nouvelles positions cristallographiques dans le groupe d’espace . Mater. Res. Bull. 1973, 8, 393–403. [Google Scholar] [CrossRef]
- Pannetier, J. Energie electrostatique des reseaux pyrochlore. J. Phys. Chem. Solids 1973, 34, 583–589. [Google Scholar] [CrossRef]
- Castro, A.; Rasines, I.; Sanchez-Martos, M.C. Novel deficient pyrochlores A(MoSb)O6 (A = Rb, Cs). J. Mater. Sci. Lett. 1987, 6, 1001–1003. [Google Scholar] [CrossRef]
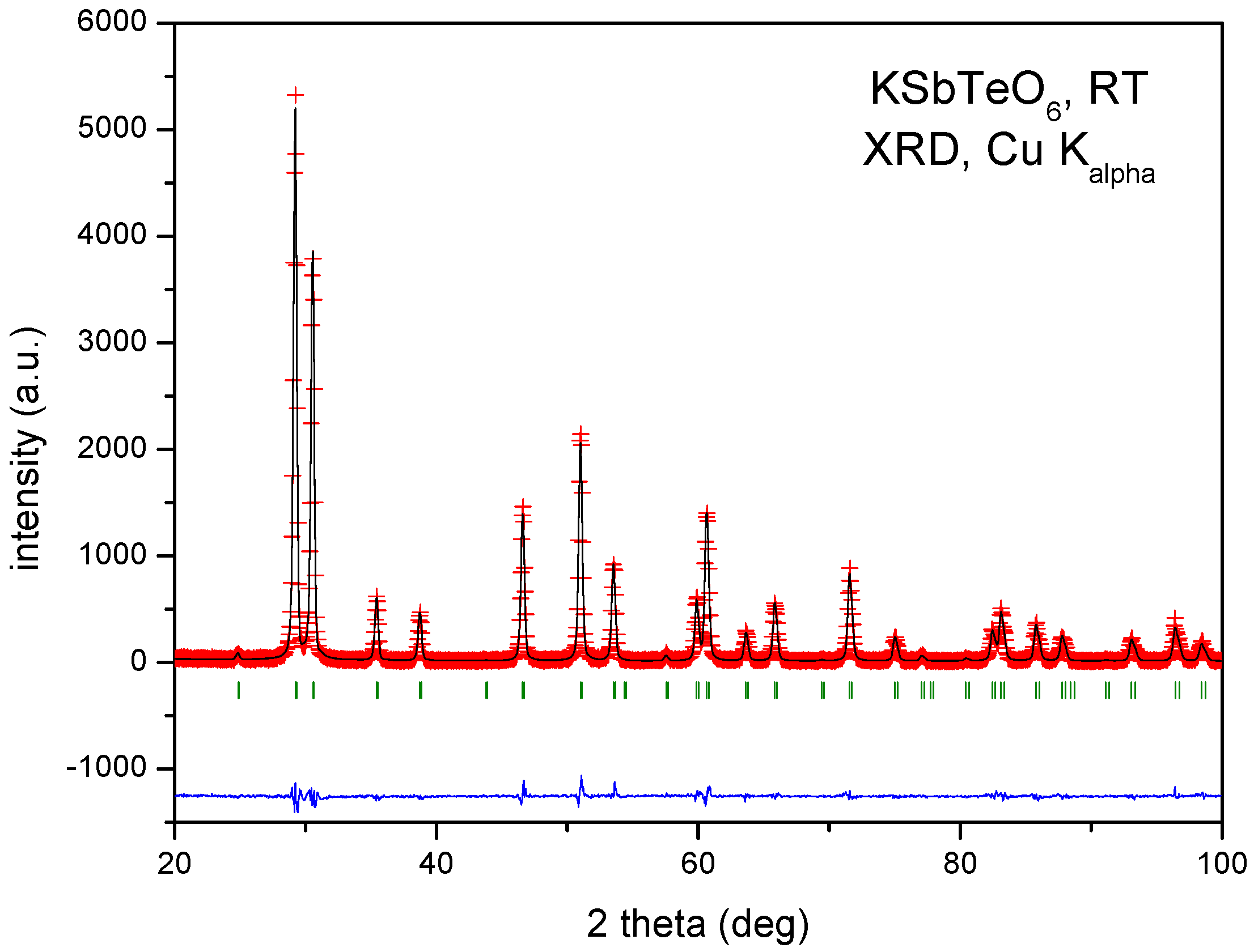
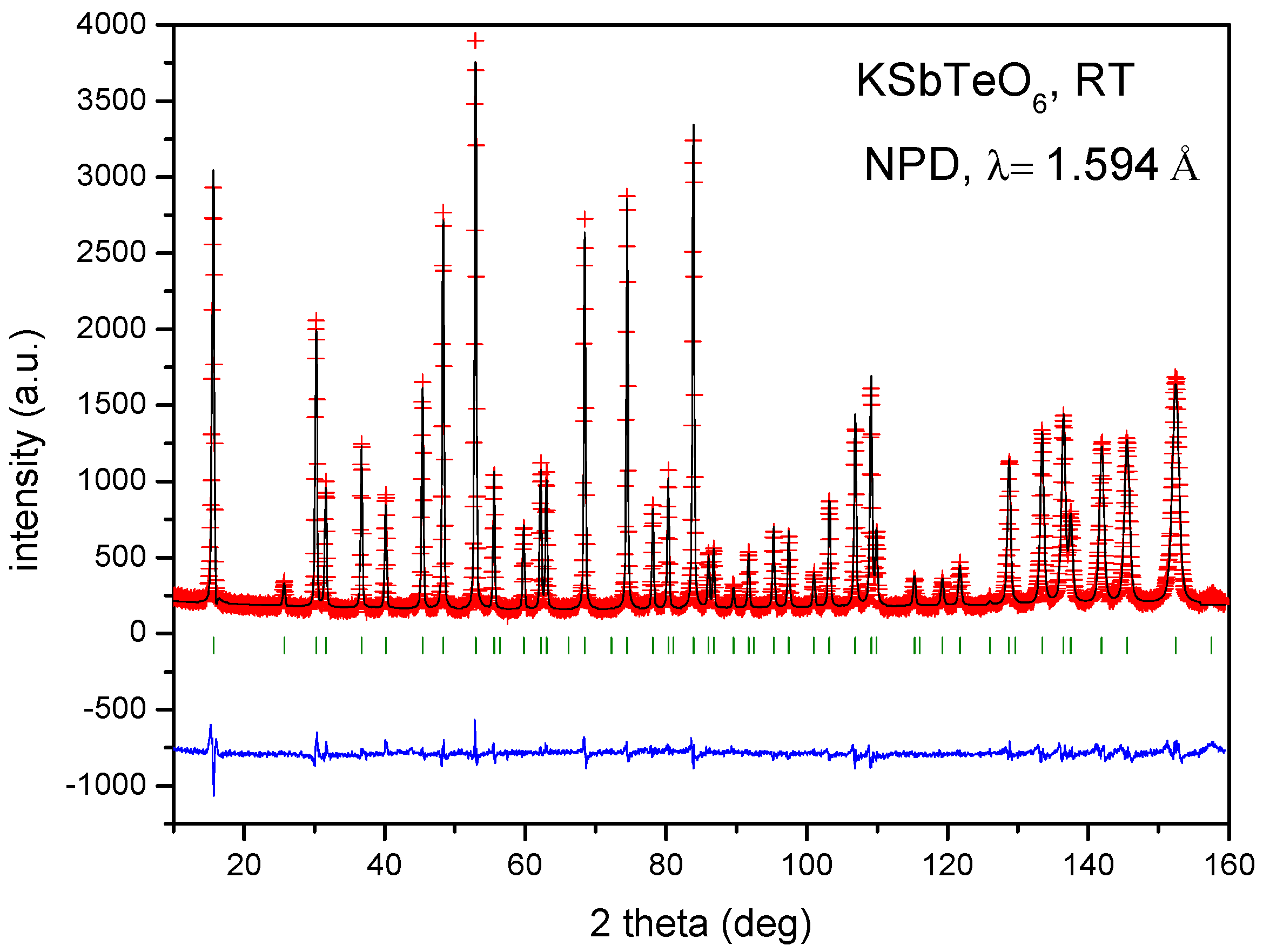
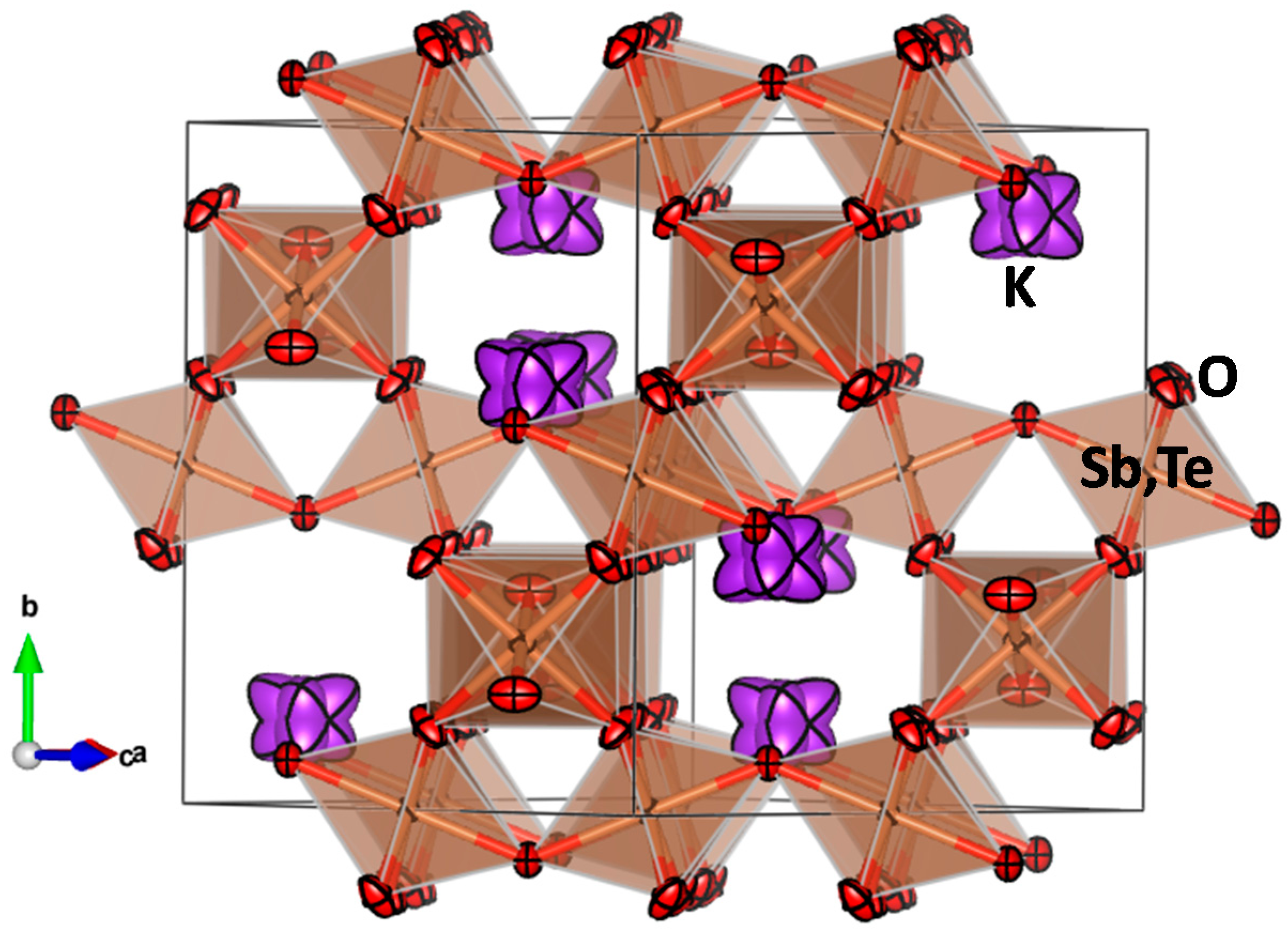

| Crystal Data | ||||||
| Cubic, | X-ray radiation, λ = 1.5418 Å | |||||
| Neutron radiation, λ = 1.595 Å | ||||||
| a = 10.1226(7) Å | Particle morphology: powder | |||||
| V = 1037.22(12) Å3 | Z = 8 | |||||
| Rietveld Agreement Factors | ||||||
| XRD data | NPD data | |||||
| Rp = 7.55% | Rp: 4.75% | |||||
| Rwp = 11.77% | Rwp: 6.27% | |||||
| Rexp = 9.11% | Rexp: 3.85% | |||||
| RBragg = 3.40% | RBragg = 3.59% | |||||
| χ2 = 1.67 | χ2 = 2.65 | |||||
| 1801 data points | 3240 data points | |||||
| Atomic Coordinates, Isotropic Atomic Displacement Parameters (Å2) and Refined Occupancy Factors | ||||||
| x | y | z | Ueq | Occupancy | ||
| K | 0.126(3) | 0.126(3) | 0.126(3) | 0.060(4) | 0.256(4) | |
| Sb1 | 0.50000 | 0.50000 | 0.50000 | 0.0037(3) | ||
| Te1 | 0.50000 | 0.50000 | 0.50000 | 0.0037(3) | ||
| O1 | 0.42760(9) | 0.12500 | 0.12500 | 0.0099(3) | ||
| Anisotropic Atomic Displacement Parameters (Å2) | ||||||
| U11 | U22 | U33 | U12 | U13 | U23 | |
| K | 0.055(3) | 0.055(3) | 0.055(3) | 0.025(8) | 0.025(8) | 0.025(8) |
| Sb | 0.0037(3) | 0.0037(3) | 0.0037(3) | −0.0004(3) | −0.0004(3) | −0.0004(3) |
| Te | 0.0037(3) | 0.0037(3) | 0.0037(3) | −0.0004(3) | −0.0004(3) | −0.0004(3) |
| O | 0.0075(4) | 0.0111(3) | 0.0111(3) | 0.0 | 0.0 | −0.0065(4) |
| Distances (Å) | |
| K-O (x3) | 3.05(3) |
| K-O′ (x3) | 3.07(3) |
| (Sb,Te)-O (x6) | 1.9338(6) |
| Angles (°) | |
| O-(Sb,Te)-O | 86.10(3) |
| 93.90(3) | |
| (Sb,Te)-O-(Sb,Te) | 135.45(2) |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, J.A.; Mayer, S.; Falcón, H.; Turrillas, X.; Fernández-Díaz, M.T. Potassium Disorder in the Defect Pyrochlore KSbTeO6: A Neutron Diffraction Study. Crystals 2017, 7, 24. https://doi.org/10.3390/cryst7010024
Alonso JA, Mayer S, Falcón H, Turrillas X, Fernández-Díaz MT. Potassium Disorder in the Defect Pyrochlore KSbTeO6: A Neutron Diffraction Study. Crystals. 2017; 7(1):24. https://doi.org/10.3390/cryst7010024
Chicago/Turabian StyleAlonso, José Antonio, Sergio Mayer, Horacio Falcón, Xabier Turrillas, and María Teresa Fernández-Díaz. 2017. "Potassium Disorder in the Defect Pyrochlore KSbTeO6: A Neutron Diffraction Study" Crystals 7, no. 1: 24. https://doi.org/10.3390/cryst7010024






