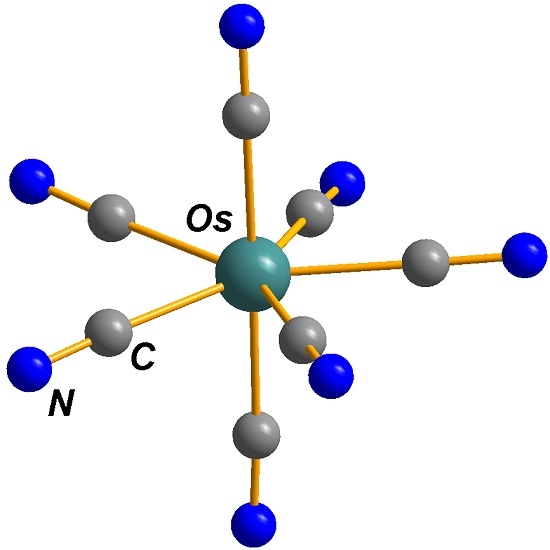
The First Homoleptic Complex of Seven-Coordinated Osmium: Synthesis and Crystallographical Evidence of Pentagonal Bipyramidal Polyhedron of Heptacyanoosmate(IV)
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Physical Methods
2.2. Synthesis
2.3. X-Ray Crystallography
3. Results and Discussion
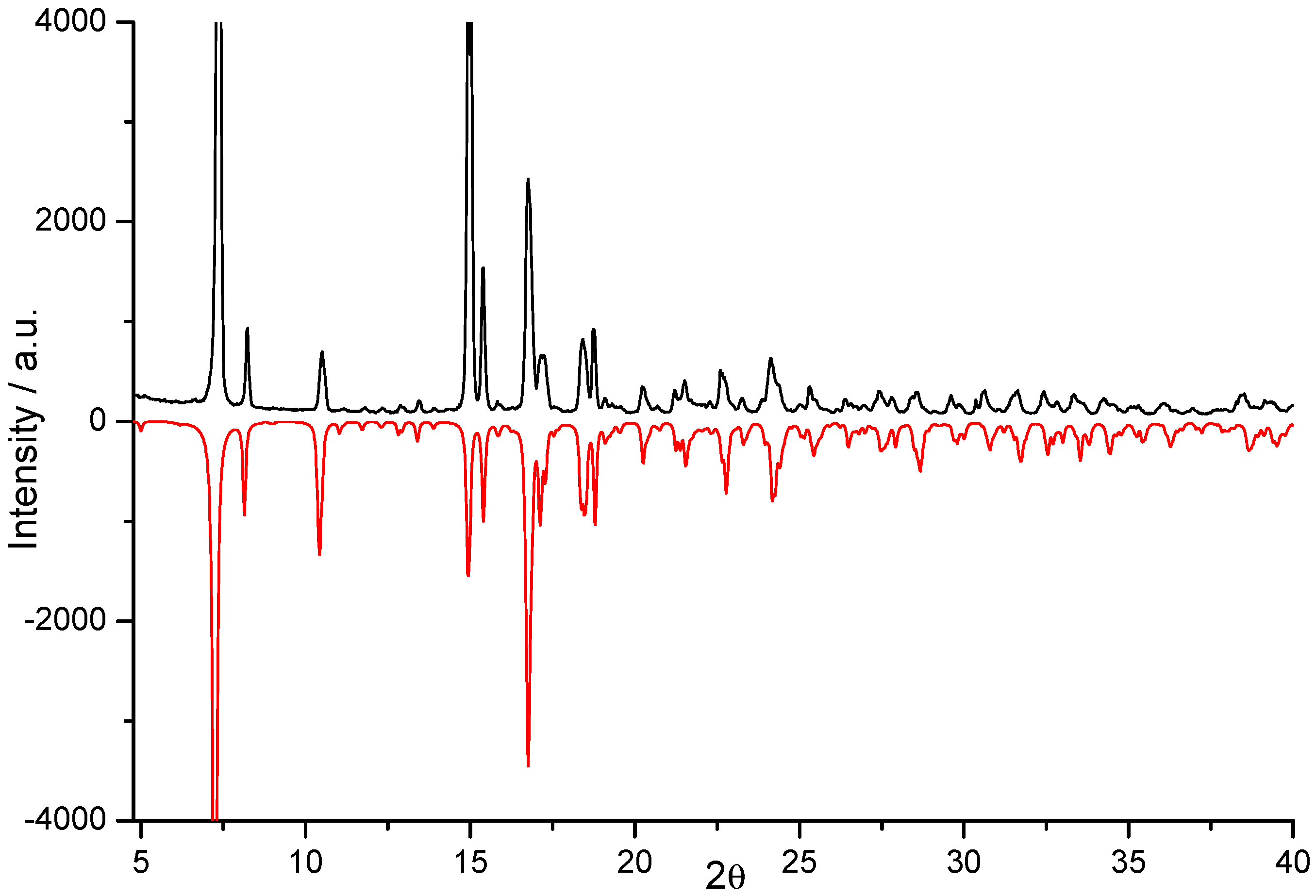
3.1. Synthesis and Characterization
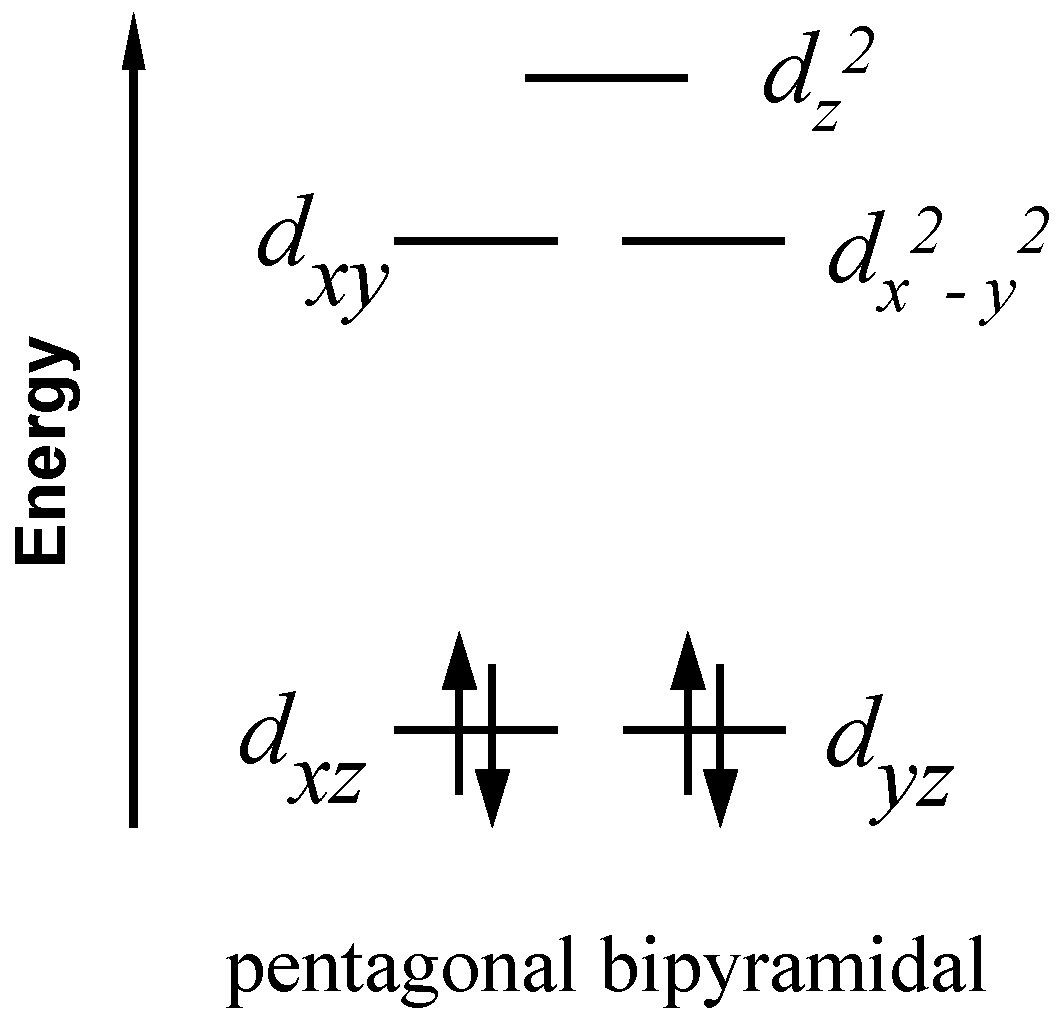
3.2. Crystal Structure Description
3.3. Magnetic Properties
4. Conclusions and Perspectives
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Werner, A. Beitrag zur Konstitution anorganischer Verbindungen. Z. Anorg. Allg. Chem. 1893, 3, 267–330. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Coordination chemistry: The scientific legacy of Alfred Werner. Chem. Soc. Rev. 2013, 42, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J. Praeparatio Caerulei Prussiaci. Philos. Trans. 1724, 33, 15–17. [Google Scholar] [CrossRef]
- Verdaguer, M.; Bleuzen, A.; Marvaud, V.; Vaissermann, J.; Seuleiman, M.; Desplanches, C.; Scuiller, A.; Train, C.; Garde, R.; Gelly, G.; et al. Molecules to build solids: high TC molecule-based magnets by design and recent revival of cyano complexes chemistry. Coord. Chem Rev. 1999, 192, 1023–1047. [Google Scholar] [CrossRef]
- Verdaguer, M.; Girolami, G.S. Magnetic Prussian Blue Analogs. In Magnetism: Molecules to Materials, V; Miller, J.S., Drillon, M., Eds.; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2005; Volume 3, pp. 283–346. [Google Scholar]
- Sato, O.; Iyoda, T.; Fujishima, A.; Hashimoto, K. Photoinduced Magnetization of a Cobalt-Iron Cyanide. Science 1996, 272, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Bleuzen, A.; Marvaud, V.; Mathoniere, C.; Sieklucka, B.; Verdaguer, M. Photomagnetism in Clusters and Extended Molecule-Based Magnets. Inorg. Chem. 2009, 48, 3453–3466. [Google Scholar]
- Carvajal, M.-A.; Caballol, R.; de Graaf, C. Insights on the photomagnetism in copper octacyanomolybdates. Dalt. Trans. 2011, 40, 7295–7303. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, J.; Cafun, J.-D.; Fornasieri, G.; Brubach, J.-B.; Creff, G.; Roy, P.; Bleuzen, A. Microscopic Origin for Multistability in a Photomagnetic CoFe Prussian Blue Analogue. Eur. J. Inorg. Chem. 2012, 3980–3983. [Google Scholar]
- Mondal, A.; Chamoreau, L.-M.; Li, Y.; Journaux, Y.; Seuleiman, M.; Lescouëzec, R. W-Co discrete complex exhibiting photo- and thermo-induced magnetization. Chem. Eur. J. 2013, 19, 7682–7685. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Available online: http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130334.htm (accessed on 28 May 2016).
- Boxhoorn, G.; Moolhuysen, J.; Coolegem, J.G.F.; Van Santen, R.A. Cyanometallates: An underestimated class of molecular sieves. J. Chem. Soc. Chem. Commun. 1985, 19, 1305–1307. [Google Scholar] [CrossRef]
- Chapman, K.W.; Southon, P.D.; Weeks, C.L.; Kepert, C.J. Reversible hydrogen gas uptake in nanoporous Prussian Blue analogues. Chem. Commun. 2005, 26, 3322–3324. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.S.; Long, J.R. Hydrogen Storage in the Dehydrated Prussian Blue Analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 2005, 127, 6506–6507. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, S.; Mallah, T.; Ouahes, R.; Veillet, P.; Verdaguer, M. A room-temperature organometallic magnet based on Prussian blue. Nature 1995, 378, 701–703. [Google Scholar] [CrossRef]
- Entley, W.R.; Girolami, G.S. High-temperature molecular magnets based on cyanovanadate building blocks: Spontaneous magnetization at 230 K. Science 1995, 268, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.M.; Girolami, G.S. Sol−Gel Synthesis of KVII[CrIII(CN)6]·2H2O: A crystalline molecule-based magnet with a magnetic ordering temperature above 100 °C. J. Am. Chem. Soc. 1999, 121, 5593–5594. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Avendaňo, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Bendix, J.; Clérac, R. Single-molecule magnet engineering: Building-block approaches. Chem. Commun. 2014, 50, 4396–4415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-X.; Breedlove, B.; Ishikawa, R.; Yamashita, M. Single-chain magnets: Beyond the Glauber model. RSC Adv. 2013, 3, 3772–3798. [Google Scholar] [CrossRef]
- Rams, M.; Peresypkina, E.V.; Mironov, V.S.; Wernsdorfer, W.; Vostrikova, K.E. Magnetic Relaxation of 1D Coordination Polymers (X)2[Mn(acacen)Fe(CN)6], X = Ph4P+, Et4N+. Inorg. Chem. 2014, 53, 10291–10300. [Google Scholar] [CrossRef] [PubMed]
- Peresypkina, E.V.; Majcher, A.; Rams, M.; Vostrikova, K.E. A single chain magnet involving hexacyanoosmate. Chem. Commun. 2014, 50, 7150–7153. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.C. The Chemistry of Cyano Complexes of the Transition Metals; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Babel, D. Magnetism and Structure: Model Studies on Transition Metal Fluorides and Cyanides. Comments Inorg. Chem. 1986, 5, 285–320. [Google Scholar] [CrossRef]
- Dunbar, K.R.; Heintz, R.A. Chemistry of Transition Metal Cyanide Compounds: Modern Perspectives. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; Volume 45, pp. 283–391. [Google Scholar]
- Shatruk, M.; Avendaňo, C.; Dunbar, K.R. Cyanide-Bridged Complexes of Transition Metals: A Molecular Magnetism Perspective. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Volume 56, pp. 155–334. [Google Scholar]
- Curtis, J.C.; Meyer, T.J. Outer-sphere charge transfer in mixed-metal ion pairs. Inorg. Chem. 1982, 21, 1562–1571. [Google Scholar] [CrossRef]
- Gentil, L.A.; Navaza, A.; Olabe, J.A.; Rigotti, G.E. The crystal and molecular structure of sodium hexacyanoosmate(II) decahydrate and related hexacyanometalate complexes. Inorg. Chim. Acta 1991, 179, 89–96. [Google Scholar] [CrossRef]
- Kang, H.W.; Moran, G.; Krausz, E. Magnetic circular dichroism spectroscopy of the hexacyano complexes of Ru(III) and Os(III). Inorg. Chim. Acta 1996, 249, 231–235. [Google Scholar] [CrossRef]
- Albores, P.; Slep, L.D.; Baraldo, L.M.; Baggio, R.; Garland, M.T.; Rentschler, E. Crystal Structure and Electronic and Magnetic Properties of Hexacyanoosmate(III). Inorg. Chem. 2006, 45, 2361–2363. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, W.; Hendrickx, M.F.A.; Ceulemans, A.A. CASPT2 Study of the Electronic Spectrum of Hexacyanoosmate(III). Inorg. Chem. 2007, 46, 8032–8037. [Google Scholar] [CrossRef] [PubMed]
- Vostrikova, K.E.; Peresypkina, E.V. Facile Preparation of Paramagnetic RuIII and OsIII Hexacyanides. Eur. J. Inorg. Chem. 2011, 6, 811–815. [Google Scholar] [CrossRef]
- Pombeiro, J.L.; Guedes da Silva, M.F.C.; Crabtree, R.H. Technetium & Rhenium: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Alberto, R. Comprehensive Coordination Chemistry II; Elsevier: Amsterdam, The Netherlands, 2004; Volume V, p. 127. [Google Scholar]
- Colton, R.; Peacock, R.D.; Wilkinson, G. 275. Complex cyanides of rhenium. J. Chem. Soc. 1960, 1374–1378. [Google Scholar] [CrossRef]
- Bennett, M.V.; Long, J.R. New Cyanometalate Building Units: Synthesis and Characterization of [Re(CN)7]3− and [Re(CN)8]3−. J. Am. Chem. Soc. 2003, 125, 2394–2395. [Google Scholar] [CrossRef] [PubMed]
- Birk, F.J.; Pinkowicz, D.; Dunbar, K.R. The Heptacyanotungstate(IV) Anion: A New 5d ransition-Metal Member of the Rare Heptacyanometallate Family of Anions. Angew. Chem. Int. Ed. 2016, 55, 1–4. [Google Scholar]
- Synthesis of the Complex Compounds of Platinum Group; Cherniaev, I.I. (Ed.) Publishing House “Nauka”: Moscow, Russia, 1964; p. 294.
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Cryst. 1995, A51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Krauss, А.; Schrader, G. Zur Kenntnis der Cyanverbindungen der Platinmetalle. III. Über die Cyanverbindungen des Osmiums. J. Pract. Chem. 1928, 119, 279–286. [Google Scholar] [CrossRef]
- Alexander, J.J.; Gray, H.B. Electronic Structures of Hexacyanometalate Complexes. J. Am. Chem. Soc. 1968, 90, 4260–4271. [Google Scholar] [CrossRef]
- Manoli, J.-M.; Potvin, C.; Brégeault, J.-M.; Griffith, W.P. Crystal structure of potassium heptacyanorhenate(III) dehydrate. J. Chem. Soc. Dalton Trans. 1980, 192–195. [Google Scholar] [CrossRef]
- Teller, R.G.; Bau, R. Crystallographic studies of transition metal hydride complexes. In Structure and Bonding; Springer-Verlag: Berlin, Germany, 1981; Volume 44, pp. 1–82. [Google Scholar]
- Moore, D.S.; Robinson, S.D. Hydrido complexes of the transition metals. Chem. Soc. Rev. 1983, 12, 415–452. [Google Scholar] [CrossRef]
- McGrady, G.S.; Guilera, G. The Multifarious World of Transition Metal Hydrides. Chem. Soc. Rev. 2003, 32, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Glemser, O.; Roesky, H.W.; Hellberg, K.-H.; Werther, H.-H. Darstellung und Eigenschaften von Osmiumheptafluorid. Chem. Ber. 1966, 99, 2652–2662. [Google Scholar] [CrossRef]
- Shorafa, H.; Seppelt, K. Osmium(VII) Fluorine Compounds. Inorg. Chem. 2006, 45, 7929–7934. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Misra, N. Theoretical investigation on the structure, stability and superhalogen properties of OsFn (n = 1–7) species. J. Fluorine Chem. 2014, 158, 65–68. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Dreiser, J.; Nehrkorn, J.; Gysler, M.; Schau-Magnussen, M.; Schnegg, A.; Holldack, K.; Bittl, R.; Piligkos, S.; Weihe, H.; et al. A linear single-molecule magnet based on [RuIII(CN)6]3−. J. Chem. Commun. 2011, 47, 6918–6920. [Google Scholar] [CrossRef]
- Dreiser, J.; Pedersen, K.S.; Schnegg, A.; Holldack, K.; Nehrkorn, J.; Sigrist, M.; Tregenna-Piggott, P.; Mutka, H.; Weihe, H.; Mironov, V.S.; et al. Three-axis anisotropic exchange coupling in the single-molecule magnets NEt4[MnIII2(5-Brsalen)2(MeOH)2MIII(CN)6] (M = Ru, Os). Chem. Eur. J. 2013, 19, 3693–3701. [Google Scholar] [CrossRef] [PubMed]
- Vostrikova, K.; Homoleptic, E. Osmium Cyanide Complexes: Synthesis and Perspective Application in Molecular Magnetism. In Osmium: Synthesis Characterization and Applications; Wise, G., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 43–78. [Google Scholar]
- Pedersen, K.S.; Sigrist, M.; Weihe, H.; Tregenna-Piggott, P.L.W.; Schau-Magnussen, M.; Dreiser, J.; Mutka, H.; Barra, A.-L.; Bendix, J. MnIII zero-field splitting parameters and weak exchange interactions in a cyanide-bridged {MnIII–IrIII–MnIII} cluster. Inorg. Chem. Commun. 2012, 24, 24–28. [Google Scholar] [CrossRef]


| Crystal Data | |
|---|---|
| Chemical formula | (C16H36N)3(C7N7Os)(H2O)0.5 |
| Mr | 1108.72 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 150 |
| a, b, c (Å) | 22.8582(3), 23.0300(4), 23.0373 (3) |
| β (°) | 90.840 (1) |
| V (Å3) | 12126.1 (3) |
| Z | 8 |
| F(000) | 4712 |
| Radiation type | Cu Kα |
| μ (mm−1) | 4.28 |
| Dcalcd (g·cm−1) | 1.215 |
| Crystal shape | rod |
| Color | pale yellow |
| Crystal size (mm) | 0.11 × 0.07 × 0.02 |
| Data Collection | |
| Super Nova diffractometer (Atlas), Single source at offset | |
| Absorption correction | Multi-scan |
| Tmin, Tmax | 0.900, 1.000 |
| Number of measured, independent and observed [I > 2σ (I)] reflections | 42839, 23015, 14167 |
| Rint | 0.056 |
| (sin θ/λ)max (Å−1) | 0.619 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.054, 0.142, 0.93 |
| No. of reflections | 23015 |
| No. of parameters | 1234 |
| No. of restraints | 15 |
| H-atom treatment | H-atom parameters constrained |
| Δ〉max, Δ〉min (e Å−3) | 4.39, −1.51 |
| Bond | Bond Length, Å | Bond | Bond Length, Å |
|---|---|---|---|
| Os1–C11 | 2.093 (7) | Os2–C21 | 2.070 (6) |
| Os1–C12 | 2.066 (8) | Os2–C22 | 2.079 (7) |
| Os1–C13 | 2.046 (8) | Os2–C23 | 2.046 (7) |
| Os1–C14 | 2.054 (7) | Os2–C24 | 2.055 (6) |
| Os1–C15 | 2.066 (8) | Os2–C25 | 2.072 (6) |
| Os1–C16 | 2.043 (8) | Os2–C26 | 2.060 (7) |
| Os1–C17 | 2.090 (8) | Os2–C27 | 2.078 (7) |
| N11–C11 | 1.124 (10) | N21–C21 | 1.155 (9) |
| N12–C12 | 1.173 (10) | N22–C22 | 1.142 (9) |
| N13–C13 | 1.164 (10) | N23–C23 | 1.171 (9) |
| N14–C14 | 1.161 (10) | N24–C24 | 1.158 (8) |
| N15–C15 | 1.141 (10) | N25–C25 | 1.153 (9) |
| N16–C16 | 1.180 (10) | N26–C26 | 1.166 (9) |
| N17–C17 | 1.114 (10) | N27–C27 | 1.145 (9) |
| Bond Angle | Angle, ° | Bond Angle | Angle, ° |
| C11–Os1–C12 | 71.6 (3) | C21–Os2–C22 | 73.3 (3) |
| C12–Os1–C13 | 71.3 (3) | C22–Os2–C23 | 72.9 (3) |
| C13–Os1–C14 | 73.9 (3) | C23–Os2–C24 | 72.9 (3) |
| C14–Os1–C15 | 73.1 (3) | C24–Os2–C25 | 72.6 (3) |
| C15–Os1–C11 | 71.9 (3) | C25–Os2–C21 | 70.9 (3) |
| C16–Os1–C17 | 179.3 (3) | C26–Os2–C27 | 178.7 (3) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peresypkina, E.V.; Gavriluta, A.; Vostrikova, K.E. The First Homoleptic Complex of Seven-Coordinated Osmium: Synthesis and Crystallographical Evidence of Pentagonal Bipyramidal Polyhedron of Heptacyanoosmate(IV). Crystals 2016, 6, 102. https://doi.org/10.3390/cryst6090102
Peresypkina EV, Gavriluta A, Vostrikova KE. The First Homoleptic Complex of Seven-Coordinated Osmium: Synthesis and Crystallographical Evidence of Pentagonal Bipyramidal Polyhedron of Heptacyanoosmate(IV). Crystals. 2016; 6(9):102. https://doi.org/10.3390/cryst6090102
Chicago/Turabian StylePeresypkina, Eugenia V., Anatolie Gavriluta, and Kira E. Vostrikova. 2016. "The First Homoleptic Complex of Seven-Coordinated Osmium: Synthesis and Crystallographical Evidence of Pentagonal Bipyramidal Polyhedron of Heptacyanoosmate(IV)" Crystals 6, no. 9: 102. https://doi.org/10.3390/cryst6090102