Metal(II) Complexes of Compartmental Polynuclear Schiff Bases Containing Phenolate and Alkoxy Groups
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Aspects
2.2. General Characteristic Properties of the Complexes
2.3. IR and UV-VIS Spectra of the Complexes
2.4. Description of the Structures
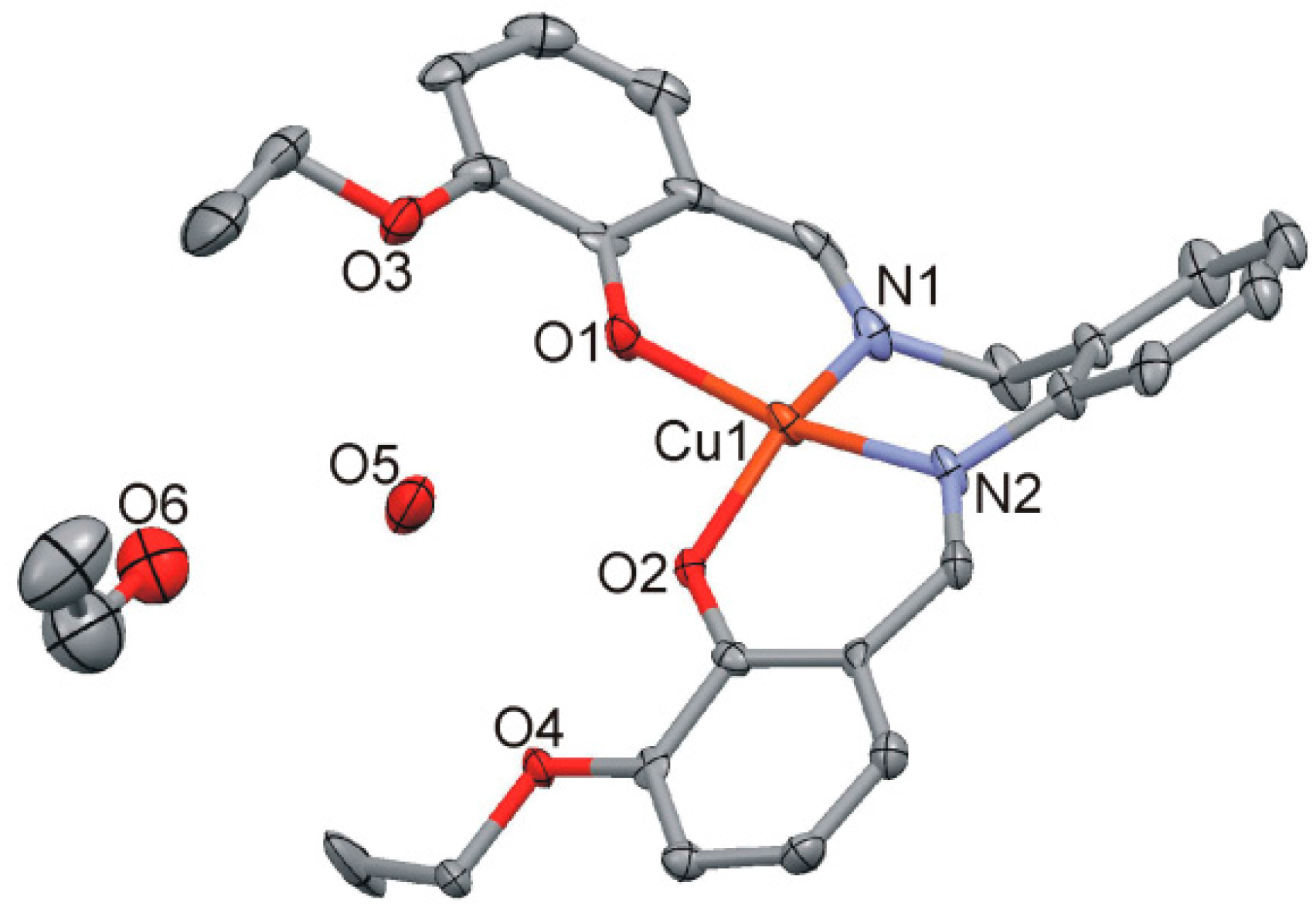
2.4.1. [Cu(LOEt-phda)(H2O)].H2O (1)
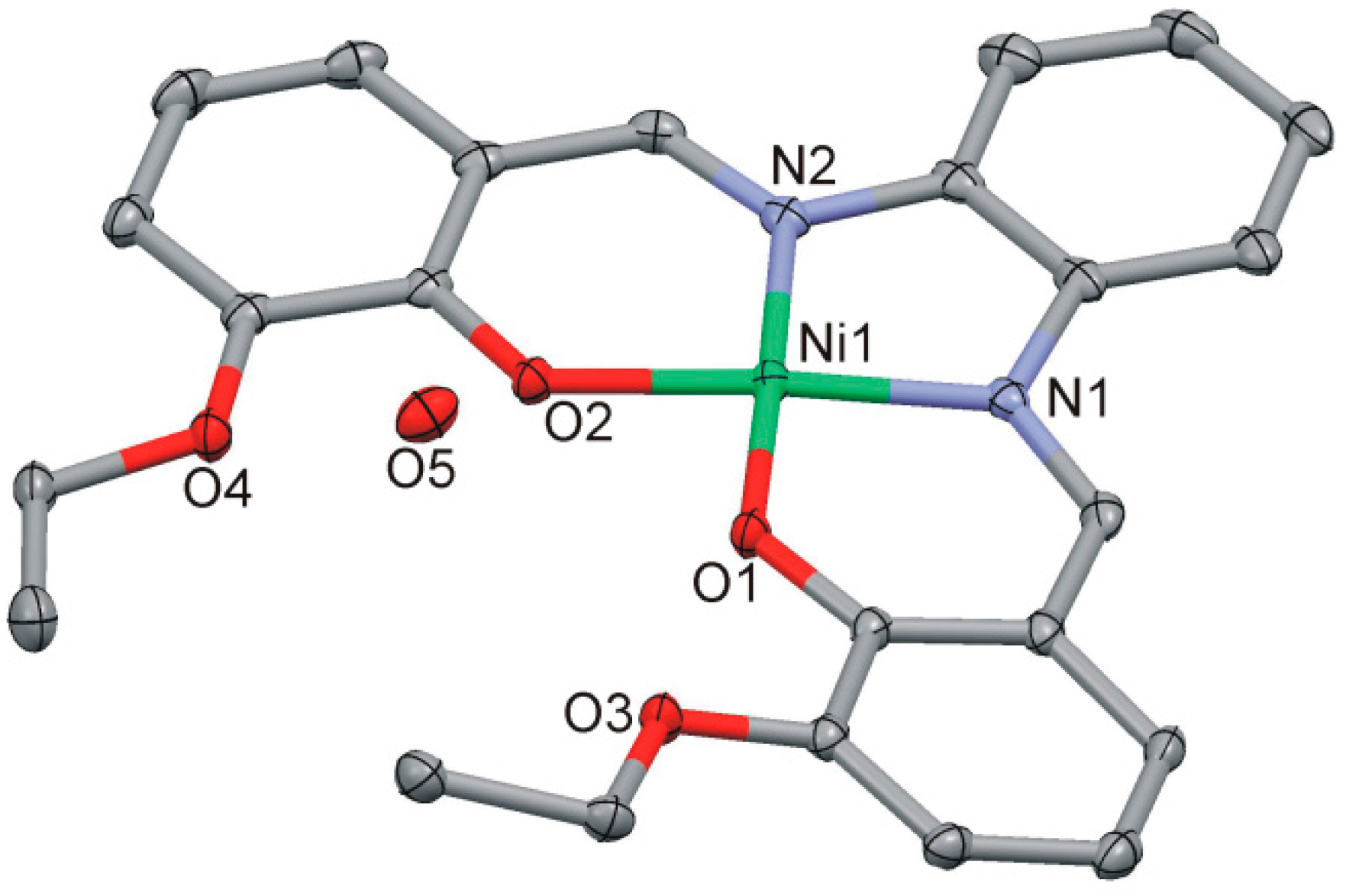
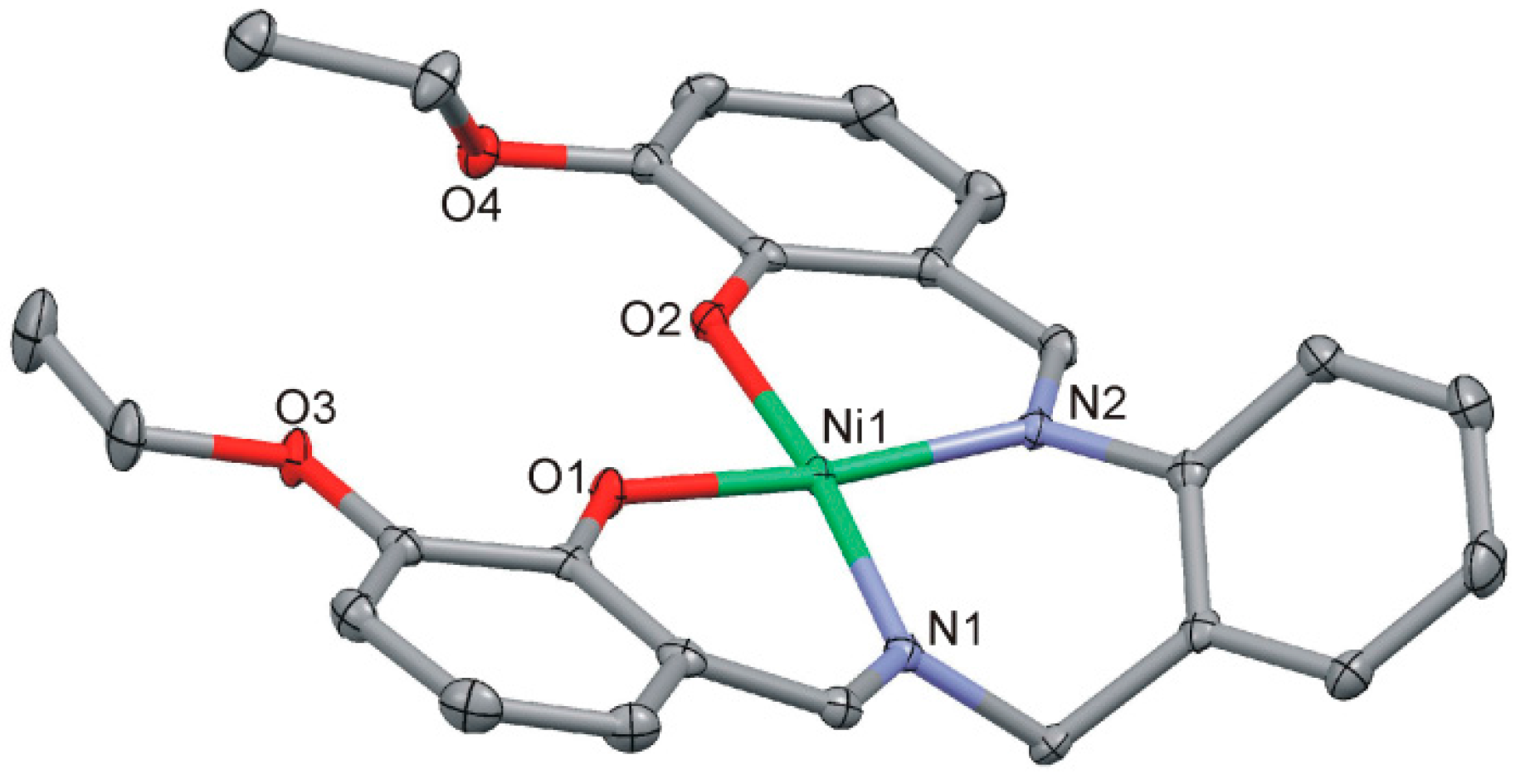
2.4.2. [Ni(LOEt-phda)].H2O (2)
2.4.3. [Cu(LOEt-ambza)].H2O·EtOH (3)
2.4.4. [Cu(LOEt-ambza)].H2O (4)
2.4.5. [Ni(LOEt-ambza)] (5)
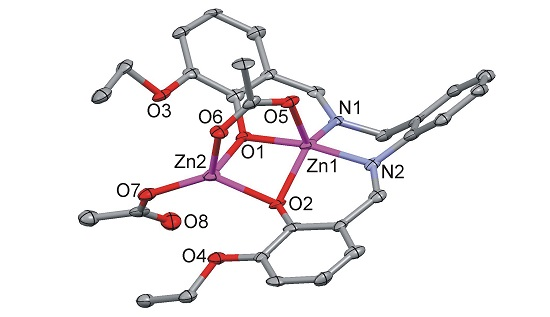
2.4.6. [Zn2(LOEt-ambza)(μ-OAc)(OAc)] (6)
3. Experimental Section
3.1. Materials and Physical Measurements
3.2. Syntheses
3.2.1. Synthesis of the Ligands
3.2.2. Synthesis of the Complexes
3.3. X-Ray Crystal Structure Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Costes, J.-P.; Laussac, J.-P.; Nicodeme, F. Complexation of a Schiff base ligand having two coordination sites (N2O2 and O2O2) with lanthanide ions (Ln = La, Pr): An NMR study. Dalton Trans. 2002, 13, 2731–2736. [Google Scholar] [CrossRef]
- Yao, H.H.; Huang, W.T.; Lo, J.M.; Liao, F.L.; Chattopadhyay, P. Copper(II) complexes with tetradentate imine-phenols: Synthesis, characterization and molecular structures. J. Coord. Chem. 2005, 58, 975–984. [Google Scholar] [CrossRef]
- Angeles, V.-F.M.; Isabel, F.-G.M.; Gonzalez-Noya, A.M.; Maneiro, M.; Bermejo, M.R.; Jesus, R.-D.M. Supramolecular networks of Mn(III)-Schiff base complexes assembled by nitrate counterions: X-ray crystal structures of 1D chains and 2D networks. Polyhedron 2012, 31, 379–385. [Google Scholar] [CrossRef]
- Maiti, M.; Thakurta, S.; Sadhukhan, D.; Pilet, G.; Rosair, G.M.; Nonat, A.; Charbonniére, L.J.; Mitra, D. Thermally stable luminescent zinc-Schiff base complexes: A thiocyanato bridged 1D coordination polymer and a supramolecular 1D polymer. Polyhedron 2013, 65, 6–15. [Google Scholar] [CrossRef]
- You, Z.-L.; lu, Y.; Zhang, N.; Ding, B.-W.; Sun, H.; Hou, P.; Wang, C. Preparation and structural characterization of hetero-dinuclear Schiff base copper(II)-zinc(II) complexes and their inhibition studies on Helicobacter pylori urease. Polyhedron 2011, 30, 2186–2194. [Google Scholar] [CrossRef]
- Thakurta, S.; Rizzoli, C.; Butcher, R.J.; Gomez-Garcia, C.J.; Garribba, E.; Mitra, S. Sterically-controlled nuclearity in new copper(II) complexes with di-compartmental ligands: Formation of antiferromagnetically coupled angular trimer and mononuclear inclusion complex. Inorg. Chim. Acta 2010, 363, 1395–1403. [Google Scholar] [CrossRef]
- Chakraborty, P.; Mohanta, S. Mononuclear and heterometallic dinuclear, trinuclear and dimer-of-dinuclear complexes derived from single- and double- compartment Schiff base ligands having a less utilized diamine. Polyhedron 2015, 87, 98–108. [Google Scholar] [CrossRef]
- Bermejo, M.R.; Fernandez, M.I.; Gonzalez-Noya, A.M.; Maneiro, M.; Pedrido, R.; Rodriguez, M.J.; Garcia-Monteagudo, J.C.; Donnadieu, B. Novel peroxidase mimics: μ-Aqua manganese-Schiff base dimers. J. Inorg. Biochem. 2006, 100, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Alsalim, T.A.; Hadi, J.S.; Al-Nasir, E.A.; Abbo, H.S.; Titinchi, S.J.J. Hydroxylation of phenol catalyzed by oxovanadium(IV) of salen-type Schiff base complexes with hydrogen peroxide. Catal. Lett. 2010, 136, 228–233. [Google Scholar] [CrossRef]
- Majumder, S.; Dutta, S.; Carrella, L.M.; Rentschler, E.; Mohanta, S. Syntheses, structures, electrochemical measurements and magnetic properties of two iron(III) complexes derived from N,N'-o-phenylenebis(3-ethoxysalicylaldimine). J. Mol. Struct. 2011, 1006, 216–222. [Google Scholar] [CrossRef]
- Majumder, S.; Hazra, S.; Dutta, S.; Biswas, P.; Mohanta, S. Syntheses, structures and electrochemistry of manganese(III) complexes derived from N,N'-o-phenylenebis(3-ethoxysalicylaldimine): Efficient catalyst for styrene epoxidation. Polyhedron 2009, 28, 2473–2479. [Google Scholar] [CrossRef]
- Ambili, K.U.; Sreejith, S.S.; Jacob, J.M.; Sithambaresan, M.; Kurup, M.R.P. 2-Ethoxy-6-({2-[(3-ethoxy-2-hydroxybenzylidene)amino]benzyl}iminomethyl)phenol. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, E68, o2482. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Dey, S.P.; Elmali, A.; Elerman, Y. Molecular structure and conformation of N-2-[3'-(methoxysalicylideneimino)benzyl]-3''-methoxysalicylideneimine. J. Mol. Struct. 2001, 562, 177–184. [Google Scholar] [CrossRef]
- Saha, S.; Biswas, D.; Chakrabarty, P.P.; Schollmeyer, D.; Jana, A.D.; Sakiyama, H.; Mikuriya, M. Quantitative estimation of the antiferromagnetic interaction between Cu(II) and Sm(II) in two dimensional heterometallic coordination polymer with isonicotinic acid and tectons. Inorg. Chem. Commun. 2013, 36, 212–215. [Google Scholar] [CrossRef]
- Bermejo, M.R.; Fernandez, M.I.; Gomez-Forneas, E.; Gonzalez-Noya, A.; Maneiro, M.; Pedrido, R.; Rodriguez, M.J. Self-assembly of dimeric MnIII-Schiff-base complexes tuned by perchlorate anions. Eur. J. Inorg. Chem. 2007, 24, 3789–3797. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Roy, S.; Chakraborty, J.; Chattopadhyay, S. Two new hetero-dinuclear nickel(II)/zinc(II) complexes with compartmental Schiff bases: Synthesis, characterization and self assembly. Polyhedron 2016, 112, 109–117. [Google Scholar] [CrossRef]
- Kargar, H. Synthesis, characterization and crystal structure of a manganese(III) Schiff base complex and investigation of its catalytic activity in the oxidation of benzylic alcohols. Transiti. Met. Chem. 2014, 39, 811–817. [Google Scholar] [CrossRef]
- Kia, R.; Kargar, H.; Zare, K.; Khan, I.U. {6,6'-Diethoxy-2,2'-[2,2-dimethylpropane-1,3-diylbis(nitrilomethylidyne)]diphenolato}(2-ethoxy-6-formylphenolato)cobalt(III)-ethanol-water(1/1/1). Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, m366–m367. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Madalan, A.M.; Maxim, C.; Andruh, M. A dinuclear iron(III) complex bridged by the dianion of trimesic acid [{Fe(3-MeOsaldmpn)(H2O)}2Htrim]·H2O. Synthesis, crystal structure and magnetic properties. Revue Roumaine de Chimie 2012, 57, 687–691. [Google Scholar]
- Kargar, H.; Kia, R.; Fun, H.-K.; Jamshidvand, A. {6,6'-Diethoxy-2,2'-[2,2-dimethylpropane-1,3-diylbis(nitrilomethylidyne)]diphenolato}copper(II) monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m515–m516. [Google Scholar] [CrossRef] [PubMed]
- Kargar, H.; Jamshidvand, A.; Fun, H.-K.; Kia, R. {6,6'-Diethoxy-2,2'-[2,2-dimethylpropane-1,3-diylbis(nitrilomethylidyne)]diphenolato}nickel(II) monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m403–m404. [Google Scholar] [CrossRef] [PubMed]
- Yeap, C.S.; Kia, R.; Kargar, H.; Fun, H.-K. {6,6'-Dimethoxy-2,2'-[2,2-dimethylpropane-1,3-diylbis(nitrilomethylidyne)]diphenolato}nickel(II) 1.78-hydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m570–m571. [Google Scholar] [CrossRef] [PubMed]
- Rayati, S.; Rafiee, N.; Wojtczak, A. cis-Dioxo-molybdenum(VI) Schiff base complexes: Synthesis, crystal structure and catalytic performance for homogeneous oxidation of olefins. Inorg. Chim. Acta 2012, 386, 27–35. [Google Scholar] [CrossRef]
- Nejo, A.A.; Kolawole, G.A.; Nejo, A.O.; Segapelo, T.V.; Muller, C.J. Synthesis, structural, and insulin-enhancing studies of oxovanadium(IV) complexes. Aust. J. Chem. 2011, 64, 1574–1579. [Google Scholar] [CrossRef]
- Arnaiz, F.J.; Costes, J.-P.; Garcia-Tojal, J. Heteronuclear d-f complexes containing binucleating ligands. In Inorganic Experiments, 3rd ed.; Woollins, J.D., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2010; pp. 283–286. [Google Scholar]
- Ishida, T.; Watanabe, R.; Fujiwara, K.; Okazawa, A.; Kojima, N.; Tanaka, G.; Yoshii, S.; Nojiri, H. Exchange coupling in TbCu and DyCu single-molecule magnets and related lanthanide and vanadium analogs. Dalton Trans. 2012, 41, 13609–13619. [Google Scholar] [CrossRef] [PubMed]
- Thevenon, A.; Garden, J.A.; White, A.J.P.; Williams, C.K. Dinuclear zinc salen catalysts for the ring opening copolymerization of epoxides and carbon dioxide or anhydrides. Inorg. Chem. 2015, 54, 11906–11915. [Google Scholar] [CrossRef] [PubMed]
- Rayati, S.; Zakavi, S.; Koliaei, M.; Wojtczak, A.; Kozakiewicz, A. Electron-rich salen-type Schiff base complexes of Cu(II) as catalysts for oxidation of cyclooctene and styrene with tert-butylhydroperoxide: A comparison with electron-deficient ones. Inorg. Chem. Commun. 2010, 13, 203–207. [Google Scholar] [CrossRef]
- Costes, J.-P.; Garcia-Tojal, J.; Tuchagues, J.-P.; Vendier, L. Structural and magnetic study of a trinuclear MnII-GdIII-MnII complex. Eur. J. Inorg. Chem. 2009, 25, 3801–3806. [Google Scholar] [CrossRef]
- Nayak, M.; Hazra, S.; Lemoine, P.; Koner, R.; Lucas, C.R.; Mohanta, S. Self-assembled [2 × 1 + 1 × 2] heterotetranuclear CuII3MnII/CuII3CoII and [2 × 2 + 1 × 3] heptanuclear CuII7 compounds derived from N,N'-o-phenylenebis(3-ethoxysalicylaldimine): Structures and magnetic properties. Polyhedron 2008, 27, 1201–1213. [Google Scholar] [CrossRef]
- Salmon, L.; Thuery, P.; Ephritikhine, M. Synthesis and crystal structure of tetra- and hexanuclear uranium(IV) complexes with hexadentate compartmental Schiff-base ligands. Dalton Trans. 2004, 24, 4139–4145. [Google Scholar] [CrossRef] [PubMed]
- Salmon, L.; Thuery, P.; Ephritikhine, M. Synthesis and crystal structure of uranium(IV) complexes with compartmental Schiff bases: From mononuclear species to tri- and tetranuclear clusters. Dalton Trans. 2004, 10, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Dahan, F.; Donnadieu, B.; Fernandez-Garcia, M.-I.; Rodriguez-Douton, M.-J. Reaction of a manganese(III)-Schiff base complex with gadolinium nitrate: Synthesis, structure and magnetic properties of an ionic species [LMn(H2O)2]2[Gd(NO3)5(MeOH)] (H2L=1,3-bis((3-methoxysalicylidene)amino)-2,2-dimethylpropane). Dalton Trans. 2003, 19, 3776–3779. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Garcia-Tojal, J. Dinuclear CoII/GdIII and CoIII/GdIII complexes derived from hexadentate schiff bases: Synthesis, structure, and magnetic properties. Chem. A Eur. J. 2002, 8, 5430–5434. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d-4f discrete complexes. Coord. Chem. Rev. 2015, 289, 74–122. [Google Scholar] [CrossRef]
- Sakamoto, S.; Fujinami, T.; Koshiro Nishi, K.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Re, N. Carbonato-bridged NiII2LnIII2 (LnIII=GdIII, TbIII, DyIII) complexes generated by atmospheric CO2 fixation and their single-molecule-magnet behavior: [(μ4-CO3)2{NiII(3-MeOsaltn)(MeOH or H2O)LnIII(NO3)}2]·solvent [3-MeOsaltn=N,N′-bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato]. Inorg. Chem. 2013, 52, 7218–7229. [Google Scholar] [PubMed]
- Ehama, K.; Ohmichi, Y.; Sakamoto, S.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Tsuchimoto, M.; Re, N. Synthesis, structure, luminescent, and magnetic properties of carbonato-bridged ZnII2LnIII2 complexes [(μ4-CO3)2{ZnIILLnLnIII(NO3)}2] (LnIII=GdIII, TbIII, DyIII; L1=N,N′-bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato, L2=N,N′-bis(3-ethoxy-2-oxybenzylidene)-1,3-propanediaminato). Inorg. Chem. 2013, 52, 12828–12841. [Google Scholar] [PubMed]
- Yang, X.; Chan, C.; Lam, D.; Schipper, D.; Stanley, J.M.; Chen, X.; Jones, R.A.; Holliday, B. J.; Wong, W.-K.; Chen, S.; et al. Anion-dependent construction of two hexanuclear 3d–4f complexes with a flexible Schiff base ligand. Dalton Trans. 2012, 41, 11449–11453. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lam, D.; Chan, C.; Stanley, J.M.; Jones, R.A.; Holliday, B.J.; Wong, W.-K. Construction of 1-D 4f and 3d–4f coordination polymers with flexible Schiff base ligands. Dalton Trans. 2011, 40, 9795–9801. [Google Scholar] [CrossRef] [PubMed]
- Kurzak, K.; Ejsmont, K.; Koprek, K. X-ray and DFT-calculated structures of a vanadyl Schiff base complex: (methanol-κO)[2-methoxy-6-({2-[(2-oxido-3-methoxybenzylidene)-amino]benzyl}iminomethyl)phenolato-κ4O1,N,N,O1']oxido-vanadium(IV) monohydrate. Acta Crystallog. Sect. C Crystal Struct. Commun. 2012, 68, m161–m165. [Google Scholar] [CrossRef] [PubMed]
- Orita, S.; Akitsu, T. Variety of crystal structures of chiral Schiff base Lu(III)-Ni(II)/ Cu(II)/Zn(II) and the related complexes. Open Chem. J. 2014, 1, 1–14. [Google Scholar]
- Hayashi, T.; Shibata, H.; Orita, S.; Akitsu, T. Variety of structure of binuclear chiral Schiff base Ce(III)/Pr(III)/Lu(III)-Ni(II)/Cu(II)/Zn(II) complexes. Eur. Chem. Bull. 2013, 2, 49–57. [Google Scholar]
- Hathaway, B.J. Comprehensive Coordination Chemistry; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; Volume 5, p. 533. [Google Scholar]
- Massoud, S.S.; Louka, F.R.; David, R.N.; Dartez, M.J.; Nguyn, Q.L.; Labry, N.J.; Fischer, R.C.; Mautner, F.A. Five-coordinate thiocyanato- and azido-metal(II) complexes based pyrazolyl ligands. Polyhedron 2015, 90, 258–265. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Obaid, Y.K.; Vicente, R.; Ribas, J.; Fischer, R.C.; Mautner, F.A. Metal ions directing the geometry and nuclearity of azido-metal(II) complexes derived from bis(2-(3,5-dimethyl-1H-pyrazol-1-yl)ethyl)amine. Dalton Trans. 2013, 42, 3968–3978. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Louka, F.R.; Le Guet, T.; Massoud, S.S. Pseudohalide copper(II) complexes derived from polypyridyl ligands. Synthesis and characterization. J. Mol. Struct. 2009, 919, 196–203. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Matović, Z.D.; Miletić, V.D.; Samardzˇić, G.; Pelosi, G.; Ianelli, S.; Snežana Trifunović, S. Square-planar copper(II) complexes with tetradentate amido-carboxylate ligands. Crystal structure of Na2[Cu(obap)]2·2H2O. Strain analysis and spectral assignments of complexes. Inorg. Chim. Acta 2005, 358, 3135–3144. [Google Scholar] [CrossRef]
- Tharmaraj, P.; Kodimunthiri, D.; Sheela, C.D.; Shanmuga Priya, C.S. Synthesis, spectral characterization, and antimicrobial activity of copper(II), cobalt(II), and nickel(II) complexes of 3-formylchromoniminopropylsilatrane. J. Coord. Chem. 2009, 62, 2220–2228. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rijin, J.V.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Bruker. SAINT v. 7.23; Bruker AXS Inc.: Madison, WI, USA, 2005. [Google Scholar]
- Bruker. APEX 2, v. 2.0-2; Bruker AXS Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Sheldrick, G.M. SADABS v. 2; University of Goettingen: Wilhelmsplatz, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, T.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mondal, S.; Sasmal, S.; Sparkes, H.A.; Howard, J.A.K.; Nayak, M.; Mohanta, S. Bis(nitrate)diaquauranyl(VI) synthon to generate [1 × 2 + 1 × 1] and [1 × 1 + 1 × 1] co-crystalized 3d⋯5f self-assemblies. CrystEngComm. 2011, 13, 1029–1036. [Google Scholar] [CrossRef]
- Shao, H.; Muduli, S.K.; Tran, P.D.; Soo., H.S. Enhancing electrocatalytic hydrogen evolution by nickel salicylaldimine complexes with alkali metal cations in aqueous media. Chem.Commun. 2016, 52, 2948–2951. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Mandal, L.; Mondal, S.; Lucas, C.R.; Mohanta, S. More surprising differences between two closely similar compartmental ligand families and another dinuclear synthon to stabilize dinuclear–mononuclear cocrystals. CrystEngComm. 2013, 15, 5888–5897. [Google Scholar] [CrossRef]
- Bhowmik, P.; Nayek, H.P.; Corbella, M.; Aliaga-Alcalde, N.; Chattopadhyay, S. Control of molecular architecture by steric factors: mononuclear vs polynuclear manganese(III) compounds with tetradentate N2O2 donor Schiff bases. Dalton Trans. 2011, 40, 7916–7926. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.; Bereau, V.; Costes, J.-P.; Duhayon, C.; Sutter, J.-P. Oligomeric and polymeric organizations of potassium salts with compartmental Schiff-base complexes as ligands. CrystEngComm. 2011, 13, 5908–5914. [Google Scholar] [CrossRef]
- Correia, I.; Pessoa, J.C.; Duarte, M.T.; da Piedade, M.F.M.; Jackush, T.; Kiss, T.; Castro, M.M.C.A.; Geraldes, C.F.G.C.; Avecilla, A. Vanadium(IV and V) complexes of Schiff bases and reduced Schiff bases derived from the reaction of aromatic o-hydroxyaldehydes and diamines: Synthesis, characterization and solution studies. Eur. J. Inorg. Chem. 2005, 4, 732–744. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, P.-F.; Gao, P.; Gao, T.; Hou, G.-F.; Li., G.-M. Salen-type lanthanide complexes with luminescence and near-infrared (NIR) properties. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1211–1218. [Google Scholar] [CrossRef]
- Gao, T.; Yan, P.-F.; Li, G.-M.; Hou, G.F.; Gao, J.-S. N,N′-Ethylene-bis(3-methoxysalicylideneimine) mononuclear (4f) and heterodinuclear (3d–4f) metal complexes: Synthesis, crystal structure and luminescent properties. Inorg. Chim. Acta 2008, 361, 2051–2058. [Google Scholar] [CrossRef]
- Hazra, S.; Sasmal, S.; Nayak, M.; Sparkes, H.A.; Howard, J.A.K.; Mohanta, S. Syntheses and crystal structures of CuIIBiIII, CuIIBaIICuII, [CuIIPbII]2 and cocrystallized (UVIO2)2.4CuII complexes: Structural diversity of the coordination compounds derived from N,N′-ethylenebis(3-ethoxysalicylaldiimine). CrystEngComm. 2010, 12, 470–477. [Google Scholar] [CrossRef]
- Moroz, O.V.; Trush, V.A.; Sliva, T.Y.; Konovalova, I.S.; Amirkhanov, V.M. Crystal structure of {μ-6,6′-dimethoxy-2,2′-[ethane-1,2-diylbis(nitrilomethanylylidene)]diphenolato}(methanol)(nitrato)nickel(II)sodium. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Vallat, R.; Ferreira, R.A.S.; Carlos, L.D.; Paz, F.A.A.; Guari, Y.; Larionova, J. A bifunctional luminescent single-ion magnet: Towards correlation between luminescence studies and magnetic slow relaxation processes. Chem. Commun. 2012, 48, 9974–9976. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Jana, A.; Fleck, M.; Hazra, S.; Mohanta, S. A unique example of a three component cocrystal of metal complexes. CrystEngComm 2010, 12, 1416–1421. [Google Scholar] [CrossRef]
- Sarkar, S.; Mohanta, S. Syntheses, crystal structures and supramolecular topologies of nickel(II)–s/p/d10/NH4+ complexes derived from a compartmental ligand. RSC Adv. 2011, 1, 640–650. [Google Scholar] [CrossRef]
- Gao, T.; Yan, P.-F.; Li, G.-M.; Zhang, J.-W.; Sun, W.-B.; Suda, M.; Einaga, Y. Correlations between structure and magnetism of three N,N′-ethylene-bis(3-methoxysalicylideneimine) gadolinium complexes. Solid State Sci. 2010, 12, 597–604. [Google Scholar] [CrossRef]
- Amirkhanov, O.V.; Moroz, O.V.; Znovjyak, K.O.; Sliva, T.Y.; Penkova, L.V.; Yushchenko, T.; Szyrwiel, L.; Konovalova, I.S.; Dyakonenko, V.V.; Shishkin, O.V.; et al. Heterobinuclear Zn–Ln and Ni–Ln complexes with Schiff-base and carbacylamidophosphate ligands: synthesis, crystal Structures, and catalytic activity. Eur. J. Inorg. Chem. 2014, 23, 3720–3730. [Google Scholar] [CrossRef]
- Sasmal, S.; Majumder, S.; Hazra, S.; Sparkes, H.A.; Howard, J.A.K.; Nayak, M.; Mohanta, S. Tetrametallic [2 × 1 + 1 × 2], octametallic double-decker–triple-decker [5 × 1 + 3 × 1], hexametallic quadruple-decker and dimetallic-based one-dimensional complexes of copper(II) and s block metal ions derived from N,N′-ethylenebis(3-ethoxysalicylaldimine). CrystEngComm. 2010, 12, 4131–4140. [Google Scholar]
- Kajiwara, T.; Nakano, M.; Takahashi, K.; Takaishi, S.; Yamashita, M. Structural design of easy-axis magnetic anisotropy and determination of anisotropic parameters of LnIII-CuII single-molecule magnets. Chem. Eur. J. 2011, 17, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Cimpoesu, F.; Dahan, F.; Ladeira, S.; Ferbinteanu, M.; Costes, J.-P. Chiral crystallization of a heterodinuclear Ni-Ln series: comprehensive analysis of the magnetic properties. Inorg. Chem. 2012, 51, 11279–11293. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Clemente-Juan, J.M.; Dahan, F.; Dumestre, F.; Tuchagues, J.-P. Dinuclear (FeII, GdIII) complexes deriving from hexadentate Schiff bases: Synthesis, structure, and mössbauer and magnetic properties. Inorg. Chem. 2002, 41, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Q.; Yan, P.-F.; Luan, F.; Li, Y.-X.; Sun, J.-W.; Chen, C.; Yang, F.; Chen, H.; Zou, X.-W.; Li, G.-M. Near-IR Luminescence and Field-Induced Single Molecule Magnet of Four Salen-type Ytterbium Complexes. Inorg.Chem. 2015, 54, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Mondal, S.; Mohanta, S. Syntheses, characterizations, and crystal structures of 3d–s/d10 metal complexes derived from two compartmental Schiff base ligands. J. Coord. Chem. 2013, 66, 152–170. [Google Scholar] [CrossRef]
- Mondal, S.; Hazra, S.; Sarkar, S.; Sasmal, S.; Mohanta, S. Syntheses, crystal structures and supramolecular topologies of copper(II)–main group metal complexes derived from N,N′-o-phenylenebis(3-ethoxysalicylaldimine). J. Mol. Struct. 2011, 1004, 204–214. [Google Scholar] [CrossRef]
- Koner, R.; Lee, G.-H.; Wang, Y.; Wei, H.H.; Mohanta, S. Two new diphenoxo-bridged discrete dinuclear CuIIGdIII compounds with cyclic diimino moieties: Syntheses, structures, and nagnetic properties. Eur. J. Inorg. Chem. 2005, 8, 1500–1505. [Google Scholar] [CrossRef]
- Sun, W.-B.; Yan, P.-F.; Jiang, S.-D.; Wang, B.-W.; Zhang, Y.-Q.; Hong-Feng, L.; Chen, P.; Wang, Z.-M.; Gao, S. High symmetry or low symmetry, that is the question–high performance Dy(III) single-ion magnets by electrostatic potential design. Chem. Sci. 2016, 7, 684–691. [Google Scholar] [CrossRef]
- Visinescu, D.; Jeon, I.-R.; Madalan, A.M.; Alexandru, M.-G.; Jurca, B.; Mathoniere, C.; Clerac, R.; Andruh, M. Self-assembly of [CuIITbIII]3+ and [W(CN)8]3− tectons: A case study of a mixture containing two complexes showing slow-relaxation of the magnetization. Dalton Trans. 2012, 41, 13578–13581. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Hino, S.; Yamashita, K.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. Correlation between slow magnetic relaxation and the coordination structures of a family of linear trinuclear Zn(II)–Ln(III)–Zn(II) complexes (Ln = Tb, Dy, Ho, Er, Tm and Yb). Dalton Trans. 2012, 41, 13640–13648. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Donnadieu, B.; Gheorghe, R.; Novitchi, G.; Tuchagues, J.-P.; Vendier, L. Di- or trinuclear 3d–4f Schiff base complexes: The Role of anions. Eur. J. Inorg. Chem. 2008, 33, 5235–5244. [Google Scholar] [CrossRef]
- Hu, K.-Q.; Jiang, X.; Wu, S.-Q.; Liu, C.-M.; Cui, A.-L.; Kou, H.Z. A trimetallic strategy towards ZnII4DyIII2CrIII2 and ZnII4DyIII2CoIII2 single-ion magnets. Dalton Trans. 2015, 44, 15413–15416. [Google Scholar] [CrossRef] [PubMed]
- Dhers, S.; Costes, J.-P.; Guionneau, P.; Paulsen, C.; Vendier, L.; Sutter, J.-P. On the importance of ferromagnetic exchange between transition metals in field-free SMMs: examples of ring-shaped hetero-trimetallic [(LnNi2){W(CN)8}]2 compounds. Chem. Commun. 2015, 51, 7875–7878. [Google Scholar] [CrossRef] [PubMed]



| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C24H24CuN2O6 | C24H24N2NiO5 | C27H32CuN2O6 |
| Formula mass | 500.00 | 479.14 | 544.10 |
| Crystal system | Tetragonal | Monoclinic | Monoclinic |
| Space group | P-421m | P21/c | P21/n |
| a/Å | 21.9174(6) | 12.9329(6) | 10.7944(6) |
| b/Å | 21.9174(6) | 14.5517(7) | 20.286(1) |
| c/Å | 4.9513(2) | 11.7485(6) | 11.7600(6) |
| α/° | 90 | 90 | 90 |
| β/° | 90 | 109.983(3) | 94.767(2) |
| γ/° | 90 | 90 | 90 |
| V/Å3 | 2378.47(16) | 2077.74(18) | 2566.2(2) |
| Z | 4 | 4 | 4 |
| T/K | 100(2) | 100(2) | 100(2) |
| μ/mm−1 | 0.959 | 0.975 | 0.895 |
| Dcalc/Mg·m−3 | 1.396 | 1.532 | 1.408 |
| Crystal size/mm | 0.24 × 0.15 × 0.11 | 0.24 × 0.18 × 0.13 | 0.16 × 0.15 × 0.11 |
| θ max/° | 30.27 | 30.02 | 26.00 |
| Data collected | 72696 | 32859 | 5073 |
| Unique refl./Rint | 3594/0.0381 | 6067/0.0600 | 5044/----- |
| Parameters/Restraints | 164/1 | 297/2 | 339/3 |
| Goodness-of-Fit on F2 | 1.082 | 0.843 | 1.155 |
| (I > 2σ(I))/wR2 (all data) | 0.0332/0.0975 | 0.0343/0.0894 | 0.0818/0.2113 |
| Residual extrema/e·Å−3 | 1.11/−0.59 | 0.56/−0.51 | 0.94/−0.73 |
| Compound | 4 | 5 | 6 |
|---|---|---|---|
| Empirical formula | C25H26CuN2O5 | C25H24N2NiO4 | C29H30N2O8Zn2 |
| Formula mass | 498.03 | 475.15 | 665.33 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/c | P21/c | P-1 |
| a/Å | 12.0486(4) | 12.0351(9) | 8.1702(15) |
| b/Å | 16.5021(5) | 11.7906(8) | 12.476(2) |
| c/Å | 11.2905(3) | 15.4288(11) | 14.531(3) |
| α/° | 90 | 90 | 70.908(8) |
| β/° | 97.206(2) | 93.783(2) | 78.161(8) |
| γ/° | 90 | 90 | 79.352(7) |
| V/Å3 | 2227.13(12) | 2184.6(3) | 1358.8(4) |
| Z | 4 | 4 | 2 |
| T/K | 100(2) | 100(2) | 100(2) |
| μ/mm−1 | 1.021 | 0.923 | 1.821 |
| Dcalc/Mg·m−3 | 1.485 | 1.445 | 1.626 |
| Crystal size/mm | 0.21 × 0.18 × 0.12 | 0.26 × 0.21 × 0.13 | 0.26 × 0.20 × 0.14 |
| θ max/° | 30.52 | 28.00 | 25.00 |
| Data collected | 86525 | 20418 | 8896 |
| Unique refl./Rint | 6786/0.0241 | 5272/0.0389 | 4772/0.0803 |
| Parameters/Restraints | 306/2 | 291/0 | 374/0 |
| Goodness-of-Fit on F2 | 1.056 | 1.047 | 1.122 |
| (I > 2σ(I))/wR2 (all data) | 0.0287/0.0919 | 0.0375/0.0885 | 0.0934/0.2488 |
| Residual extrema/e.Å−3 | 0.54/−0.34 | 0.96/−0.59 | 1.89/−1.44 |
| Complex | Dihedral angle(s) (°) | O2-O2 bond distance (Ǻ) |
|---|---|---|
| 3 | 118.2, 35.5 | 5.873 |
| 4 | 64.2, 49.4 | 5.818 |
| 5 | 128.0, 129.0 | 5.177 |
| 6 | 69.7, 31.8 | 5.468 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, F.A.; Fischer, R.C.; Spell, M.; Acevedo, A.R.; Tran, D.H.; Massoud, S.S. Metal(II) Complexes of Compartmental Polynuclear Schiff Bases Containing Phenolate and Alkoxy Groups. Crystals 2016, 6, 91. https://doi.org/10.3390/cryst6080091
Mautner FA, Fischer RC, Spell M, Acevedo AR, Tran DH, Massoud SS. Metal(II) Complexes of Compartmental Polynuclear Schiff Bases Containing Phenolate and Alkoxy Groups. Crystals. 2016; 6(8):91. https://doi.org/10.3390/cryst6080091
Chicago/Turabian StyleMautner, Franz A., Roland C. Fischer, Mark Spell, Andres R. Acevedo, Diana H. Tran, and Salah S. Massoud. 2016. "Metal(II) Complexes of Compartmental Polynuclear Schiff Bases Containing Phenolate and Alkoxy Groups" Crystals 6, no. 8: 91. https://doi.org/10.3390/cryst6080091