Control of Cellular Arrangement by Surface Topography Induced by Plastic Deformation
Abstract
:1. Introduction
2. Results
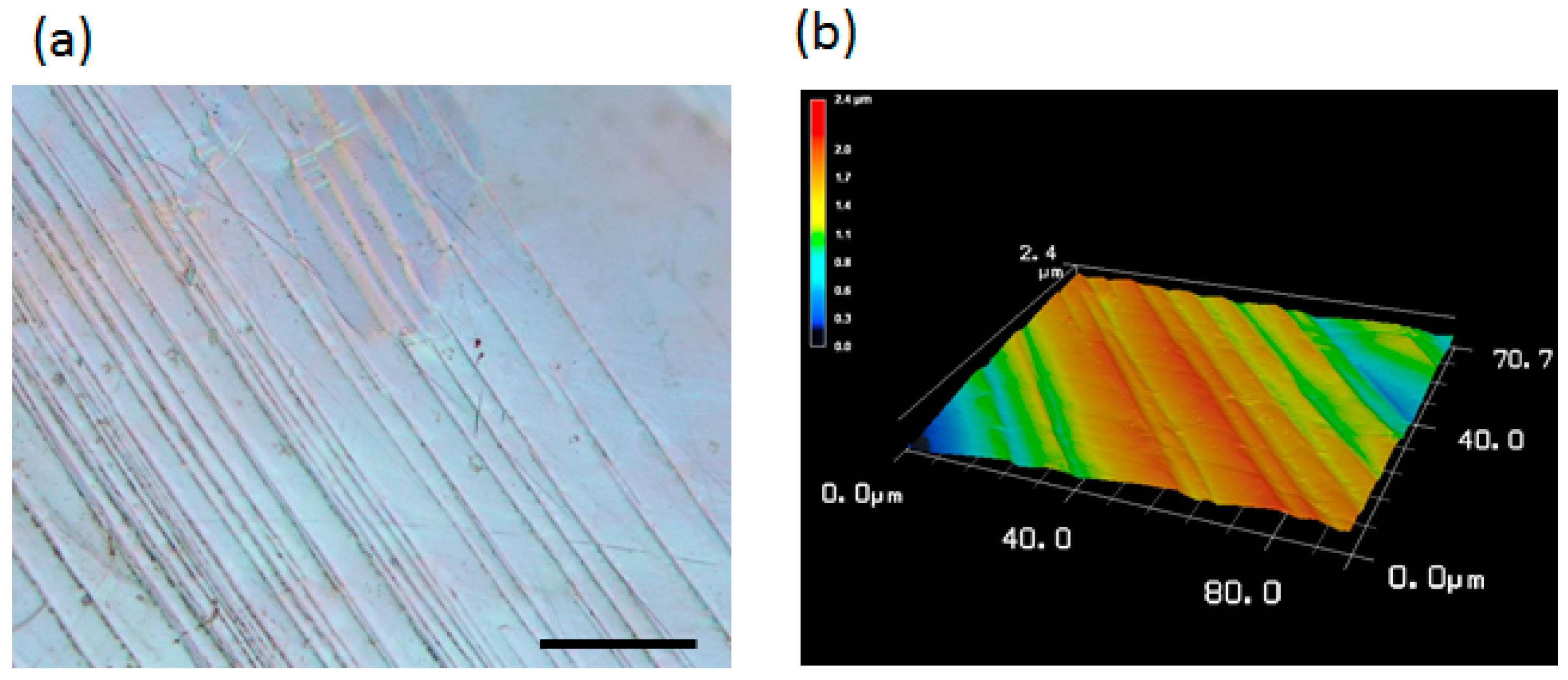
2.1. Surface Topography Induced by Plastic Deformation
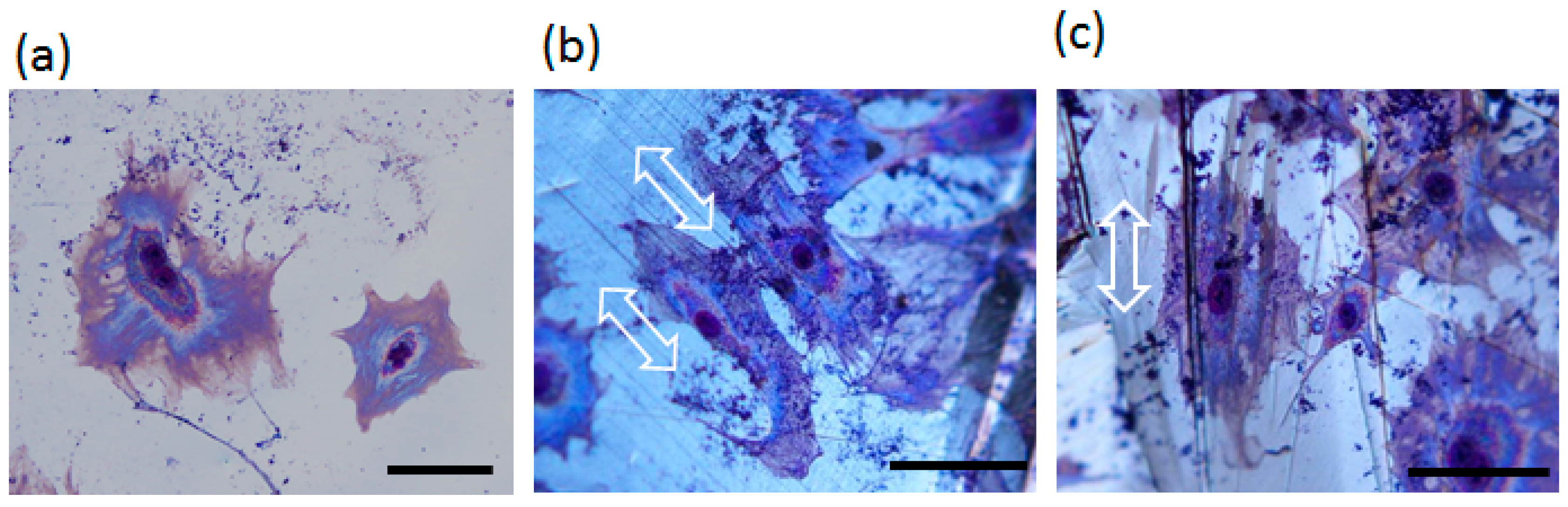
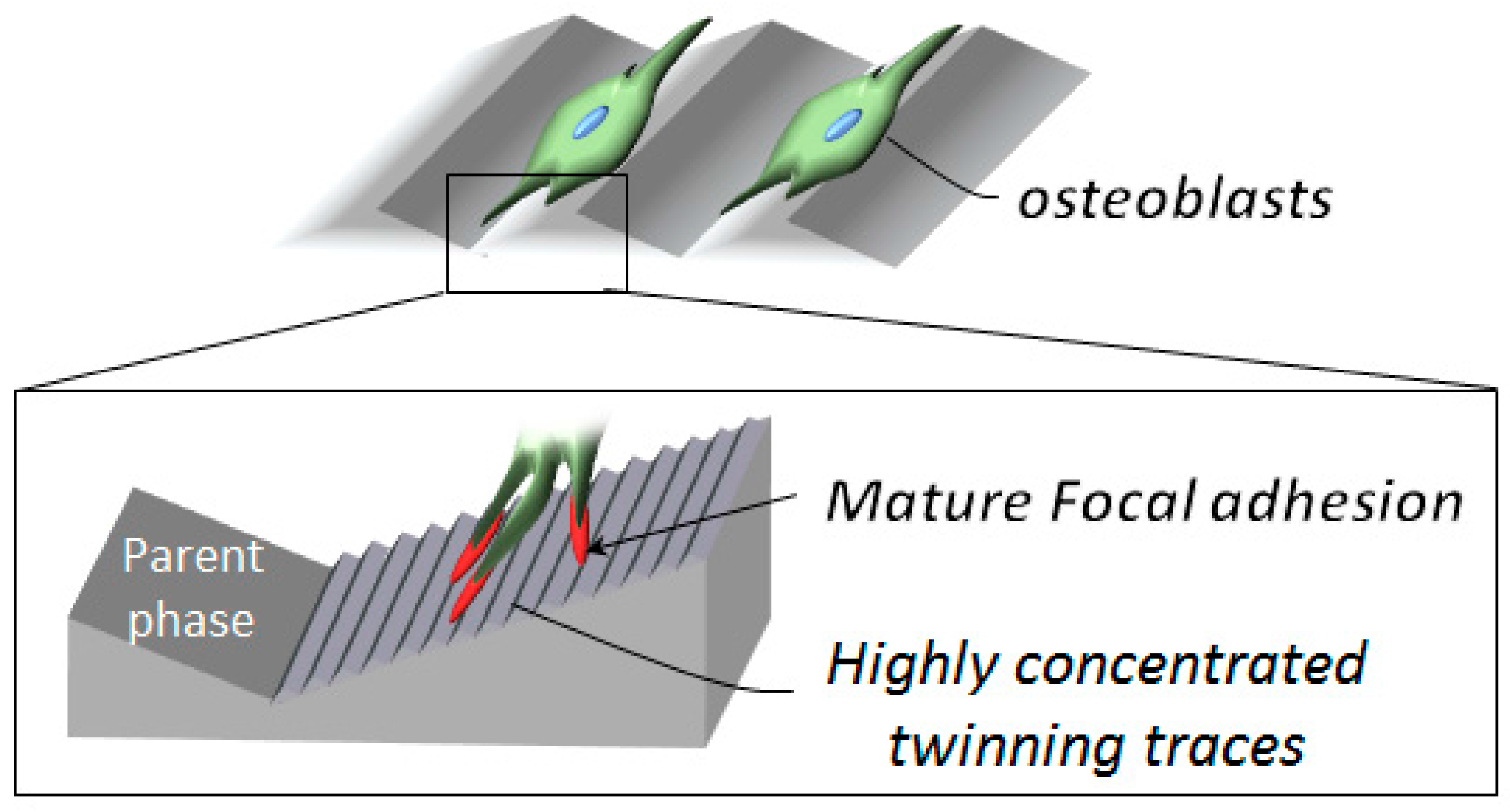
2.2. Cell Orientation
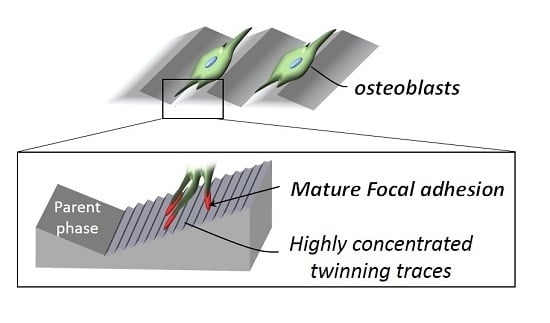
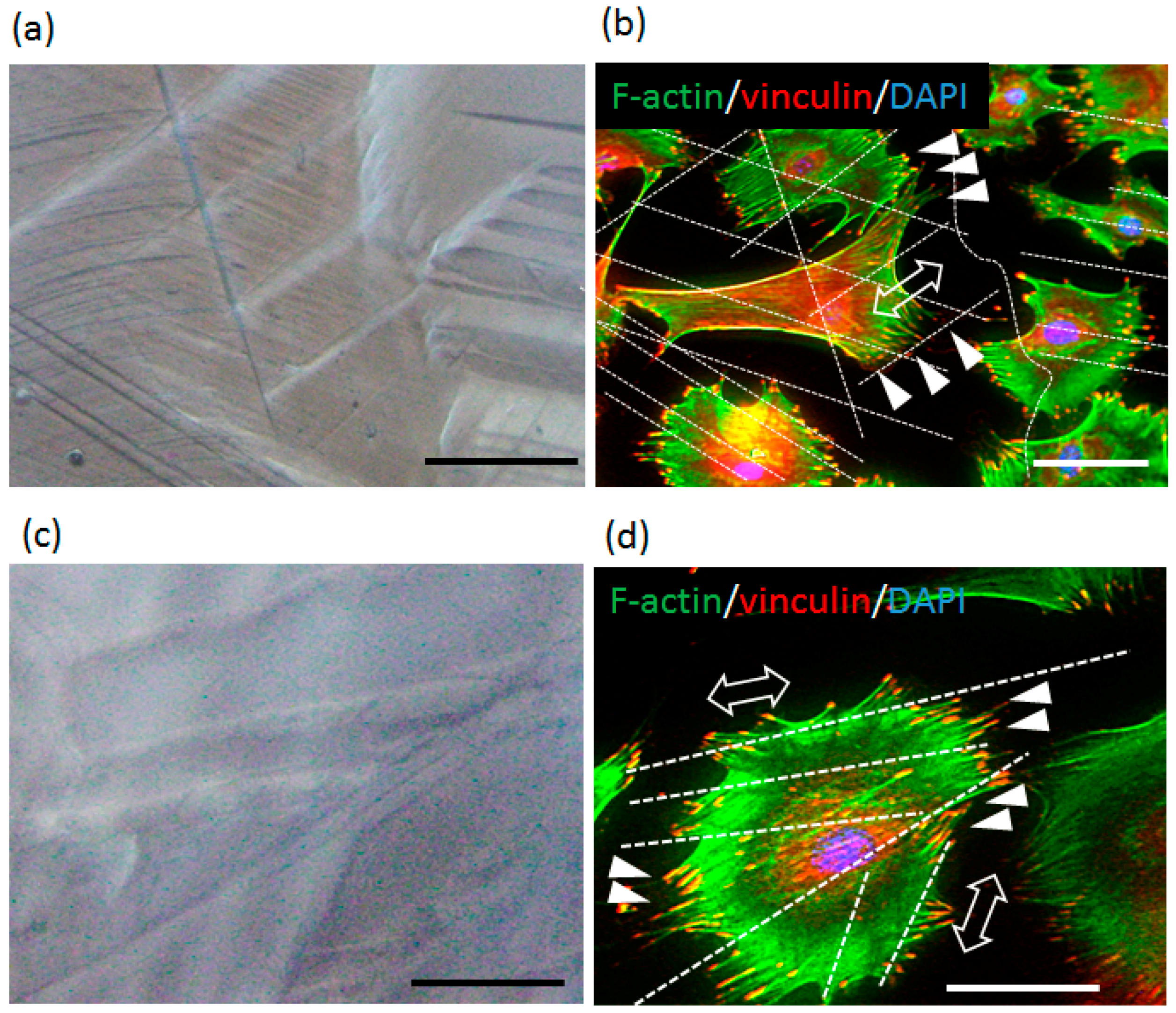
2.3. Plastic Deformation-Induced Cytoskeletal Rearrangement
3. Discussion
4. Materials and Methods
4.1. Deformation of α-Titanium Polycrystals
4.2. Surface Characterization
4.3. Cell Culture
4.4. Optical Images of Cultured Cells
4.5. Fluorescent Images of Cultured Cells
5. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Landis, W.J. The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 1995, 16, 533–544. [Google Scholar] [CrossRef]
- Onsager, L. The effects of shape on the interactions of colloidal particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M. Liquid crystallinity in condensed type I collagen solutions.a clue to the packing of collagen in extracellular matrices. J. Mol. Biol. 1992, 224, 861–873. [Google Scholar] [CrossRef]
- Campi, G.; Fratini, M.; Bukreeva, I.; Ciasca, G.; Burghammer, M.; Brun, F.; Tromba, G.; Mastrogiacomo, M.; Cedola, A. Imaging collagen packing dynamics during mineralization of engineered bone tissue. Acta Biomater. 2015, 23, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Nakano, T.; Umakoshi, Y.; Yamamoto, M.; Tabata, Y. Degree of biological apatite c-axis orientation rather than bone mineral density controls mechanical function in bone regenerated using rBMP-2. J. Bone Miner. Res. 2013, 28, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Kaibara, K.; Ishimoto, T.; Tabata, Y.; Umakoshi, Y. Biological apatite (BAp) crystallographic orientation and texture as a new index for assessing the microstructure and function of bone regenerated by tissue engineering. Bone 2012, 51, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Matsugaki, A.; Aramoto, G.; Ninomiya, T.; Sawada, H.; Hata, S.; Nakano, T. Abnormal arrangement of a collagen/apatite extracellular matrix orthogonal to osteoblast alignment is constructed by a nanoscale periodic surface structure. Biomaterials 2015, 37, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Matsugaki, A.; Fujiwara, N.; Nakano, T. Continuous cyclic stretch induces osteoblast alignment and formation of anisotropic collagen fiber matrix. Acta Biomater. 2013, 9, 7227–7235. [Google Scholar] [CrossRef] [PubMed]
- Matsugaki, A.; Isobe, Y.; Saku, T.; Nakano, T. Quantitative regulation of bone-mimetic, oriented collagen/apatite matrix structure depends on the degree of osteoblast alignment on oriented collagen substrates. J. Biomed. Mater. Res. 2015, 103, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Kaibara, K.; Tabata, Y.; Nagata, N.; Enomoto, S.; Marukawa, E.; Umakoshi, Y. Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam X-ray diffractometer system. Bone 2002, 31, 479–487. [Google Scholar] [CrossRef]
- Matsugaki, A.; Aramoto, G.; Nakano, T. The alignment of MC3T3-E1 osteoblasts on steps of slip traces introduced by dislocation motion. Biomaterials 2012, 33, 7327–7335. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Davidson, L.; Asashima, M.; Keller, R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr. Biol. 2005, 2615, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Kurihara, H.; Kuno, K.; Yokoyama, H.; Wada, T.; Kurihara, Y.; Imai, T.; Wang, Y.; Ogata, M.; Nishimatsu, H.; et al. ADAMTS-1: A metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J. Clin. Investig. 2000, 105, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.H.; Lee, J.K. Deformation twinning in h.c.p. metals and alloys. Philos. Mag. A 1991, 63, 987–1000. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keel, P.J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 2004, 1692, 103–119. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsugaki, A.; Nakano, T. Control of Cellular Arrangement by Surface Topography Induced by Plastic Deformation. Crystals 2016, 6, 73. https://doi.org/10.3390/cryst6060073
Matsugaki A, Nakano T. Control of Cellular Arrangement by Surface Topography Induced by Plastic Deformation. Crystals. 2016; 6(6):73. https://doi.org/10.3390/cryst6060073
Chicago/Turabian StyleMatsugaki, Aira, and Takayoshi Nakano. 2016. "Control of Cellular Arrangement by Surface Topography Induced by Plastic Deformation" Crystals 6, no. 6: 73. https://doi.org/10.3390/cryst6060073