Bottom-Up Assembly and Applications of Photonic Materials
Abstract
:1. Introduction
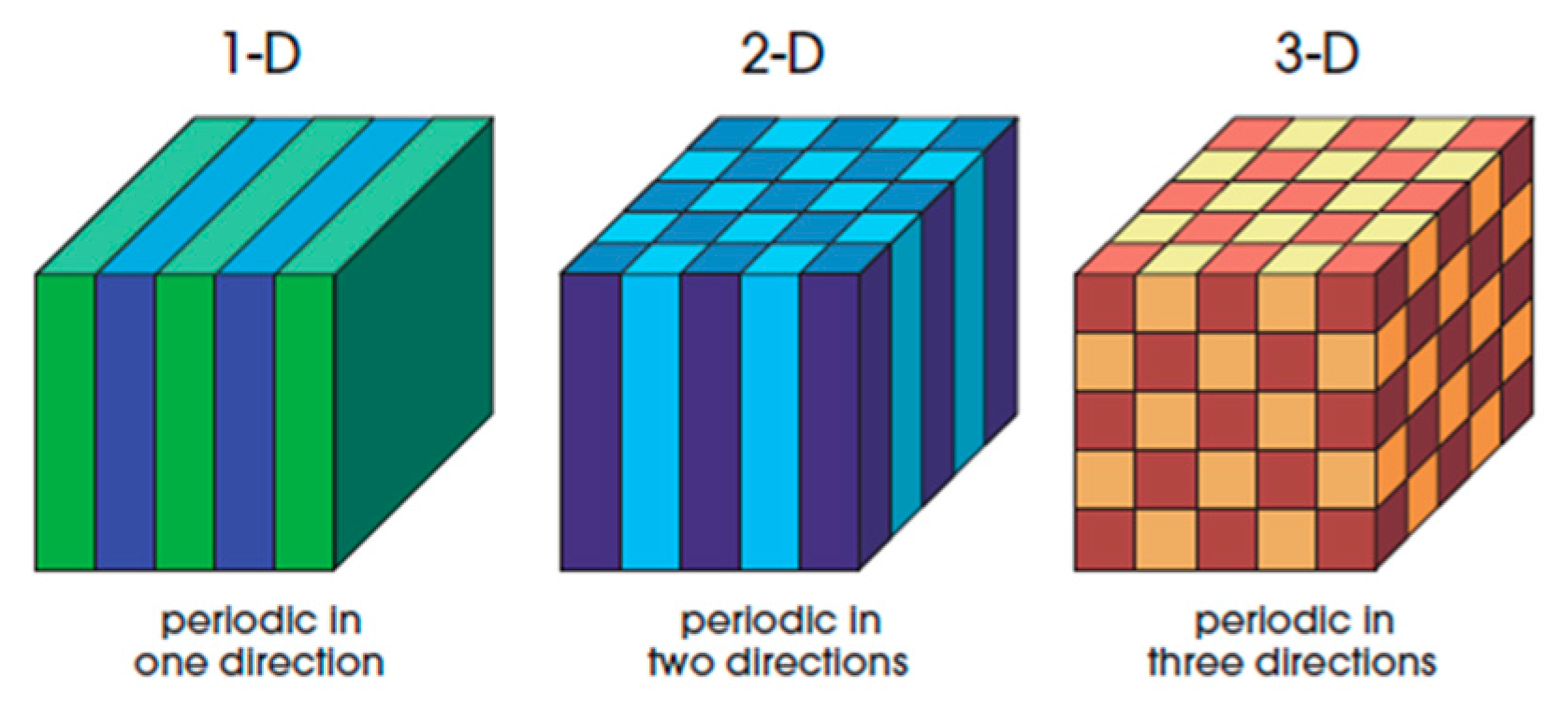
2. Concepts
3. Fabrication Approaches
3.1. Top-Down vs. Bottom-Up
3.2. Colloidal Assembly
4. Post-Assembly Modification
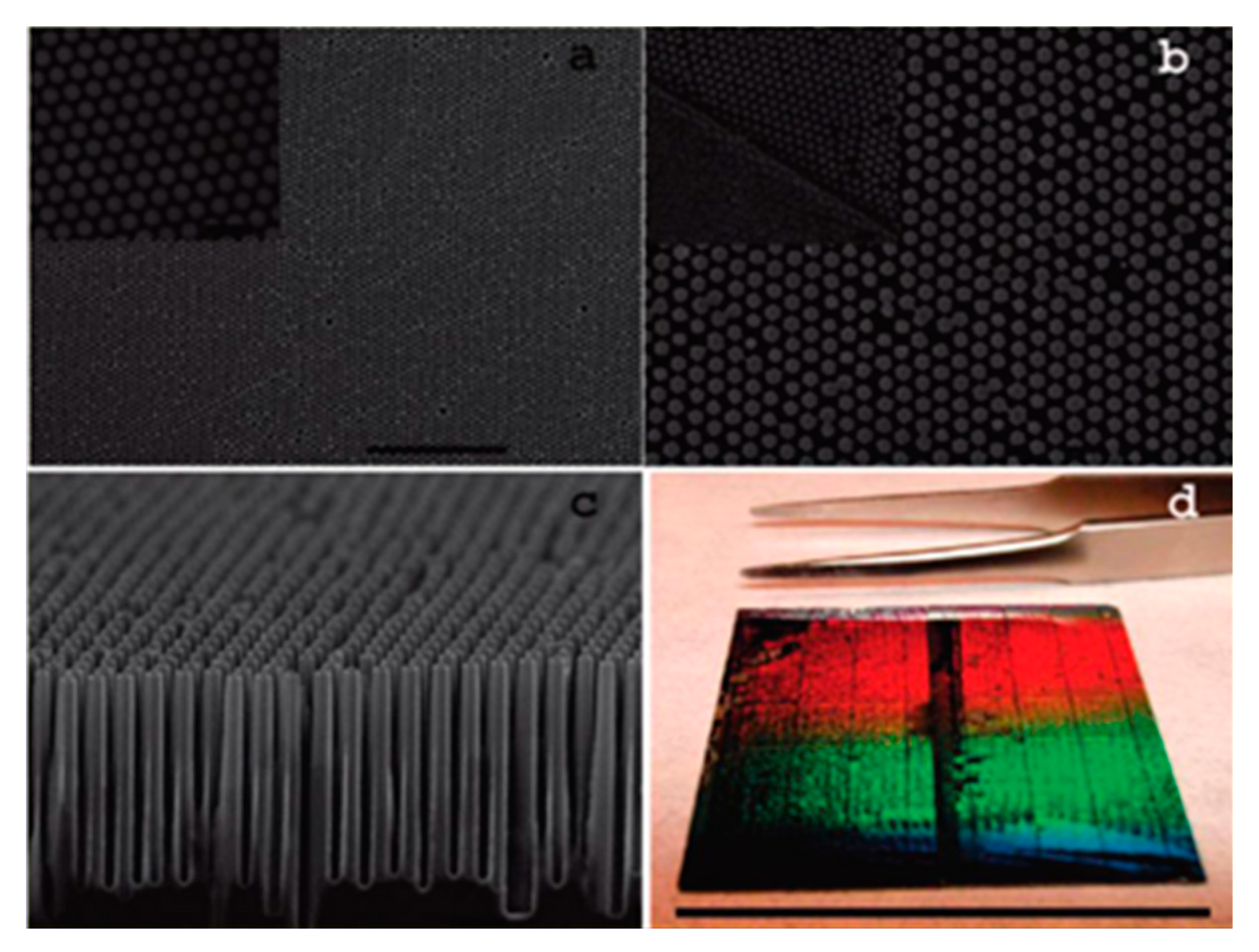
4.1. Nanosphere Lithography
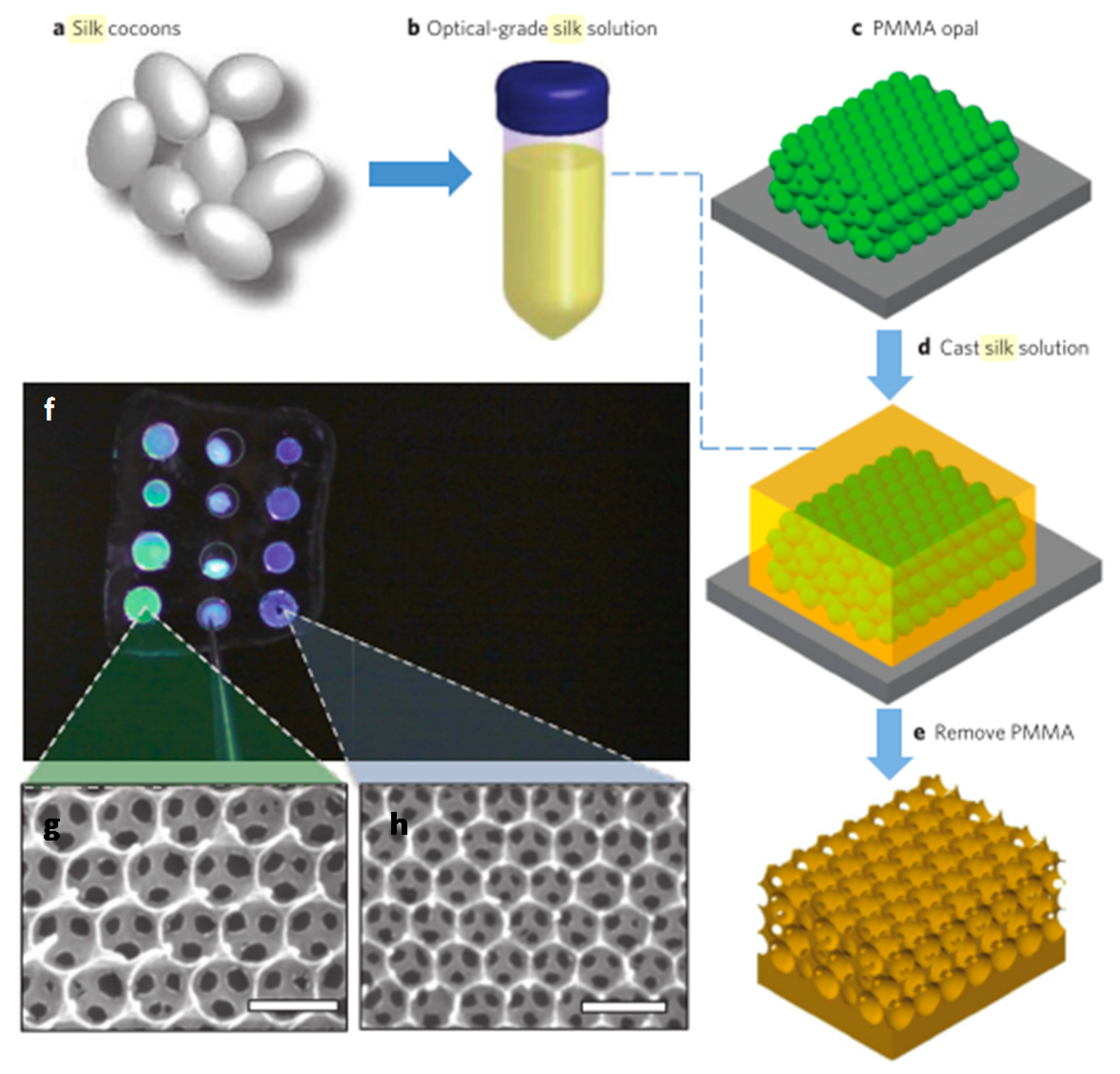
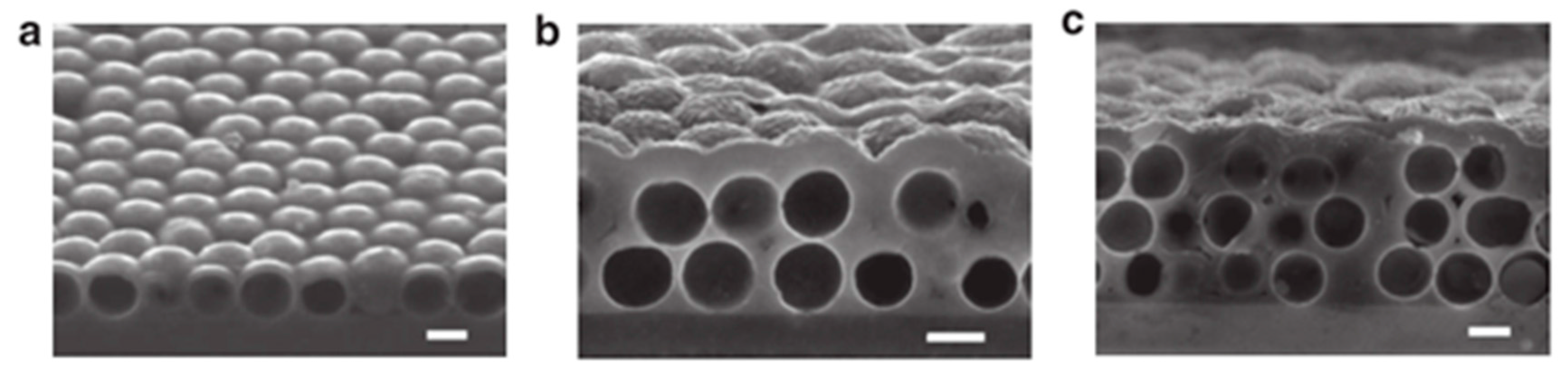
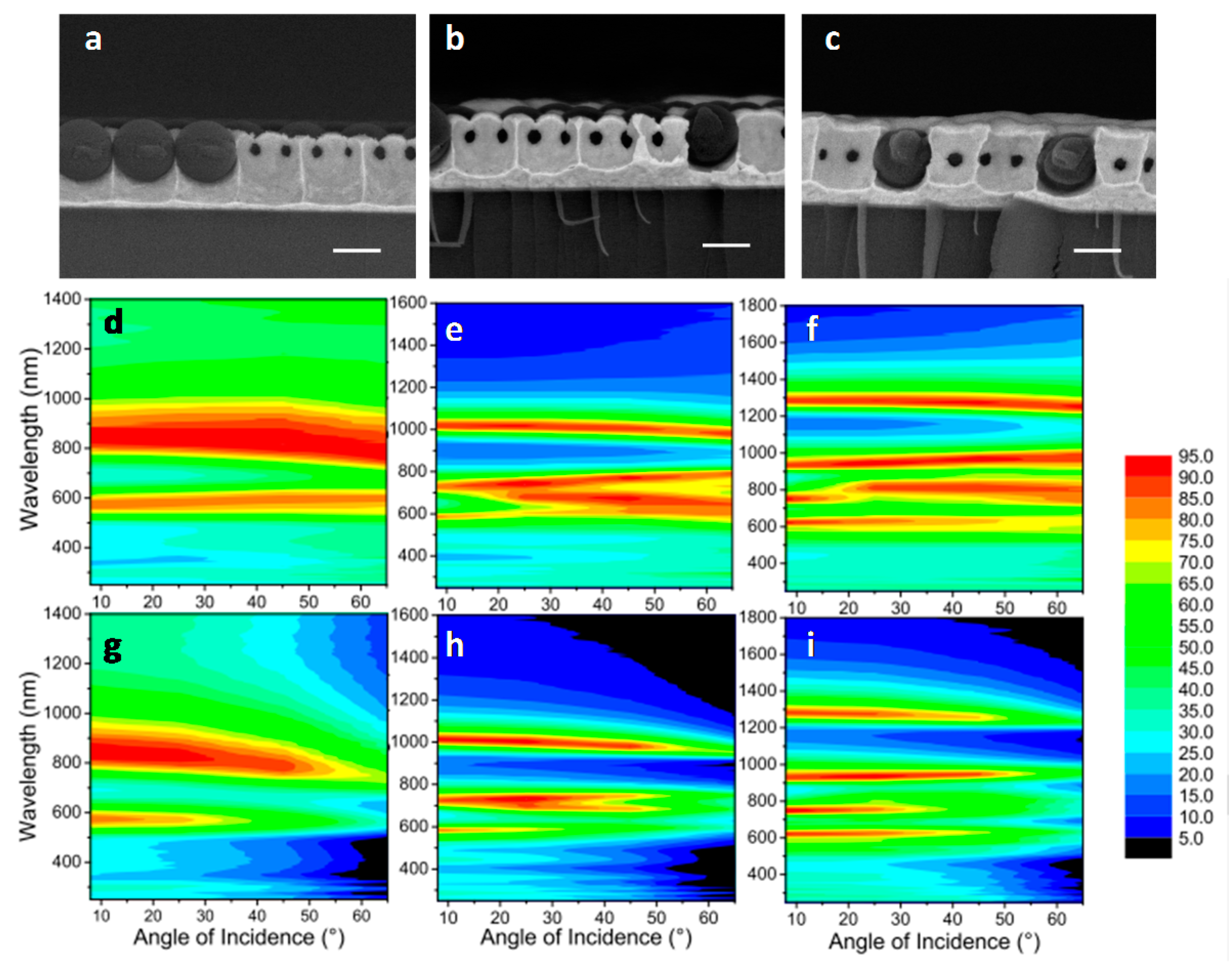
4.2. Inverse Opals
5. Applications
5.1. Photon Management in Photovoltaics
5.2. Light Emitting Diodes
5.3. Sensors
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Committee on Nanophotonics Accessibility and Applicability; Air Force Studies Board; Division on Engineering and Physical Sciences; National Research Council. Nanophotonics: Accessibility and Applicability; National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Hu, E.L.; Brongersma, M.; Baca, A. Applications: Nanophotonics and Plasmonics. In Nanotechnology Research Directions for Societal Needs in 2020; Springer: Dordrecht, The Netherlands, 2008; pp. 318–340. [Google Scholar]
- Cong, H.; Yu, B.; Tang, J.; Li, Z.; Liu, X. Current status and future developments in preparation and application of colloidal crystals. Chem. Soc. Rev. 2013, 42, 7774–7800. [Google Scholar] [CrossRef] [PubMed]
- Galisteo-Lõpez, J.F.; Ibisate, M.; Sapienza, R.; Froufe-Pérez, L.S.; Blanco, Ú.; Lõpez, C. Self-assembled photonic structures. Adv. Mater. 2011, 23, 30–69. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, S.Y.; Yang, S.-M.; Yi, G.-R. Self-assembled colloidal structures for photonics. NPG Asia Mater. 2011, 3, 25–33. [Google Scholar] [CrossRef]
- Li, F.; Josephson, D.P.; Stein, A. Colloidal assembly: The road from particles to colloidal molecules and crystals. Angew. Chem. Int. Ed. 2011, 50, 360–388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, T. A comparison of chemical sensors based on the different ordered inverse opal films. Sens. Actuators B Chem. 2008, 131, 190–195. [Google Scholar] [CrossRef]
- López, C. Materials aspects of photonic crystals. Adv. Mater. 2003, 15, 1679–1704. [Google Scholar] [CrossRef]
- Stein, A.; Wilson, B.E.; Rudisill, S.G. Design and functionality of colloidal-crystal-templated materials—Chemical applications of inverse opals. Chem. Soc. Rev. 2013, 42, 2763–2803. [Google Scholar] [CrossRef] [PubMed]
- Tétreault, N.; Míguez, H.; Ozin, G.A. Silicon inverse opal—A platform for photonic bandgap research. Adv. Mater. 2004, 16, 1471–1476. [Google Scholar] [CrossRef]
- Vogel, N.; Weiss, C.K.; Landfester, K. From soft to hard: The generation of functional and complex colloidal monolayers for nanolithography. Soft Matter 2012, 8, 4044–4061. [Google Scholar] [CrossRef]
- Von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up assembly of photonic crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Qi, L. Two-dimensionally patterned nanostructures based on monolayer colloidal crystals: Controllable fabrication, assembly, and applications. Nano Today 2011, 6, 608–631. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic crystals for chemical sensing and biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef] [PubMed]
- Joannopoulos, J.D.; Johnson, S.G.; Winn, J.N.; Meade, R.D. Photonic Crystals—Molding the Flow of Light, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localisation of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Song, K.; Clays, K.; Tung, C.H. Controlled directionality of ellipsoids in monolayer and multilayer colloidal crystals. Langmuir 2010, 26, 11544–11549. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Song, K.; Clays, K.; Tung, C.-H. Fabrication of 3D photonic crystals of ellipsoids: Convective self-assembly in magnetic field. Adv. Mater. 2009, 21, 1936–1940. [Google Scholar] [CrossRef]
- Walker, D.A.; Kowalczyk, B.; de la Cruz, M.O.; Grzybowski, B.A. Electrostatics at the nanoscale. Nanoscale 2011, 3, 1316–1344. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.J.M.; Wilmer, C.E.; Soh, S.; Grzybowski, B.A. Nanoscale forces and their uses in self-assembly. Small 2009, 5, 1600–1630. [Google Scholar] [CrossRef] [PubMed]
- Velev, O.D.; Gupta, S. Materials fabricated by micro- and nanoparticle assembly—The challenging path from science to engineering. Adv. Mater. 2009, 21, 1897–1905. [Google Scholar] [CrossRef]
- Davis, K.E.; Russel, W.B.; Glantschnig, W.J. Disorder-to-order transition in settling suspensions of colloidal silica: X-ray measurements. Science 1989, 245, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Pusey, P.N.; van Megen, W. Phase behaviour of concentrated suspensions of nearly hard colloidal spheres. Nature 1986, 320, 340–342. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, X.S. Flow-controlled vertical deposition method for the fabrication of photonic crystals. Langmuir 2004, 20, 1524–1526. [Google Scholar] [CrossRef] [PubMed]
- Fortes, L.M.; Gonçalves, M.C.; Almeida, R.M. Processing optimization and optical properties of 3-D photonic crystals. J. Non Cryst. Solids 2009, 355, 1189–1192. [Google Scholar] [CrossRef]
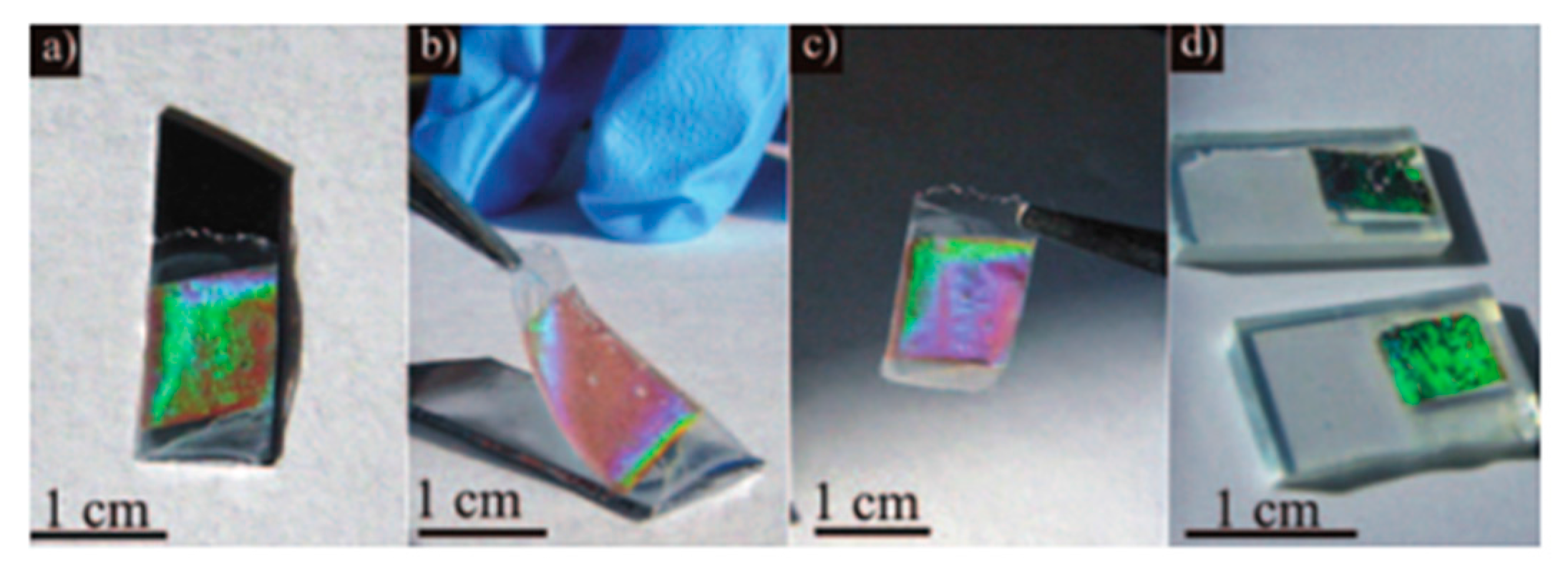
- Fortes, L.M.; Gonçalves, M.C.; Almeida, R.M. Flexible photonic crystals for strain sensing. Opt. Mater. 2011, 33, 408–412. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Wang, C.; Li, H.; Dong, F.; Yang, B.; Yu, K.; Lin, Q. Centrifugation-induced water-tunable photonic colloidal crystals with narrow diffraction bandwidth and highly sensitive detection of SCN. ACS Appl. Mater. Interfaces 2013, 5, 1990–1996. [Google Scholar]
- Ko, Y.G.; Shin, D.H.; Lee, G.S.; Choi, U.S. Fabrication of colloidal crystals on hydrophilic/hydrophobic surface by spin-coating. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 188–194. [Google Scholar] [CrossRef]
- Arutinov, G.; Brichkin, S.B.; Razumov, V.F. Self-assembling of polystyrene microsphere monolayers by spin-coating. Nanotechnol. Russ. 2010, 5, 67–72. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Ogi, T.; Iskandar, F.; Okuyama, K. Highly ordered porous monolayer generation by dual-speed spin-coating with colloidal templates. Chem. Eng. J. 2011, 167, 409–415. [Google Scholar] [CrossRef]
- Jiang, P.; McFarland, M.J. Large-scale fabrication of wafer-size colloidal crystals, macroporous polymers and nanocomposites by spin-coating. J. Am. Chem. Soc. 2004, 126, 13778–13786. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Prasad, T.; McFarland, M.J.; Colvin, V.L. Two-dimensional non close-packed colloidal crystals formed by spincoating. Appl. Phys. Lett. 2006, 89, 011908. [Google Scholar] [CrossRef]
- Jiang, P.; McFarland, M.J. Wafer-scale periodic nanohole arrays templated from two-dimensional nonclose-packed colloidal crystals. J. Am. Chem. Soc. 2005, 127, 3710–3711. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.; Khunsin, W.; Osiak, M.; Blömker, M.; Torres, C.M.S.; O’Dwyer, C. Ordered 2D colloidal photonic crystals on gold substrates by surfactant-assisted fast-rate dip coating. Small 2014, 10, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.-H.; Mayer, T.S.; Khoo, I.-C.; Divliansky, I.B.; Abrams, N.; Mallouk, T.E. Self-assembly of three-dimensional photonic-crystals with air-core line defects. J. Mater. Chem. 2002, 12, 3637–3639. [Google Scholar] [CrossRef]
- Deleuze, C.; Sarrat, B.; Ehrenfeld, F.; Perquis, S.; Derail, C.; Billon, L. Photonic properties of hybrid colloidal crystals fabricated by a rapid dip-coating process. Phys. Chem. Chem. Phys. 2011, 13, 10681–10689. [Google Scholar] [CrossRef] [PubMed]
- Amos, R.; Rarity, J.; Tapster, P.; Shepherd, T.; Kitson, S. Fabrication of large-area face-centered-cubic hard-sphere colloidal crystals by shear alignment. Phys. Rev. E 2000, 61, 2929–2935. [Google Scholar] [CrossRef]
- Hur, J.; Won, Y.-Y. Fabrication of high-quality non-close-packed 2D colloid crystals by template-guided Langmuir-Blodgett particle deposition. Soft Matter 2008, 4, 1261–1269. [Google Scholar] [CrossRef]
- Reculusa, S.; Ravaine, S. Synthesis of colloidal crystals of controllable thickness through the Langmuir-Blodgett technique. Chem. Mater. 2003, 15, 598–605. [Google Scholar] [CrossRef]
- Heim, M.; Reculusa, S.; Ravaine, S.; Kuhn, A. Engineering of complex macroporous materials through controlled electrodeposition in colloidal superstructures. Adv. Funct. Mater. 2012, 22, 538–545. [Google Scholar] [CrossRef]
- Szamocki, R.; Reculusa, S.; Ravaine, S.; Bartlett, P.N.; Kuhn, A.; Hempelmann, R. Tailored mesostructuring and biofunctionalization of gold for increased electroactivity. Angew. Chem. Int. Ed. 2006, 45, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Reculusa, S.; Massé, P.; Ravaine, S. Three-dimensional colloidal crystals with a well-defined architecture. J. Colloid Interface Sci. 2004, 279, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Goerres, S.; Landfester, K.; Weiss, C.K. A convenient method to produce close- and non-close-packed monolayers using direct assembly at the air-water interface and subsequent plasma-induced size reduction. Macromol. Chem. Phys. 2011, 212, 1719–1734. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, M. Fabrication of large scale two-dimensional colloidal crystal of polystyrene particles by an interfacial self-ordering process. J. Colloid Interface Sci. 2011, 361, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.R.; Moon, J.H.; Yoon, S.; Park, C.R.; Do, Y.R. Fabrication of wafer-scale polystyrene photonic crystal multilayers via the layer-by-layer scooping transfer technique. J. Mater. Chem. 2011, 21, 14167–14172. [Google Scholar] [CrossRef]
- Gao, P.; He, J.; Zhou, S.; Yang, X.; Li, S.; Sheng, J.; Wang, D.; Yu, T.; Ye, J.; Cui, Y. Large-area nanosphere self-assembly by a micro-propulsive injection method for high throughput periodic surface nanotexturing. Nano Lett. 2015, 15, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Asher, S.A. Synthesis and utilization of monodisperse hollow polymeric particles in photonic crystals. J. Am. Chem. Soc. 2004, 126, 7940–7945. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Friedman, G.; Humfeld, K.D.; Majetich, S.A.; Asher, S.A. Superparamagnetic photonic crystals. Adv. Mater. 2001, 13, 1681–1684. [Google Scholar] [CrossRef]
- Xu, X.; Friedman, G.; Humfeld, K.D.; Majetich, S.A.; Asher, S.A. Synthesis and utilization of monodisperse superparamagnetic colloidal particles for magnetically controllable photonic crystals. Chem. Mater. 2002, 14, 1249–1256. [Google Scholar] [CrossRef]
- Fang, Y.; Phillips, B.M.; Askar, K.; Choi, B.; Jiang, P.; Jiang, B. Scalable bottom-up fabrication of colloidal photonic crystals and periodic plasmonic nanostructures. J. Mater. Chem. C 2013, 1, 6031–6047. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-crystal colloidal multilayers of controlled thickness. Chem. Mater. 1999, 2132–2140. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Y.J.; Leong, E.S.P.; Teng, J.; Lu, X. Highly ordered and gap controllable two-dimensional non-close-packed colloidal crystals and plasmonic-photonic crystals with enhanced optical transmission. J. Mater. Chem. 2012, 24668–24675. [Google Scholar] [CrossRef]
- Massé, P.; Pouclet, G.; Ravaine, S. Periodic distribution of planar defects in colloidal photonic crystals. Adv. Mater. 2008, 20, 584–587. [Google Scholar] [CrossRef]
- Zheng, H.; Vallée, R.; Almeida, R.M.; Rivera, T.; Ravaine, S. Quasi-total omnidirectional light absorption in nanostructured gold films. Appl. Phys. A 2014, 117, 471–475. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, Z.; Shan, L.; Zheng, L.; Wei, T.; Yan, Q. Layer-by-Layer Approach to (2 + 1)D Photonic Crystal Superlattice with Enhanced Crystalline Integrity. Small 2015, 11, 4910–4921. [Google Scholar] [CrossRef] [PubMed]
- Hulteen, J.C.; van Duyne, R.P. Nanosphere lithography: A materials general fabrication process for periodic particle array surfaces. J. Vac. Sci. Technol. A Vac. Surf Film 1995, 13, 1553–1558. [Google Scholar] [CrossRef]
- Colson, P.; Henrist, C.; Cloots, R. Nanosphere lithography: A powerful method for the controlled manufacturing of nanomaterials. J. Nanomater. 2013, 948510. [Google Scholar] [CrossRef]
- Li, Y.; Duan, G.; Liu, G.; Cai, W. Physical processes-aided periodic micro/nanostructured arrays by colloidal template technique: Fabrication and applications. Chem. Soc. Rev. 2013, 42, 3614–3627. [Google Scholar] [CrossRef] [PubMed]
- Hulteen, J.C.; Treichel, D.A.; Smith, M.T.; Duval, M.L.; Jensen, T.R.; van Duyne, R.P. Nanosphere lithography: Size-tunable silver nanoparticle and surface cluster arrays. J. Phys. Chem. B 1999, 103, 3854–3863. [Google Scholar] [CrossRef]
- Haynes, C.L.; van Duyne, R.P. Nanosphere lithography: A versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B 2001, 105, 5599–5611. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, W.; Chen, K.J. Application of two-dimensional polystyrene arrays in the fabrication of ordered silicon pillars. J. Alloy. Compd. 2008, 450, 512–516. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Sangar, A.; Kazemi-Zanjani, N.; Torchio, P.; Merlen, A.; Lagugné-Labarthet, F. Optical properties of silver and gold tetrahedral nanopyramid arrays prepared by nanosphere lithography. J. Phys. Chem. C 2013, 117, 14778–14786. [Google Scholar] [CrossRef]
- Zheng, H.; Vallée, R.; Ly, I.; Almeida, R.M.; Rivera, T.; Ravaine, S. Morphological Design of Gold Nanopillar Arrays and Their Optical Properties. J. Phys. Chem. C 2016, 120, 1178–1185. [Google Scholar] [CrossRef]
- Bae, C.; Moon, J.; Shin, H.; Kim, J.; Sung, M.M. Fabrication of monodisperse asymmetric colloidal clusters by using contact area lithography (CAL). J. Am. Chem. Soc. 2007, 129, 14232–14239. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, J.; Ye, X.; Li, Y.; Ma, Y.; Qi, L. Heterostructured TiO2 nanorod@nanobowl arrays for efficient photoelectrochemical water splitting. Small 2016, 12, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Kosiorek, A.; Kandulski, W.; Glaczynska, H.; Giersig, M. Fabrication of nanoscale rings, dots, and rods by combining shadow nanosphere lithography and annealed polystyrene nanosphere masks. Small 2005, 1, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.S.; Friesen, S.A.; Mallouk, T.E. Wafer-scale fabrication of plasmonic crystals from patterned silicon templates prepared by nanosphere lithography. Nano Lett. 2013, 13, 2623–2627. [Google Scholar] [CrossRef] [PubMed]
- Lodewijks, K.; Verellen, N.; van Roy, W.; Moshchalkov, V.; Borghs, G.; van Dorpe, P. Self-assembled hexagonal double fishnets as negative index materials. Appl. Phys. Lett. 2011, 98, 091101. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, F.; Liu, M.; Liu, D.; Men, D.; Cai, W.; Duan, G.; Li, Y. Spherical Nanoparticle Arrays with Tunable Nanogaps and Their Hydrophobicity Enhanced Rapid SERS Detection by Localized Concentration of Droplet Evaporation. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Hang, L.; Zhao, Y.; Zhang, H.; Liu, G.; Cai, W.; Li, Y.; Qu, L. Copper nanoparticle @ graphene composite arrays and their enhanced catalytic performance. Acta Mater. 2016, 105, 59–67. [Google Scholar] [CrossRef]
- Kuo, C.W.; Shiu, J.Y.; Chen, P. Size- and shape-controlled fabrication of large-area periodic nanopillar arrays. Chem. Mater. 2003, 15, 2917–2920. [Google Scholar] [CrossRef]
- Ji, W.Y.; Jae, I.S.; Ho, M.A.; Tae, G.K. Fabrication of nanometer-scale pillar structures by using nanosphere lithography. J. Korean Phys. Soc. 2011, 58, 994–997. [Google Scholar] [CrossRef]
- Garnett, E.; Yang, P. Light trapping in silicon nanowire solar cells. Nano Lett. 2010, 10, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.L.; Nikolić, R.J.; Reinhardt, C.E.; Wang, T.F. Fabrication of nanopillars by nanosphere lithography. Nanotechnology 2006, 17, 1339–1343. [Google Scholar] [CrossRef]
- Halpern, A.R.; Corn, R.M. Lithographically patterned electrodeposition of gold, silver, and resonances. ACS Nano 2013, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, W.; Zhu, Z.; Zhang, H.; Liu, B.; Shi, W.; Qin, X.; Cheng, C. Highly-ordered silicon inverted nanocone arrays with broadband light antireflectance. Nanoscale Res. Lett. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, M.; Zhou, F.; Liu, D.; Liu, G.; Duan, G.; Cai, W.; Li, Y. Physical deposition improved SERS stability of morphology controlled periodic micro/nanostructured arrays based on colloidal templates. Small 2015, 11, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Braun, P.V.; Wiltzius, P. Macroporous materials—Electrochemically grown photonic crystals. Curr. Opin. Colloid Interface Sci. 2002, 7, 116–123. [Google Scholar] [CrossRef]
- Colodrero, S.; Calvo, M.E.; Míguez, H. Solar Energy; Rugescu, R.D., Ed.; InTech: Rijeka, Croatia, 2010. [Google Scholar]
- Mihi, A.; López-Alcaraz, F.J.; Míguez, H. Full spectrum enhancement of the light harvesting efficiency of dye sensitized solar cells by including colloidal photonic crystal multilayers. Appl. Phys. Lett. 2006, 88, 193110. [Google Scholar] [CrossRef] [Green Version]
- Mihi, A.; Míguez, H. Origin of light-harvesting enhancement in colloidal-photonic-crystal-based dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 15968–15976. [Google Scholar] [CrossRef] [PubMed]
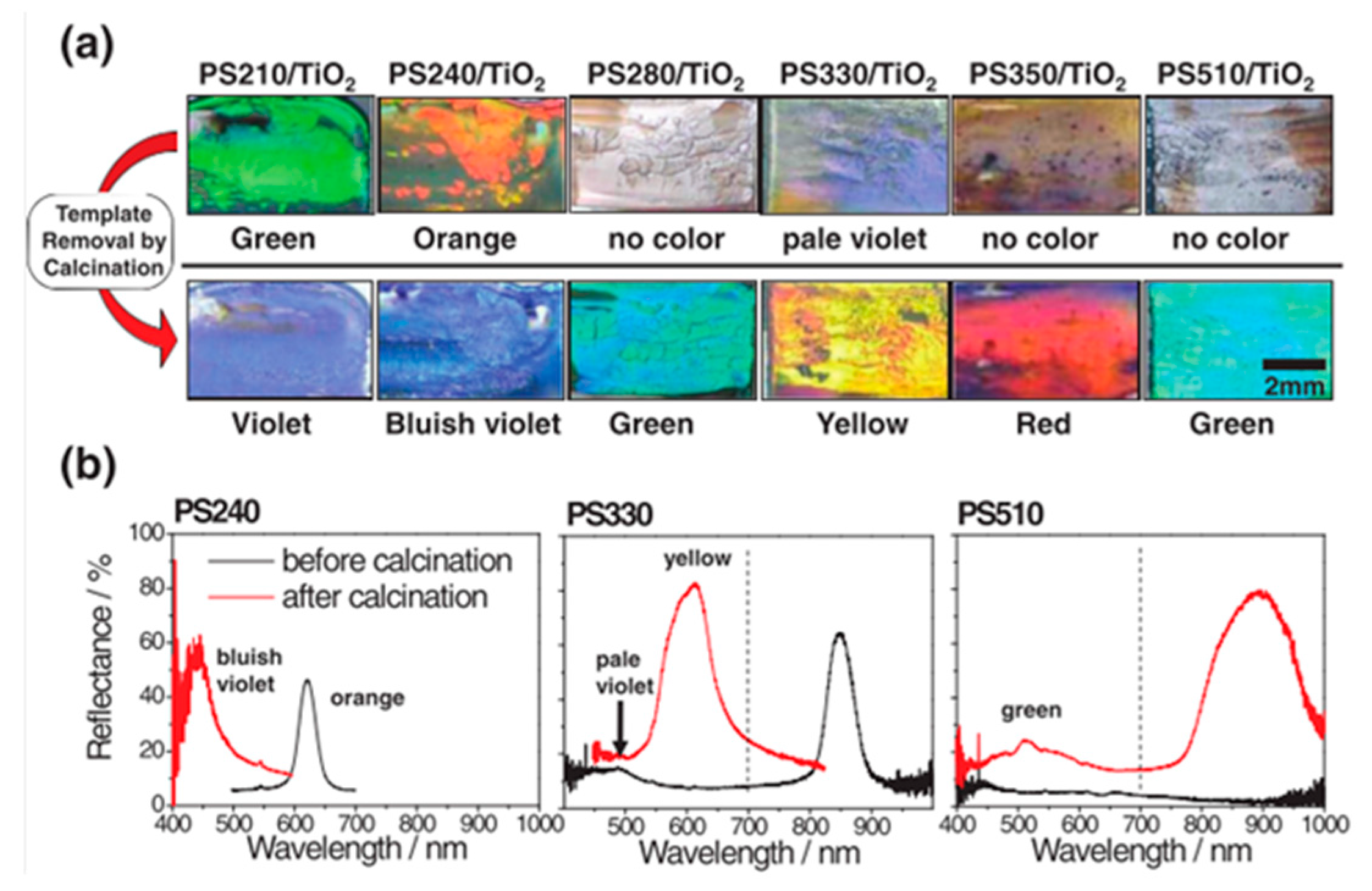
- Moon, J.H.; Cho, Y.; Yang, S. Room temperature chemical vapor deposition for fabrication of titania inverse opals: Fabrication, morphology analysis and optical characterization. Bull. Korean Chem. Soc. 2009, 30, 2245–2248. [Google Scholar]
- Ma, Y.; Chen, J.-F.; Ren, Y.; Tao, X. Transition metal-doped titania inverse opals: Fabrication and characterization. Colloids Surf. A Physicochem. Eng. Asp. 2010, 370, 129–135. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhu, Y.; Chen, H.; Li, Z.; Ding, J.; Chi, Y. Fabrication of anatase titania inverse opal films using polystyrene templates. Superlattices Microstruct. 2006, 40, 155–160. [Google Scholar] [CrossRef]
- Cai, Z.; Teng, J.; Xiong, Z.; Li, Y.; Li, Q.; Lu, X.; Zhao, X.S. Fabrication of TiO2 binary inverse opals without overlayers via the sandwich-vacuum infiltration of precursor. Langmuir 2011, 27, 5157–5164. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Marichy, C.; Dechézelles, J.-F.; Willinger, M.-G.; Pinna, N.; Ravaine, S.; Vallée, R. Nonaqueous sol-gel chemistry applied to atomic layer deposition: Tuning of photonic band gap properties of silica opals. Nanoscale 2010, 2, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhao, X.S. Opal and inverse opal fabricated with a flow-controlled vertical deposition method. Langmuir 2005, 21, 4717–4723. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C.; Fortes, L.M.; Almeida, R.M.; Chiasera, A.; Chiappini, A.; Ferrari, M.; Bhaktha, S. Photoluminescence in Er3+/Yb3+-doped silica-titania inverse opal structures. J. Sol Gel Sci. Technol. 2010, 55, 52–58. [Google Scholar] [CrossRef]
- Clara Gonçalves, M.; Fortes, L.M.; Almeida, R.M.; Chiasera, A.; Chiappini, A.; Ferrari, M. 3D rare earth-doped colloidal photonic crystals. Opt. Mater. 2009, 31, 1315–1318. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Y.J.; Teng, J.; Lu, X. Fabrication of large domain crack-free colloidal crystal heterostructures with superposition bandgaps using hydrophobic polystyrene spheres. ACS Appl. Mater. Interfaces 2012, 4, 5562–5569. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X. Electrodeposition zinc-oxide inverse opal and its application in hybrid photovoltaics. Sol. Energy Mater. Sol. Cells 2008, 92, 357–362. [Google Scholar] [CrossRef]
- Braun, P.V.; Wiltzius, P. Electrochemically grown photonic crystals. Nature 1999, 402, 603–604. [Google Scholar] [CrossRef]
- Yu, X.; Lee, Y.-J.; Furstenberg, R.; White, J.O.; Braun, P.V. Filling fraction dependent properties of inverse opal metallic photonic crystals. Adv. Mater. 2007, 19, 1689–1692. [Google Scholar] [CrossRef]
- Zheng, H.; Vallée, R.; Almeida, R.M.; Rivera, T.; Ravaine, S. Quasi-omnidirectional total light absorption in nanostructured gold surfaces. Opt. Mater. Express 2014, 4, 1236–1242. [Google Scholar] [CrossRef]
- Teperik, T.V.; García de Abajo, F.J.; Borisov, A.G.; Abdelsalam, M.; Bartlett, P.N.; Sugawara, Y.; Baumberg, J.J. Omnidirectional absorption in nanostructured metal surfaces. Nat. Photonics 2008, 2, 299–301. [Google Scholar] [CrossRef]
- Cole, R.M.; Mahajan, S.; Bartlett, P.N.; Baumberg, J.J. Engineering SERS via absorption control in novel hybrid Ni/Au nanovoids. Opt. Express 2009, 17, 13298–13308. [Google Scholar] [CrossRef] [PubMed]
- Perez, N.; Huls, A.; Puente, D.; Gonzalezvinas, W.; Castano, E.; Olaizola, S. Fabrication and characterization of silver inverse opals. Sens. Actuators B Chem. 2007, 126, 86–90. [Google Scholar] [CrossRef]
- Kim, S.; Mitropoulos, A.N.; Spitzberg, J.D.; Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk inverse opals. Nat. Photonics 2012, 6, 818–823. [Google Scholar] [CrossRef]
- Puzzo, D.P.; Arsenault, A.C.; Manners, I.; Ozin, G.A. Electroactive Inverse Opal: A Single Material for All Colors. Angew. Chem. Int. Ed. 2009, 48, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Yang, L.; Song, Y. Superoleophilic and superhydrophobic inverse opals for oil sensors. Adv. Funct. Mater. 2008, 18, 3258–3264. [Google Scholar] [CrossRef]
- Xu, X.; Goponenko, A.V.; Asher, S.A. Polymerized polyHEMA photonic crystals: pH and ethanol sensor materials. J. Am. Chem. Soc. 2008, 130, 3113–3119. [Google Scholar] [CrossRef] [PubMed]
- Cassagneau, T.; Caruso, F. Conjugated Polymer Inverse Opals for Potentiometric Biosensing. Adv. Mater. 2002, 14, 1837–1841. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.; Kim, C.; Cho, C.-Y.; Moon, J.H. Facile fabrication of sub-100 nm mesoscale inverse opal films and their application in dye-sensitized solar cell electrodes. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- King, J.S.; Graugnard, E.; Summers, C.J. TiO2 inverse opals fabricated using low-temperature atomic layer deposition. Adv. Mater. 2005, 17, 1010–1013. [Google Scholar] [CrossRef]
- Mihi, A.; Zhang, C.; Braun, P.V. Transfer of preformed three-dimensional photonic crystals onto dye-sensitized solar cells. Angew. Chem. Int. Ed. 2011, 50, 5712–5715. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Zucca, M.; Ferroni, M.; Bontempi, E.; Depero, L.E. Tailoring the Pore Size and Architecture of CeO2/TiO2 Core/Shell Inverse Opals by Atomic Layer Deposition. Small 2009, 5, 336–340. [Google Scholar] [CrossRef] [PubMed]
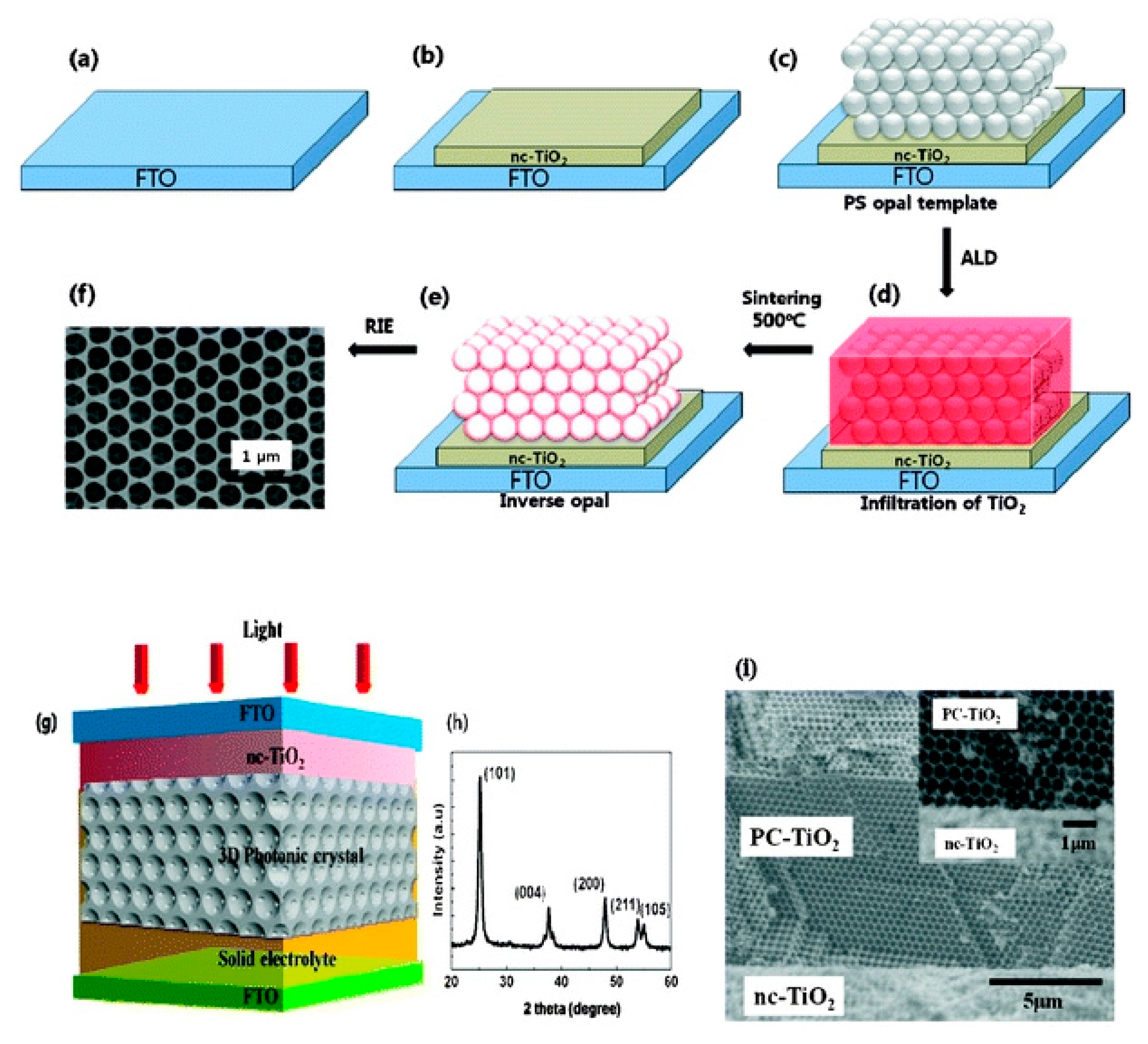
- Guldin, S.; Hüttner, S.; Kolle, M.; Welland, M.E.; Müller-Buschbaum, P.; Friend, R.H.; Steiner, U.; Tétreault, N. Dye-sensitized solar cell based on a three-dimensional photonic crystal. Nano Lett. 2010, 10, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Pikul, J.H.; Gang Zhang, H.; Cho, J.; Braun, P.V.; King, W.P. High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 2013, 4, 1732. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.N.; Baumberg, J.J.; Coyle, S.; Abdelsalam, M.E. Optical properties of nanostructured metal films. Faraday Discuss. 2004, 125, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Szamocki, R.; Velichko, A.; Holzapfel, C.; Mücklich, F.; Ravaine, S.; Garrigue, P.; Sojic, N.; Hempelmann, R.; Kuhn, A. Macroporous ultramicroelectrodes for improved electroanalytical measurements. Anal. Chem. 2007, 79, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Szamocki, R.; Massé, P.; Ravaine, S.; Ravaine, V.; Hempelmann, R.; Kuhn, A. Multicomponent macroporous materials with a controlled architecture. J. Mater. Chem. 2009, 19, 409–414. [Google Scholar] [CrossRef]
- Liu, W.; Zou, B.; Zhao, J.; Cui, H. Optimizing sol–gel infiltration for the fabrication of high-quality titania inverse opal and its photocatalytic activity. Thin Solid Films 2010, 518, 4923–4927. [Google Scholar] [CrossRef]
- Yan, Q.; Teh, L.K.; Shao, Q.; Wong, C.C.; Chiang, Y.-M. Layer transfer approach to opaline hetero photonic crystals. Langmuir 2008, 24, 1796–1800. [Google Scholar] [CrossRef] [PubMed]
- Wehrspohn, R.B.; Üpping, J. 3D photonic crystals for photon management in solar cells. J. Opt. 2012, 14, 024003. [Google Scholar] [CrossRef]
- Ko, D.-H.; Tumbleston, J.R.; Gadisa, A.; Aryal, M.; Liu, Y.; Lopez, R.; Samulski, E.T. Light-trapping nano-structures in organic photovoltaic cells. J. Mater. Chem. 2011, 21, 16293–16303. [Google Scholar] [CrossRef]
- Mokkapati, S.; Catchpole, K.R. Nanophotonic light trapping in solar cells. J. Appl. Phys. 2012, 112, 101101. [Google Scholar] [CrossRef]
- Polman, A.; Atwater, H.A. Photonic design principles for ultrahigh-efficiency photovoltaics. Nat. Mater. 2012, 11, 174–177. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.G.; Kherani, N.P.; Chutinan, A.; Ozin, G.A.; John, S.; Zukotynski, S. Silicon photovoltaics using conducting photonic crystal back-reflectors. Adv. Mater. 2008, 20, 1577–1582. [Google Scholar] [CrossRef]
- Bermel, P.; Luo, C.; Zeng, L.; Kimerling, L.C.; Joannopoulos, J.D. Improving thin-film crystalline silicon solar cell efficiencies with photonic crystals. Opt. Express 2007, 15, 16986–17000. [Google Scholar] [CrossRef] [PubMed]
- Suezaki, T.; Chen, J.I.L.; Hatayama, T.; Fuyuki, T.; Ozin, G.A. Electrical properties of p-type and n-type doped inverse silicon opals—Towards optically amplified silicon solar cells. Appl. Phys. Lett. 2010, 96, 242102. [Google Scholar] [CrossRef]
- Chen, C.-H.; Juan, P.-C.; Liao, M.-H.; Tsai, J.-L.; Hwang, H.-L. The effect of surface treatment on omni-directional efficiency of the silicon solar cells with micro-spherical texture/ITO stacks. Sol. Energy Mater. Sol. Cells 2011, 95, 2545–2548. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, J.; Narasimhan, V.K.; Ruan, Z.; Xie, C.; Fan, S.; Cui, Y. Broadband light management using low-Q whispering gallery modes in spherical nanoshells. Nat. Commun. 2012, 2, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Teperik, T.; Popov, V.; García de Abajo, F. Void plasmons and total absorption of light in nanoporous metallic films. Phys. Rev. B 2005, 71, 085408. [Google Scholar] [CrossRef]
- Teperik, T.V.; Popov, V.V.; García de Abajo, F.J.; Abdelsalam, M.; Bartlett, P.N.; Kelf, T.A.; Sugawara, Y.; Baumberg, J.J. Strong coupling of light to flat metals via a buried nanovoid lattice: The interplay of localized and free plasmons. Opt. Express 2006, 14, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Almeida, R.M.; Rivera, T.; Ravaine, S. Fabrication of broadband omnidirectional non-reflective gold surfaces by electrodeposition. Adv. Device Mater. 2015, 1, 11–16. [Google Scholar] [CrossRef]
- Shin, J.-H.; Kang, J.-H.; Jin, W.-M.; Park, J.H.; Cho, Y.-S.; Moon, J.H. Facile synthesis of TiO2 inverse opal electrodes for dye-sensitized solar cells. Langmuir 2011, 27, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Mandlmeier, B.; Szeifert, J.M.; Fattakhova-Rohlfing, D.; Amenitsch, H.; Bein, T. Formation of interpenetrating hierarchical titania structures by confined synthesis in inverse opal. J. Am. Chem. Soc. 2011, 133, 17274–17282. [Google Scholar] [CrossRef] [PubMed]
- Somani, P.R.; Dionigi, C.; Murgia, M.; Palles, D.; Nozar, P.; Ruani, G. Solid-state dye PV cells using inverse opal TiO2 films. Sol. Energy Mater. Sol. Cells 2005, 87, 513–519. [Google Scholar] [CrossRef]
- Lee, S.-H.A.; Abrams, N.M.; Hoertz, P.G.; Barber, G.D.; Halaoui, L.I.; Mallouk, T.E. Coupling of titania inverse opals to nanocrystalline titania layers in dye-sensitized solar cells. J. Phys. Chem. B 2008, 112, 14415–14421. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.G.; Woo, K.; Kim, J.; Lee, H.; Lee, W. Rapid fabrication of an inverse opal TiO2 photoelectrode for DSSC using a binary mixture of TiO2 nanoparticles and polymer microspheres. Adv. Funct. Mater. 2011, 21, 3094–3103. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
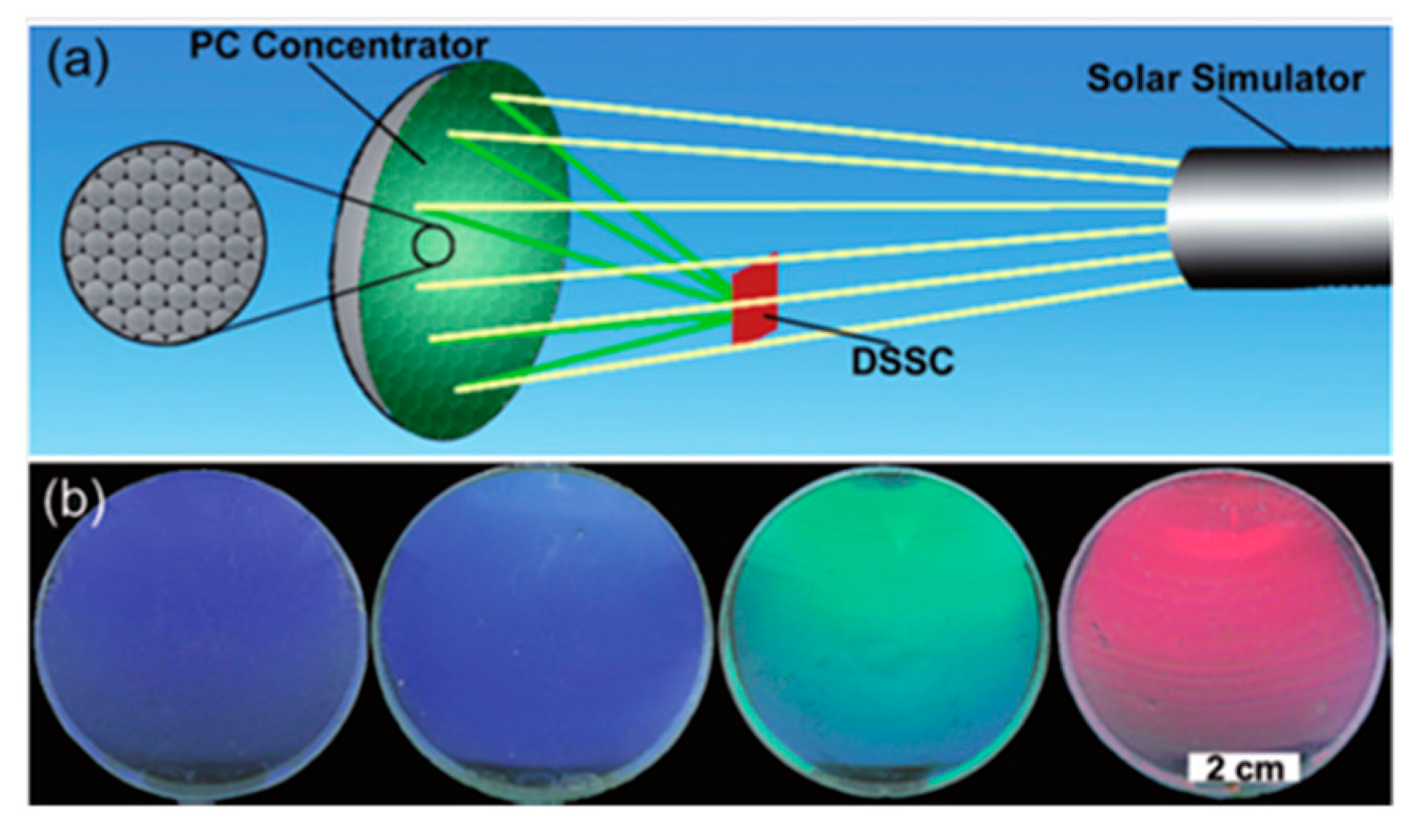
- Zhang, Y.; Wang, J.; Zhao, Y.; Zhai, J.; Jiang, L.; Song, Y.; Zhu, D. Photonic crystal concentrator for efficient output of dye-sensitized solar cells. J. Mater. Chem. 2008, 18, 2650–2650. [Google Scholar] [CrossRef]
- Hwang, D.-K.; Lee, B.; Kim, D.-H. Efficiency enhancement in solid dye-sensitized solar cell by three-dimensional photonic crystal. RSC Adv. 2013, 3, 3017–3023. [Google Scholar] [CrossRef]
- Hsieh, M.Y.; Wang, C.Y.; Chen, L.Y.; Lin, T.P.; Ke, M.Y.; Cheng, Y.W.; Yu, Y.C.; Chen, C.P.; Yeh, D.M.; Lu, C.F.; et al. Improvement of external extraction efficiency in GaN-based LEDs by SiO2 nanosphere lithography. IEEE Electron Device Lett. 2008, 29, 658–660. [Google Scholar] [CrossRef]
- Kim, B.J.; Jung, H.; Shin, J.; Mastro, M.A.; Eddy, C.R.; Hite, J.K.; Kim, S.H.; Bang, J.; Kim, J. Enhancement of light extraction efficiency of ultraviolet light emitting diodes by patterning of SiO2 nanosphere arrays. Thin Solid Films 2009, 517, 2742–2744. [Google Scholar] [CrossRef]
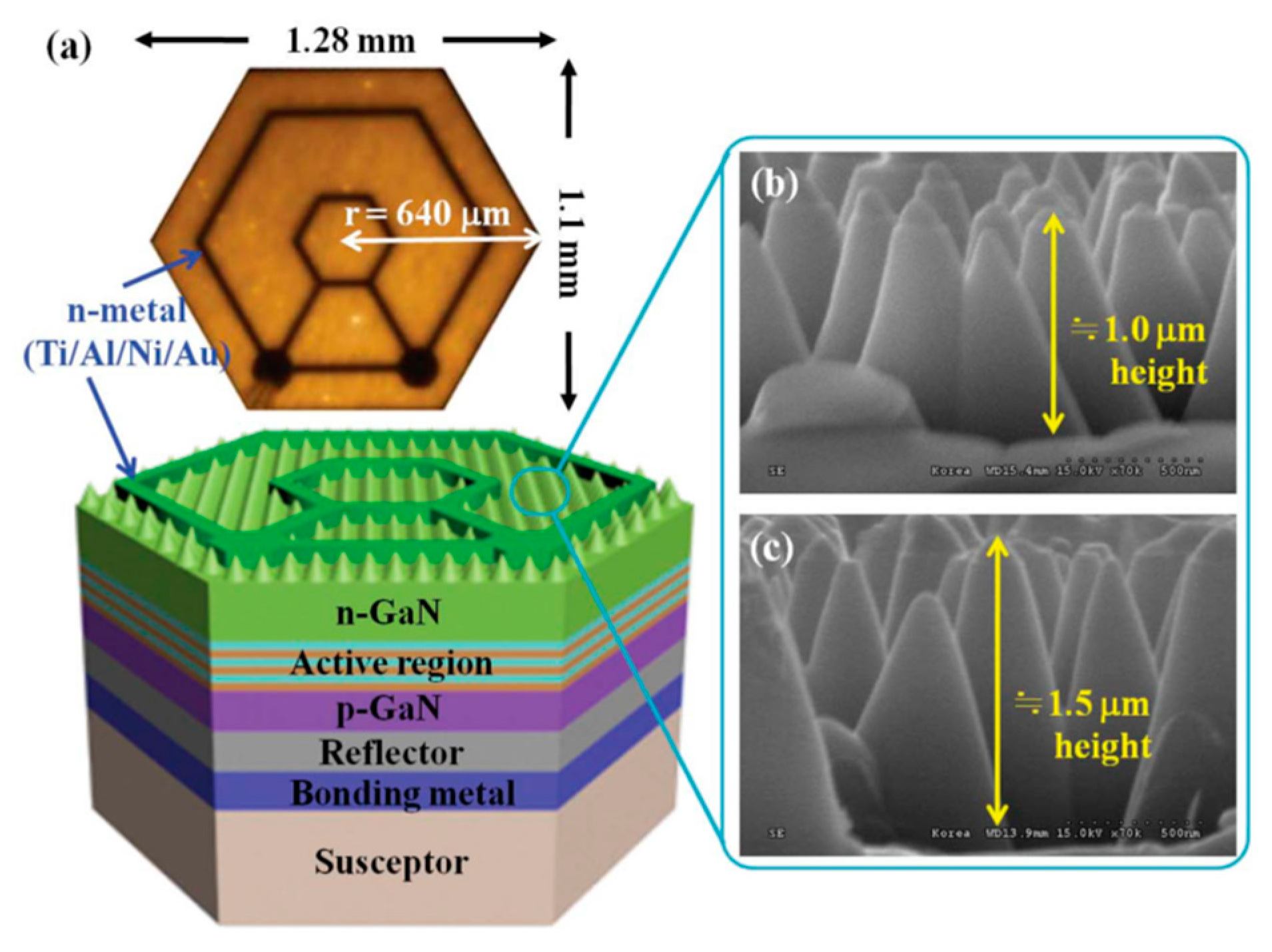
- An, H.M.; Sim, J.I.; Shin, K.S.; Sung, Y.M.; Kim, T.G. Increased light extraction from vertical GaN light-emitting diodes with ordered, cone-shaped deep-pillar nanostructures. IEEE J. Quantum Electron. 2012, 48, 891–896. [Google Scholar] [CrossRef]
- Li, K.H.; Zang, K.Y.; Chua, S.J.; Choi, H.W. III-nitride light-emitting diode with embedded photonic crystals. Appl. Phys. Lett. 2013, 102, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dylewicz, R.; Khokhar, A.Z.; Wasielewski, R.; Mazur, P.; Rahman, F. Nanotexturing of GaN light-emitting diode material through mask-less dry etching. Nanotechnology 2011, 22, 055301. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.N.; Leung, C.H.; Lai, P.T.; Choi, H.W. Photonic crystal light-emitting diodes fabricated by microsphere lithography. Nanotechnology 2008, 19, 255302. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Wilhelm, S.; Hirsch, T.; Wolfbeis, O.S. Optical sensing of the ionic strength using photonic crystals in a hydrogel matrix. ACS Appl. Mater. Interfaces 2013, 5, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Kirchinger, M.; Hirsch, T.; Wolfbeis, O. Photonic Crystal-Based Sensing and Imaging of Potassium Ions. Chemosensors 2014, 2, 207–218. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic crystal based sensor for organic solvents and for solvent-water mixtures. Sensors (Basel) 2012, 12, 16954–16963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenzl, C.; Genslein, C.; Zöpfl, A.; Baeumner, A.J.; Hirsch, T. A photonic crystal based sensing scheme for acetylcholine and acetylcholinesterase inhibitors. J. Mater. Chem. B 2015, 3, 2089–2095. [Google Scholar] [CrossRef]
- Hoi, S.K.; Chen, X.; Kumar, V.S.; Homhuan, S.; Sow, C.H.; Bettiol, A.A. A microfluidic chip with integrated colloidal crystal for online optical analysis. Adv. Funct. Mater. 2011, 21, 2847–2853. [Google Scholar] [CrossRef]
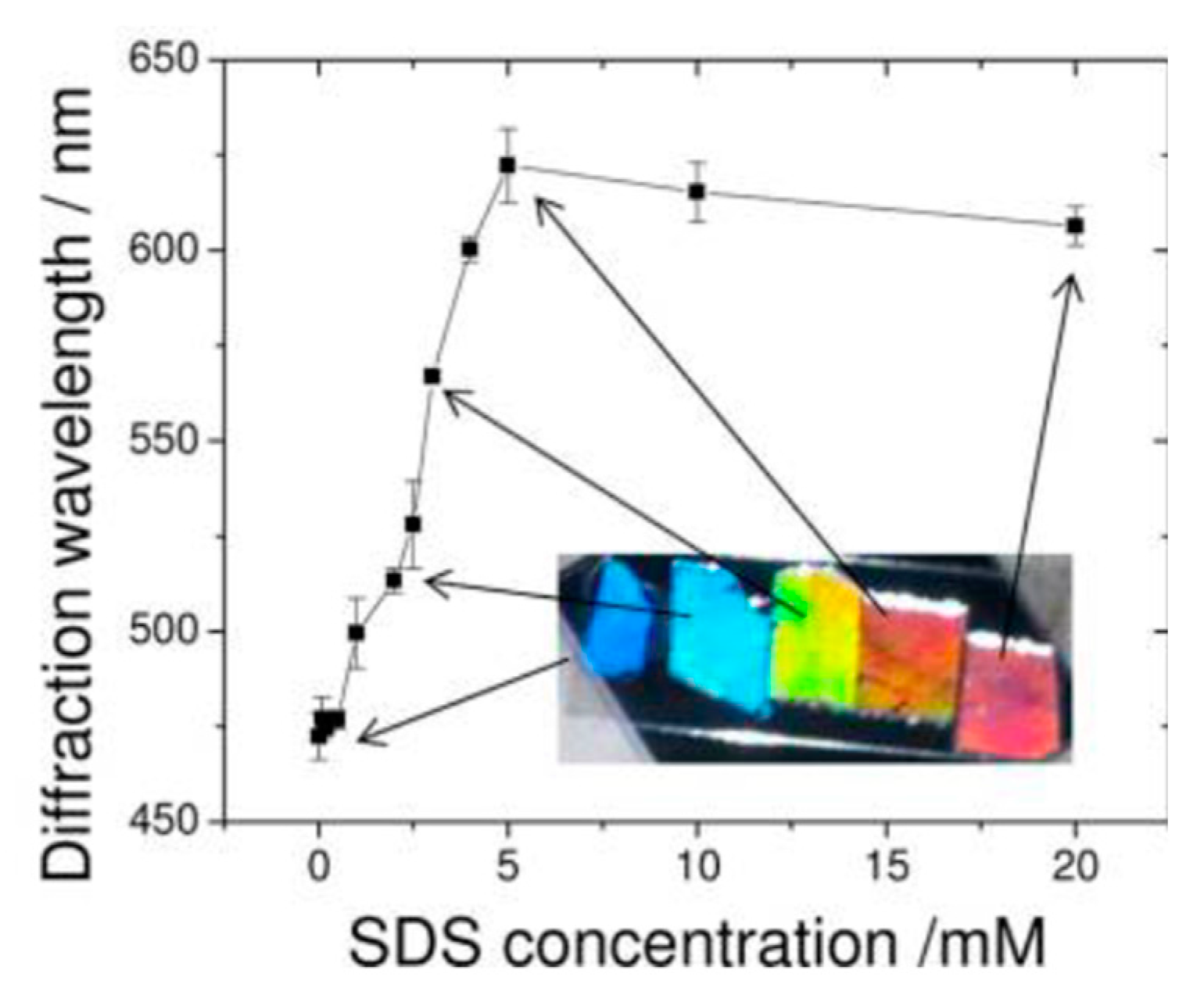
- Zhang, J.T.; Smith, N.; Asher, S.A. Two-dimensional photonic crystal surfactant detection. Anal. Chem. 2012, 84, 6416–6420. [Google Scholar] [CrossRef] [PubMed]
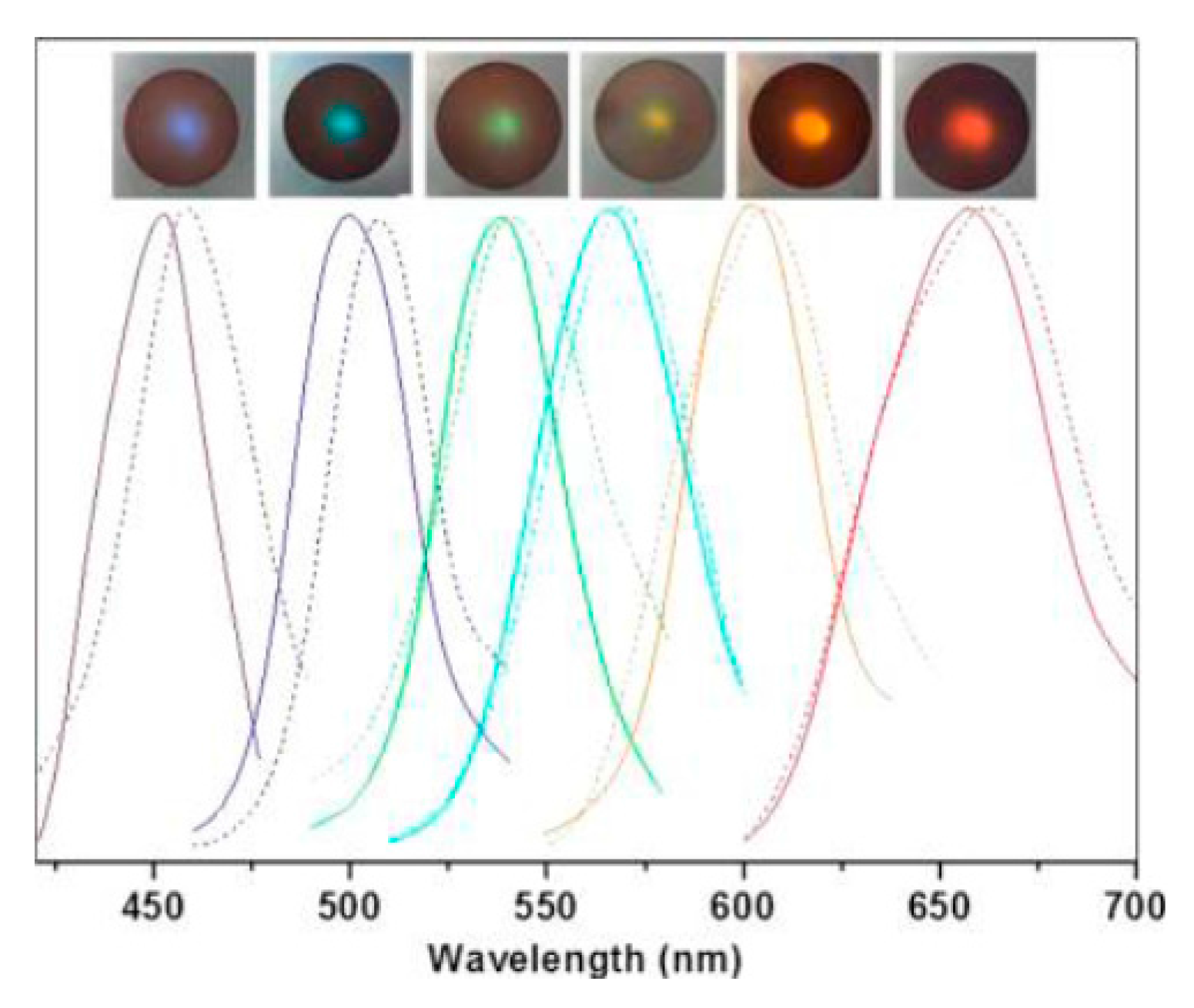
- Xu, H.; Cao, K.D.; Ding, H.B.; Zhong, Q.F.; Gu, H.C.; Xie, Z.Y.; Zhao, Y.J.; Gu, Z.Z. Spherical porphyrin sensor array based on encoded colloidal crystal beads for VOC vapor detection. ACS Appl. Mater. Interfaces 2012, 4, 6752–6757. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Farha, O.K.; Kreno, L.E.; Schoenecker, P.M.; Walton, K.S.; van Duyne, R.P.; Hupp, J.T. Fabrication of metal-organic framework-containing silica-colloidal crystals for vapor sensing. Adv. Mater. 2011, 23, 4449–4452. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Yanagida, Y.; Hatsuzawa, T. Colorimetric detection of volatile organic compounds using a colloidal crystal-based chemical sensor for environmental applications. Sens. Actuators B Chem. 2007, 125, 589–595. [Google Scholar] [CrossRef]
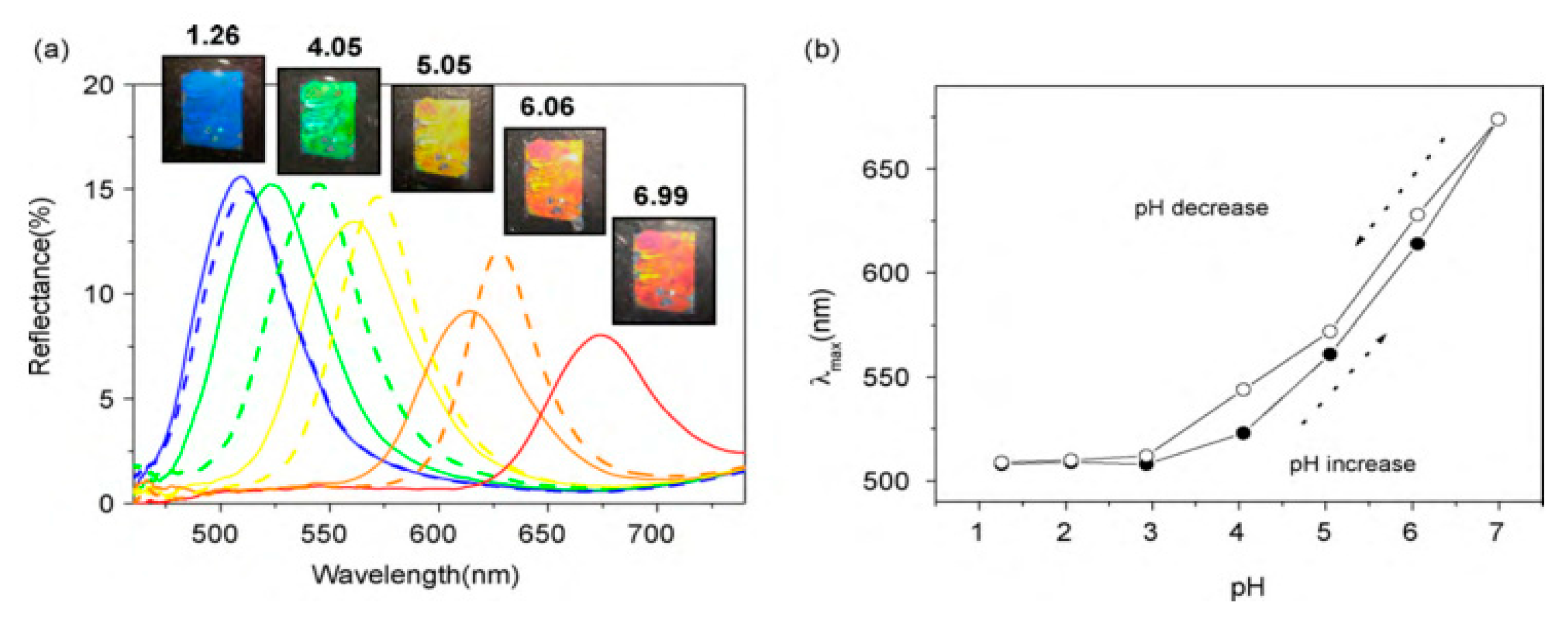
- Shin, J.; Braun, P.V.; Lee, W. Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sens. Actuators B Chem. 2010, 150, 183–190. [Google Scholar] [CrossRef]
- Li, C.; Lotsch, B.V. Stimuli-responsive 2D polyelectrolyte photonic crystals for optically encoded pH sensing. Chem. Commun. 2012, 48, 6169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, M.; Kataoka, K.; Seki, T.; Takeoka, Y. Confined stimuli-responsive polymer gel in inverse opal polymer membrane for colorimetric glucose sensor. Langmuir 2009, 25, 8349–8356. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, X.; Zhang, W.; Zhang, Y.; Li, G. pH and ionic strength responsive photonic polymers fabricated by using colloidal crystal templating. Colloid Polym. Sci. 2008, 286, 113–118. [Google Scholar] [CrossRef]
- Griffete, N.; Frederich, H.; Maître, A.; Chehimi, M.M.; Ravaine, S.; Mangeney, C. Photonic crystal pH sensor containing a planar defect for fast and enhanced response. J. Mater. Chem. 2011, 21, 13052–13055. [Google Scholar] [CrossRef]
- Griffete, N.; Frederich, H.; Maître, A.; Schwob, C.; Ravaine, S.; Carbonnier, B.; Chehimi, M.M.; Mangeney, C. Introduction of a planar defect in a molecularly imprinted photonic crystal sensor for the detection of bisphenol A. J. Colloid Interface Sci. 2011, 364, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Frederich, H.; Ravaine, S.; Chehimi, M.M.; Mangeney, C. Inverse Opals of Molecularly Imprinted Hydrogels for the Detection of Bisphenol A and pH Sensing. Langmuir 2012, 28, 1005–1012. [Google Scholar]
- Cai, Z.; Kwak, D.H.; Punihaole, D.; Hong, Z.; Velankar, S.S.; Liu, X.; Asher, S.A. A photonic crystal protein hydrogel sensor for Candida albicans. Angew. Chem. Int. Ed. 2015, 54, 13036–13040. [Google Scholar] [CrossRef] [PubMed]
- Men, D.; Zhang, H.; Hang, L.; Liu, D.; Li, X.; Cai, W.; Xiong, Q.; Li, Y. Optical sensor based on hydrogel films with 2D colloidal arrays attached on both the surfaces: Anti-curling performance and enhanced optical diffraction intensity. J. Mater. Chem. C 2015, 3, 3659–3665. [Google Scholar] [CrossRef]
- Men, D.; Zhou, F.; Hang, L.; Li, X.; Duan, G.; Cai, W.; Li, Y. A functional hydrogel film attached with a 2D Au nanosphere array and its ultrahigh optical diffraction intensity as a visualized sensor. J. Mater. Chem. C 2016, 4, 2117–2122. [Google Scholar] [CrossRef]

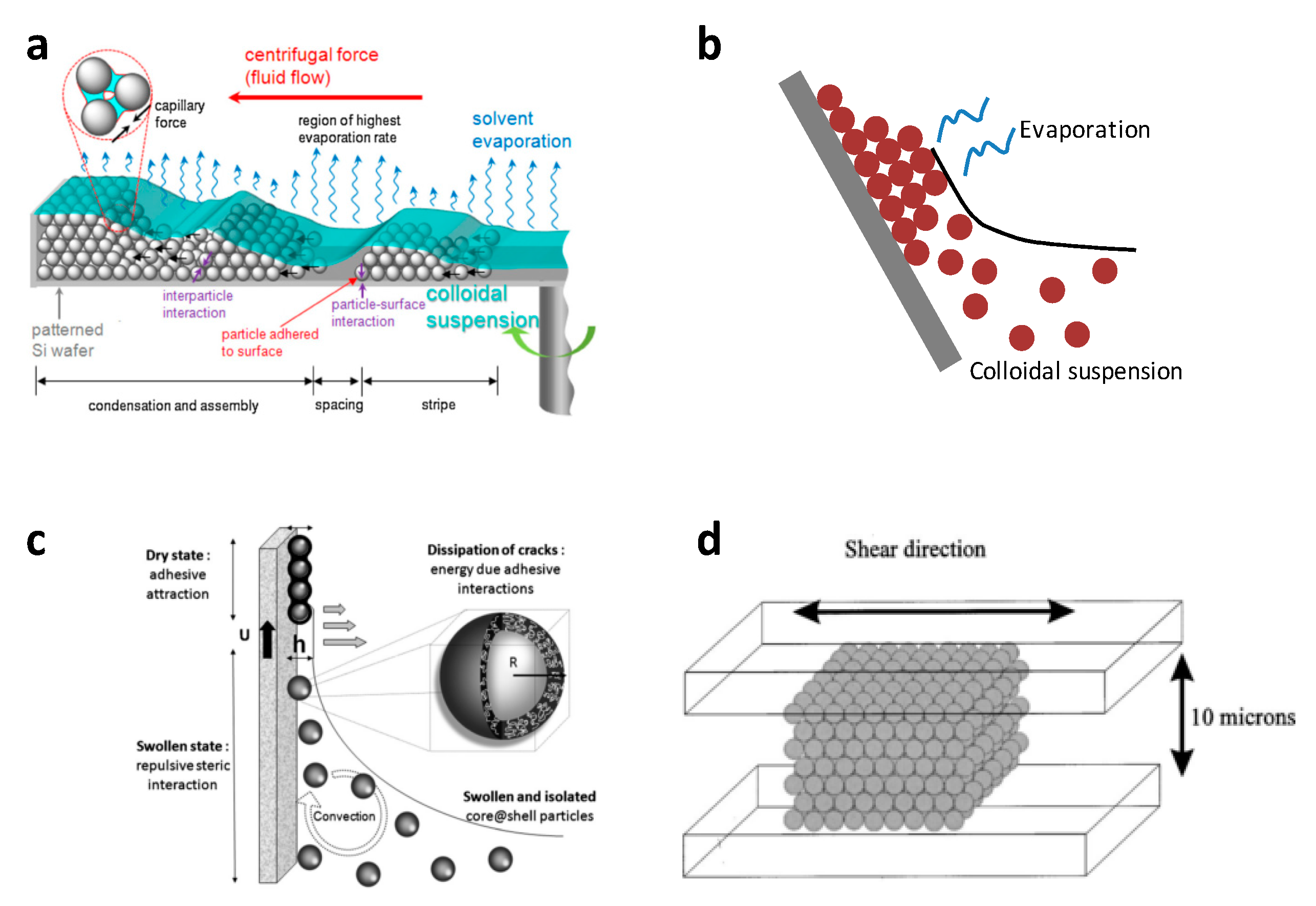
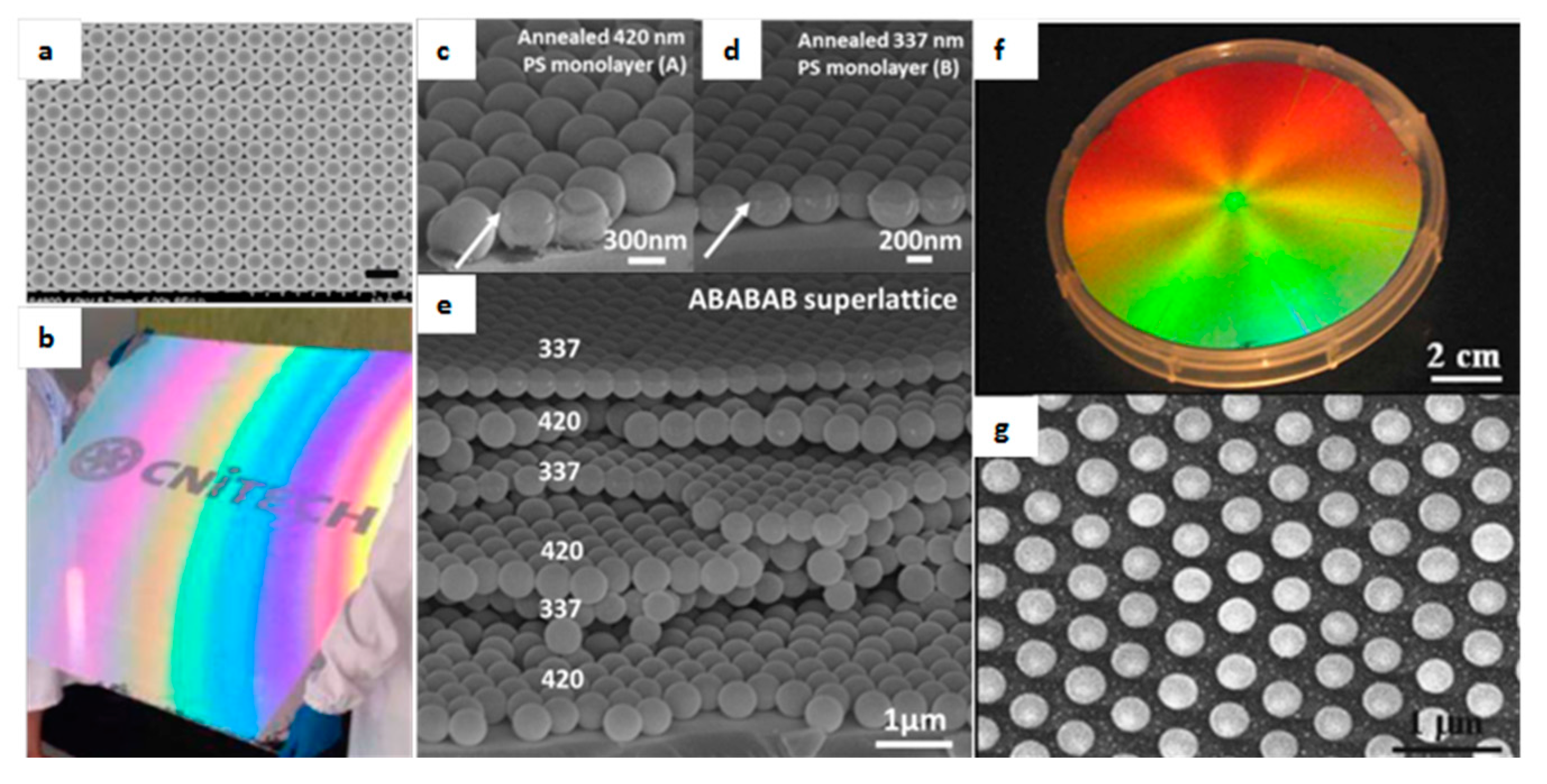
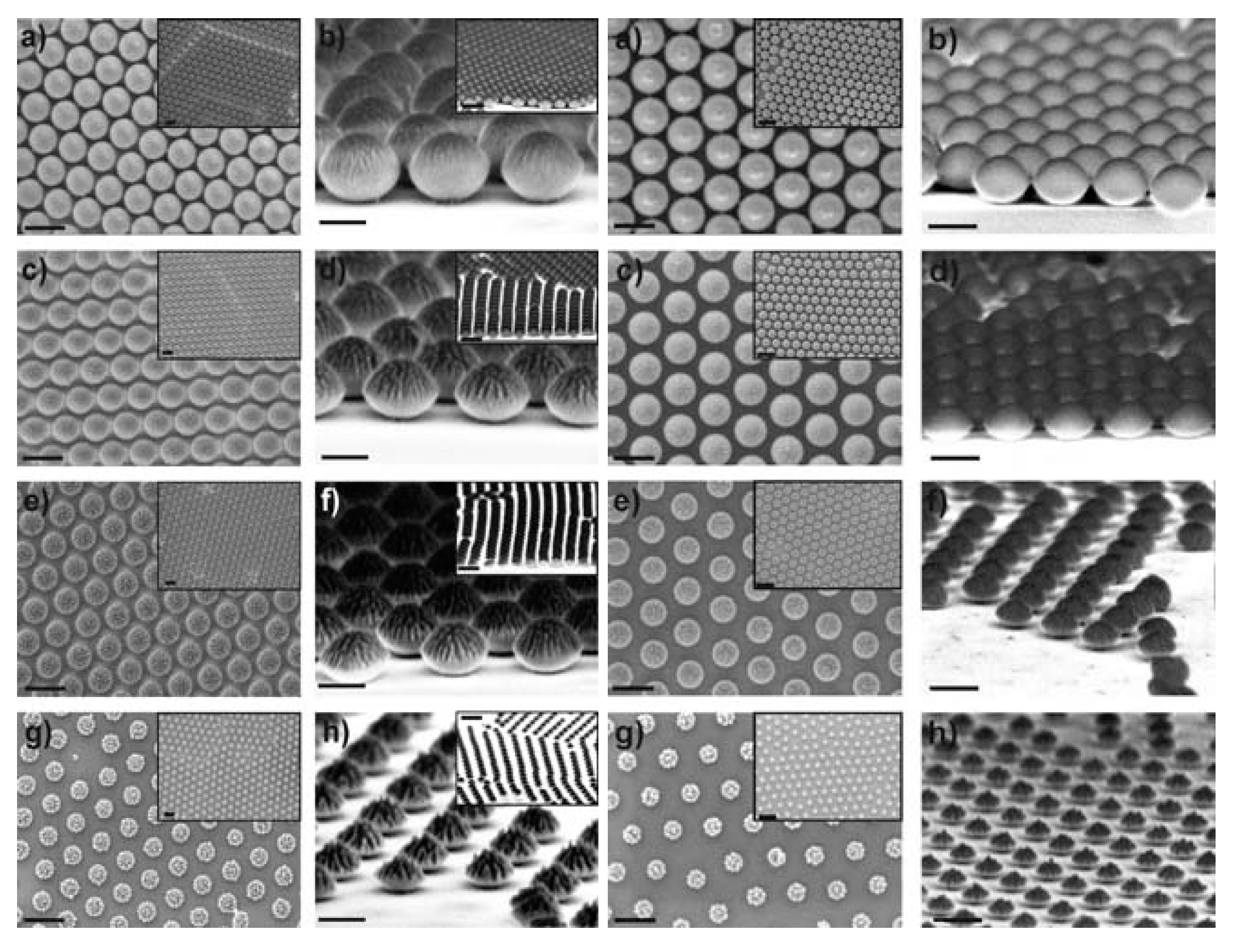
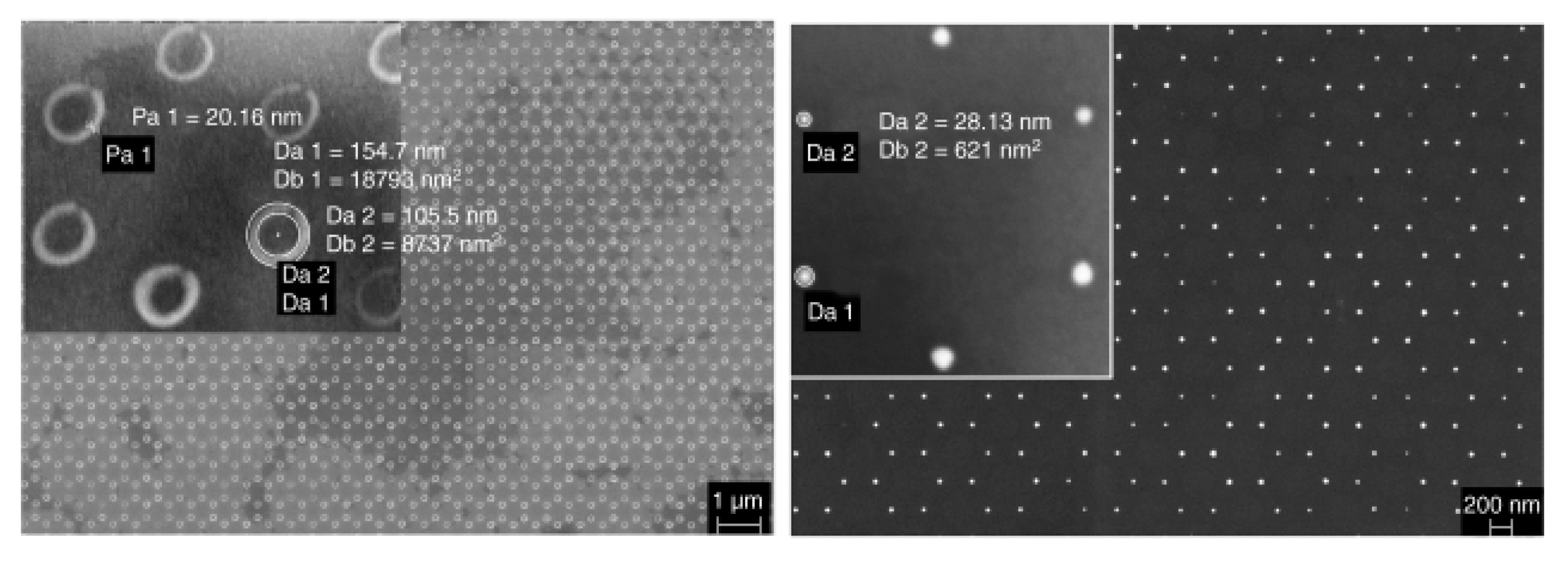
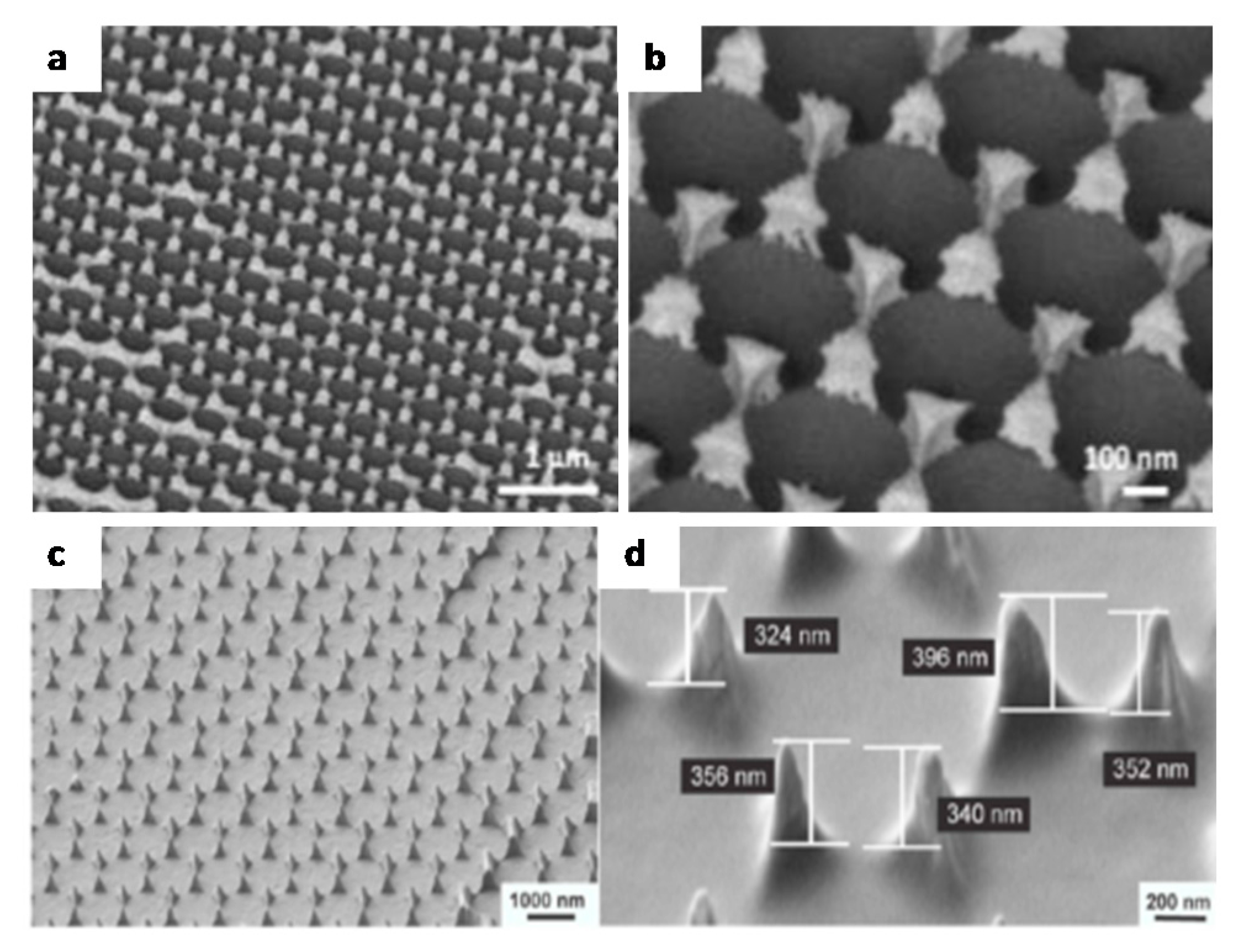
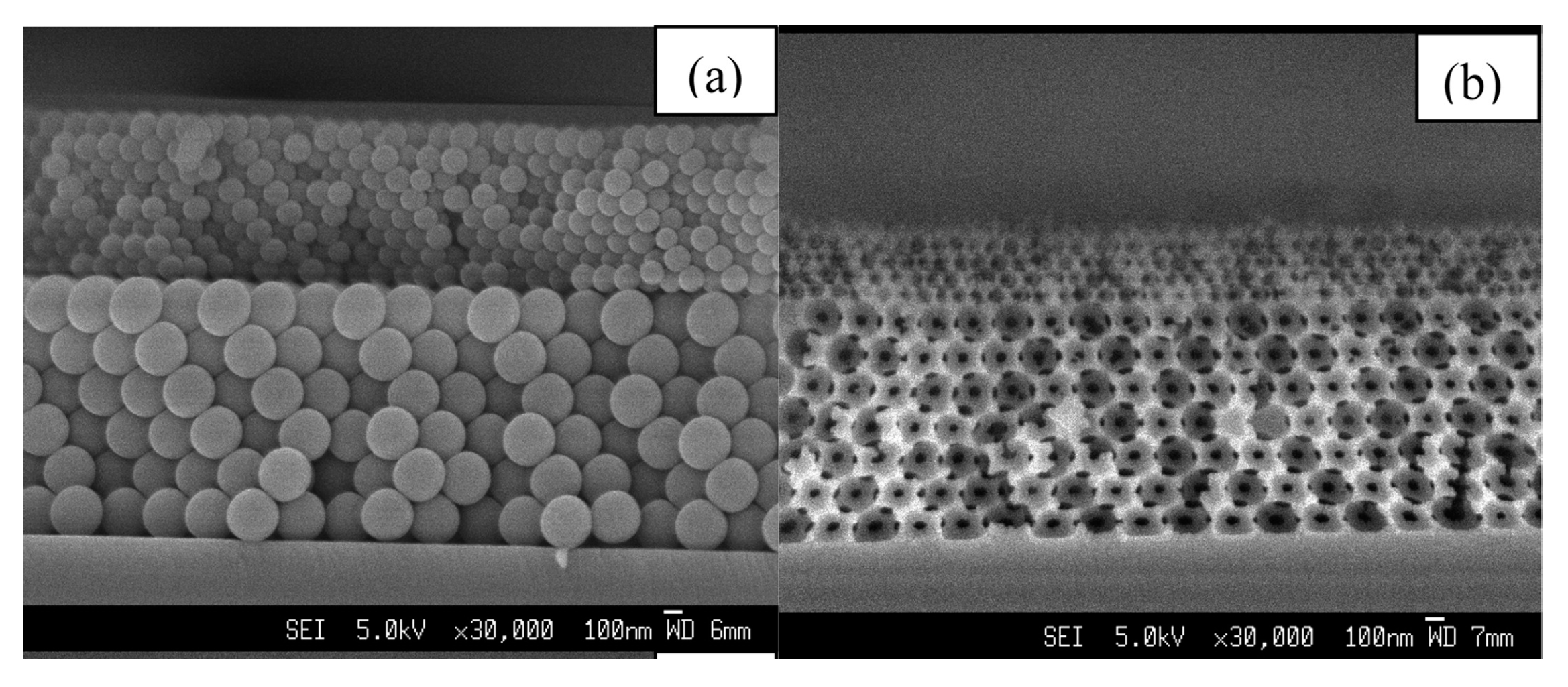

| Method | Remarks |
|---|---|
| Drop casting (Sedimentation) [24,25] | Simple but slow process. Patches of colloidal crystals formed. Difficult to control exact conditions |
| Vertical deposition [26,27,28] | Requires very good control of evaporation conditions (i.e., temperature and humidity) for a good deposition. Slow process (days). Very good quality of colloidal crystals formed under the proper conditions Gradient in the thickness of colloidal crystal formed |
| Centrifugation [29] | Simple and fast process. Generally big bulky colloidal crystals formed. |
| Spin-coating [30,31,32,33,34,35] | Simple and fast process. Monolayer formation possible. Patches of small coating area of monolayers. |
| Dip-coating [36,37,38] | Can control thickness of layers by the speed of withdrawal. Gradient in layer thickness. [27] |
| Shear ordering [39] | Requires very good control of process parameters Slow process Makes thin films |
| Langmuir-Blodgett [40,41,42,43,44] | Monolayer compressed on water surface by mobile arms. Short range order of closed packed regions within the monolayer. Monolayer transfer onto substrate can be repeated to deposit multilayers exactly as desired. Takes time for preparation of equipment and spreading of particles. |
| Direct assembly on water surface [45,46,47,48] | Simple and fast process. Good two-dimensional closed pack array on water surface. One monolayer at a time can be transferred. Can be repeated to deposit multilayers exactly as desired. |
| Magnetic self-assembly [49,50,51] | Requires highly-charged monodisperse magnetic colloidal particles Self-assembly occurs inside liquid medium Can be controlled by external magnetic field |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Ravaine, S. Bottom-Up Assembly and Applications of Photonic Materials. Crystals 2016, 6, 54. https://doi.org/10.3390/cryst6050054
Zheng H, Ravaine S. Bottom-Up Assembly and Applications of Photonic Materials. Crystals. 2016; 6(5):54. https://doi.org/10.3390/cryst6050054
Chicago/Turabian StyleZheng, Hanbin, and Serge Ravaine. 2016. "Bottom-Up Assembly and Applications of Photonic Materials" Crystals 6, no. 5: 54. https://doi.org/10.3390/cryst6050054