2.1. Crystal Structures
Arbidol is a relatively strong base with the pK
a value equaling 6.0 (for the dimethylamino group) [
27]. It is widely accepted in the literature that whether an API and a guest molecule form a salt or cocrystal can be predicted in terms of the ∆pK
a rule (ΔpK
a = pK
a(base) − pK
a(acid)) [
28,
29,
30]. When the ΔpK
a is greater than 4, the components tend to form a salt. If ΔpK
a ≤ −1, a cocrystal is likely to be formed. In the ΔpK
a range between −1 and 4, however, the ionization state of molecules in a crystal remains unpredictable. The difference between pK
a of arbidol and first ionization constants of maleic acid (pK
a,1 = 1.9; pK
a,2 = 6.1) is equal to 4.1, which suggests proton transfer and salt formation. For the arbidol with fumaric acid (pK
a = 3.0; pK
a,2 = 4.4) and succinic acid (pK
a,1 = 4.2; pK
a,2 = 5.6) pairs, the ΔpK
a values are found to be 3.0 and 1.8, respectively, which are inside the range of the salt−cocrystal continuum.
Crystallographic data are summarized in
Table 1, and the molecular packing arrangements in the crystal forms are shown in
Figure 2,
Figure 3 and
Figure 4 (asymmetric units with displacement ellipsoids are shown in
Figure S1).
Table 1.
Crystallographic data for arbidol multicomponent crystals. Arb: Arbidol; Mlc: Maleic acid; Fum: Fumaric acid; Suc: Succinic acid.
Table 1.
Crystallographic data for arbidol multicomponent crystals. Arb: Arbidol; Mlc: Maleic acid; Fum: Fumaric acid; Suc: Succinic acid.
| Compound Reference | [Arb+Mlc] (1:1) | [Arb+Fum] (2:1) | [Arb+Suc] (1:1) |
|---|
| Chemical formula | C22H26BrN2O3S·C4H3O4 | 2(C22H26BrN2O3S)·C4H2O4 | C22H25BrN2O3S·C4H6O4 |
| Formula Mass | 593.48 | 1070.89 | 595.50 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| a/Å | 8.3812(12) | 15.9728(12) | 10.4180(11) |
| b/Å | 13.0305(18) | 14.0080(10) | 14.7052(15) |
| c/Å | 13.2656(19) | 22.8933(17) | 17.8673(18) |
| α/° | 64.054(2) | 90.00 | 90.00 |
| β/° | 83.078(2) | 109.716(1) | 99.129(2) |
| γ/° | 86.643(2) | 90.00 | 90.00 |
| Unit cell volume/Å3 | 1293.2(3) | 4822.0(6) | 2702.6(5) |
| Temperature/K | 180(2) | 183(2) | 180(2) |
| Space group | P | P21/n | P21/n |
| Z | 2 | 4 | 4 |
| No. of reflections measured | 12386 | 49265 | 25637 |
| No. of independent reflections | 5634 | 11638 | 5895 |
| Rint | 0.0276 | 0.0314 | 0.0448 |
| Final R1 values (I > 2σ(I)) | 0.0337 | 0.0317 | 0.0303 |
| Final wR2(F2) values (I > 2σ(I)) | 0.0759 | 0.0715 | 0.0696 |
| Final R1 values (all data) | 0.0477 | 0.0507 | 0.0419 |
| Final wR2(F2) values (all data) | 0.0804 | 0.0786 | 0.0735 |
| Goodness of fit on F2 | 1.071 | 1.011 | 1.055 |
| Largest diff. peak & hole, e·Å−3 | 0.523/−0.305 | 0.519/−0.494 | 0.342/−0.290 |
| CCDC number | 1433018 | 1433017 | 1433016 |
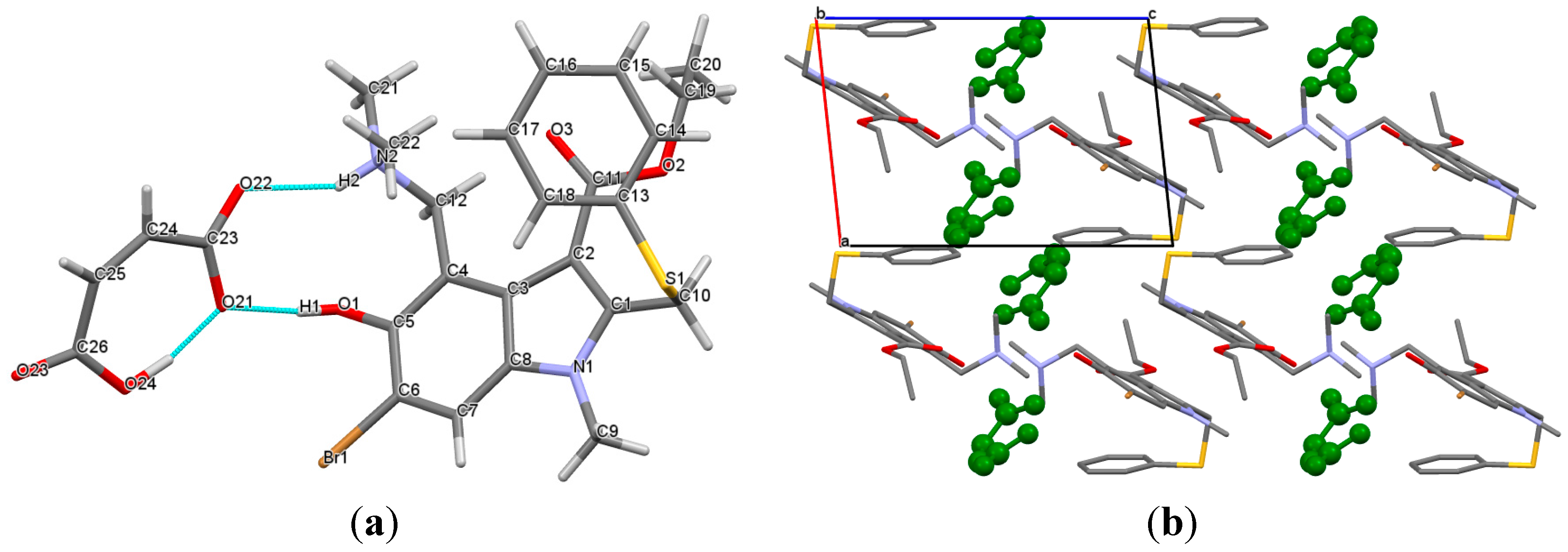
As expected, arbidol and maleic acid form a salt, which crystallizes in the triclinic
Pī space group with one arbidol cation and one maleate anion in the asymmetric unit. The structure is in good agreement with that reported by Orola
et al., who, however, collected diffraction data at 293 K [
22]. In this work, single-crystal X-ray experiment was carried out at low temperature (180 K), and the crystal structure of the [
Arb +
Mlc] salt was refined.
Figure 2a shows that the arbidol and maleate ions are connected by two different types of hydrogen bonds,
i.e., the charge-assisted N
2+–H
2···O
22‒ and conventional O
1–H
1···O
21 H-bonds, to form a discrete dimeric unit with
graph set notation [
31,
32]. The packing arrangement of the
Arb molecules in the crystal can be described as alternating layers containing the
π-stacks of the indole moieties (3.449 Å) and phenyl rings. The latter form planar layers extended along the (100) planes (
Figure 2b). The maleate ions are located between the adjacent
Arb units to form layers in the (011) planes.
Figure 2.
(a) Hydrogen bonded molecular unit in the [Arb + Mlc] crystal; (b) molecular packing projections for [Arb + Mlc]. Maleic acid molecules are colored in green. H atoms are omitted.
Figure 2.
(a) Hydrogen bonded molecular unit in the [Arb + Mlc] crystal; (b) molecular packing projections for [Arb + Mlc]. Maleic acid molecules are colored in green. H atoms are omitted.
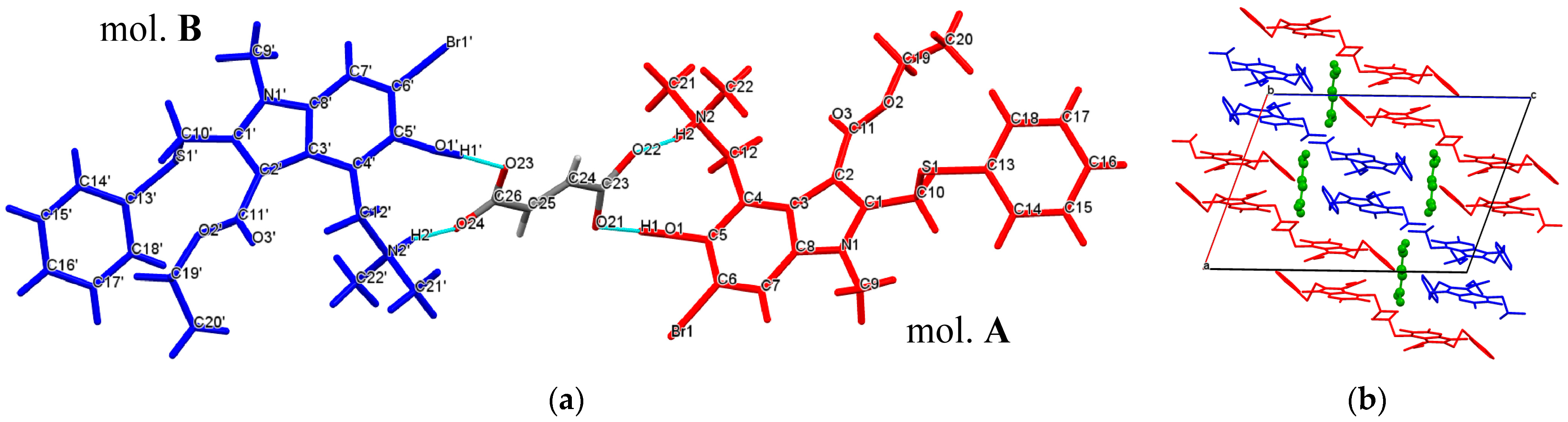
The asymmetric unit of the [
Arb +
Fum] salt contains one fumarate anion and two conformationally distinct cations of arbidol (mol.
A and mol.
B) (
Figure 3a). Each fumarate ion accepts complementary N
2+–H
2···O
22‒ (N
2ʹ
+–H
2ʹ···O
24‒) and O
1–H
1···O21 (O
1ʹ–H
1ʹ···O
23) hydrogen bonds from two
Arb ions, constructing a trimeric unit assembled via
motives. It should be noted that the carboxylic groups of fumaric acid in the salt are found to be fully deprotonated, despite the fact that the second acidity of the acid is appreciably weaker than the first one, and the ΔpK
a is less than three. In the [
Arb +
Fum] crystal, the
Arb molecules are arranged into layers of symmetry-inequivalent molecules, so that each layer contains the molecules of one conformation only. Inside the layer, the
Arb species are generally held by
π-π interactions between the indole fragments (3.521 Å and 3.411 Å), while the neighboring layers are linked to each other through the hydrogen bonded fumarate ions (
Figure 3b).
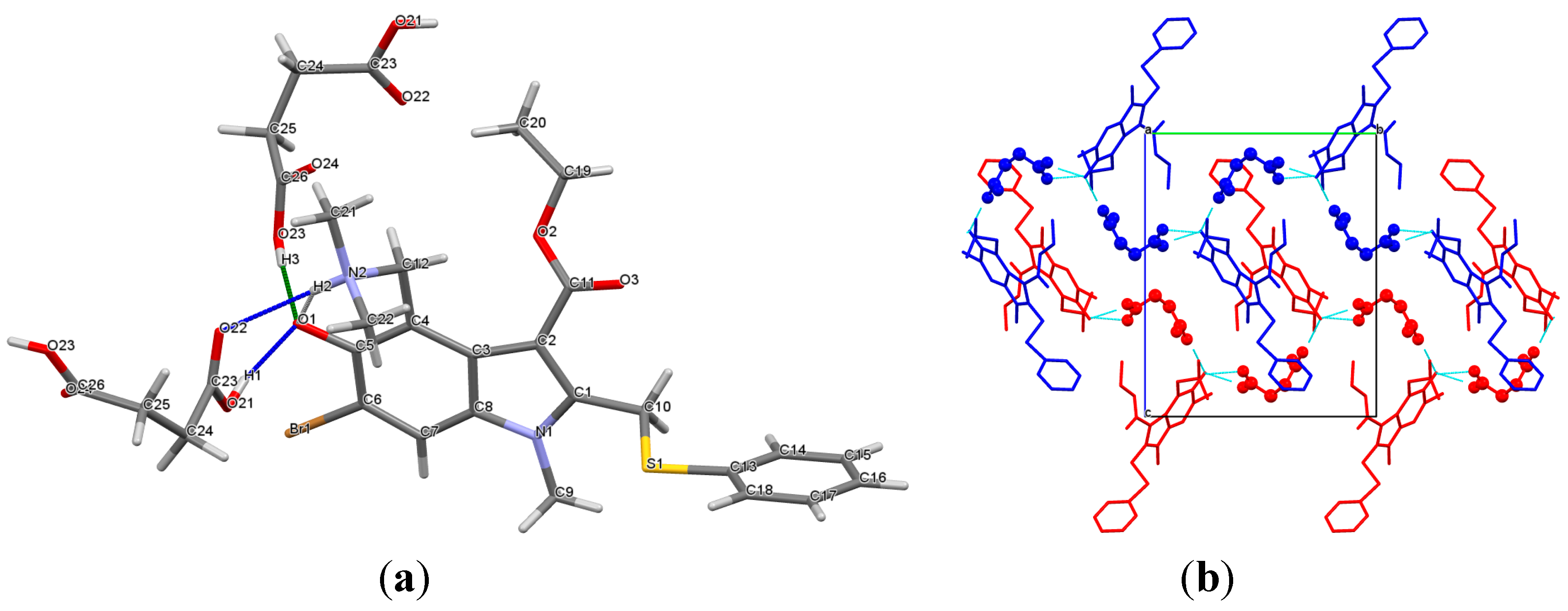
The crystal structure of 1:1 arbidol and succinic acid ([
Arb +
Suc]) is interesting, both chemically and crystallographically. This crystalline complex should be attributed to a cocrystal rather than a salt. Indeed, in the crystal, the
Arb molecule is found to be in its zwitterionic form, while an intermolecular charge transfer between the components does not occur. To the best of our knowledge, the
Arb zwitterion has not been previously seen in any of the drug crystal forms, and the [
Arb +
Suc] cocrystal seems to be the first found instance. Cocrystals with zwitterionic co-formers have been reported in the literature, and the most numerous examples can be found among amino acids [
33]. Multicomponent crystals containing zwitterionic API molecule and neutral co-former are less frequent, but also known and described [
34,
35,
36,
37].
The formation of the
Arb zwitterion is associated with an intramolecular proton transfer from the hydroxy-group to the dimethylamino fragment to form an S(6) H-bonded ring. Being released from the proton, the carbonyl O
1 atom accepts two O
21–H
1…O
1 and O
23–H
3…O
1 hydrogen bonds from the neighboring molecules of succinic acid (
Figure 4a). Meanwhile, the quaternary nitrogen atom (N
2) acts as an H-bond donor, which is responsible for the
ring motive formation between the
Arb and succinic acid molecules. In the [
Arb +
Suc] cocrystal, the hydrogen bond network between the components is extended along the b-axis to form a ribbon-like structure, assembled via the C(9) H-bond motives (
Figure 4b). The neighboring ribbons are held on account of
π-π contacts and other weak van der Waals interactions.
Figure 3.
(a) Hydrogen bonded trimeric molecular unit in the [Arb + Fum] crystal, A and B denote two conformationally distinct cations of arbidol; (b) packing organization of [Arb + Fum]. The layers of conformationally distinct Arb molecules are shown in red and blue colors. Fumaric acid molecules are colored in green. H atoms are omitted.
Figure 3.
(a) Hydrogen bonded trimeric molecular unit in the [Arb + Fum] crystal, A and B denote two conformationally distinct cations of arbidol; (b) packing organization of [Arb + Fum]. The layers of conformationally distinct Arb molecules are shown in red and blue colors. Fumaric acid molecules are colored in green. H atoms are omitted.
Figure 4.
(a) Hydrogen-bonded molecular unit in the [Arb + Suc] crystal; (b) molecular packing arrangement of the [Arb + Suc] crystal. The neighboring ribbons of the H-bonded Arb and Suc molecules are colored in red and blue. H atoms are omitted.
Figure 4.
(a) Hydrogen-bonded molecular unit in the [Arb + Suc] crystal; (b) molecular packing arrangement of the [Arb + Suc] crystal. The neighboring ribbons of the H-bonded Arb and Suc molecules are colored in red and blue. H atoms are omitted.
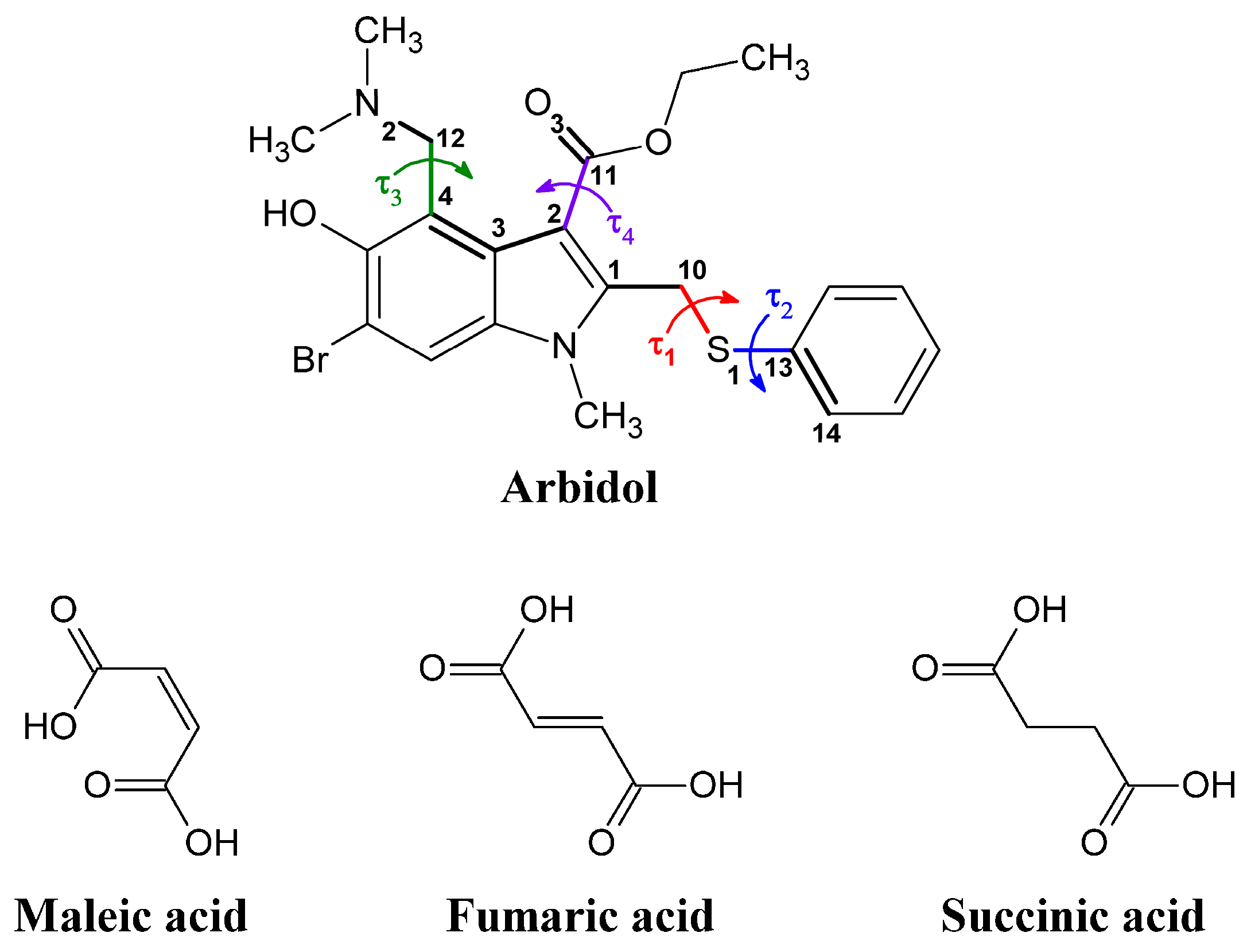
2.2. Conformational Analysis
Arbidol is considered to be a flexible molecule. The diversity of its conformational states can be described in terms of at least four torsion angles, namely τ
1 (∠C
1-C
10-S
1-C
13), τ
2 (∠C
10-S
1-C
13-C
14), τ
3 (∠C
3-C
4-C
12-N
2), and τ
4 (∠C
3-C
2-C
11-O
3) (see
Figure 1). The values of the selected torsion angles for the arbidol ions in all the known solid forms are collected in
Table 2.
Table 2.
Selected torsion angles τ1, τ2, τ3, and τ4 for arbidol ion (zwitterion) in the known crystal forms.
Table 2.
Selected torsion angles τ1, τ2, τ3, and τ4 for arbidol ion (zwitterion) in the known crystal forms.
| -- | τ1,°a
(∠C1-C10-S1-C13) | τ2,°
(∠C10-S1-C13-C14) | τ3,°
(∠C3-C4-C12-N2) | τ4,°
(∠C3-C2-C11-O3) |
|---|
| [Arb + Mlc] (1:1) | −62.3 | −88.9 | −110.5 | 18.4 |
| [Arb + Fum] (2:1) mol. A | 177.0 | −64.4 | −100.6 | −22.4 |
| [Arb + Fum] (2:1) mol. B | −134.5 | −60.8 | −102.7 | −20.5 |
| [Arb + Suc] (1:1) | −170.5 | −21.3 | −126.4 | 164.0 |
| [Arb + Salicylic acid] (1:1)b | −83.0 | −117.5 | −108.3 | 21.3 |
| [Arb + Salicylic+CHCl3] (1:1:1)b | −58.9 | −105.7 | −97.9 | −30.9 |
| [Arb + Gentisic acid] (1:1)b | −45.6 | −62.8 | −101.3 | 30.2 |
| [Arb + Glutaric acid] (1:1)b | −170.2 | −34.6 | −81.4 | −14.7 |
| [Arb+HCl+H2O] (1:1) | −108.9 | 141.4 | −78.7 | 17.7 |
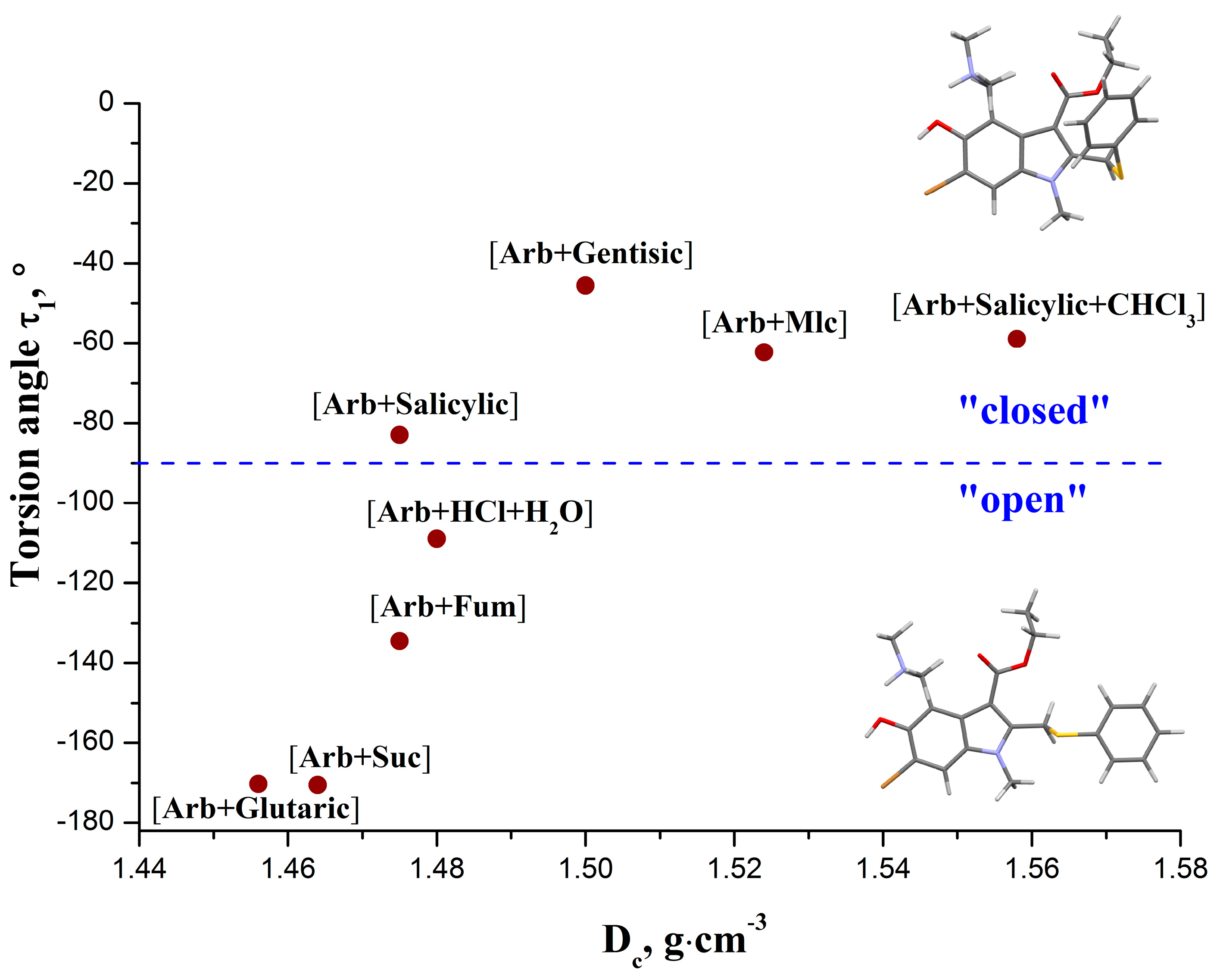
Table 2 indicates that the torsion angle τ
1 (∠C
1-C
10-S
1-C
13), which determines the orientation of the phenyl ring in relation to the indole moiety, is the most widely varying molecule fragment. Moreover, it is the τ
1 angle that determines the overall geometry of the
Arb molecule, to a large extent. If |τ
1| < 90°, the molecule has a “closed” conformation. This type of conformation is observed in the crystals of [
Arb +
Mlc acid], [
Arb +
Salicylic acid], [
Arb +
Salicylic +
CHCl3], and [
Arb +
Gentisic acid]. On the other hand, the molecular conformations with |τ
1| > 90° may be defined as “open”. The structures that adopt “open” conformation of
Arb are [
Arb +
Fum], [
Arb +
Suc], [
Arb +
Glutaric acid], and [
Arb +
HCl +
H2O].
A considerable variation is also found for the torsion angle τ2 (∠C10-S1-C13-C14), which is responsible for the rotation of the phenyl ring around the S1-C13 bond in general. The τ2 values change from ca. −20° to −120° (an exception is observed for [Arb + HCl + H2O]).
The third flexible torsion angle τ
3 (∠C
3-C
4-C
12-N
2) in the
Arb molecule refers to the conformation of the dimethylamino group. In most cases, the protonated quaternary nitrogen atom points toward the ‒O
1H
1 hydroxy-group, so that |τ
3| > 90° ([
Arb +
BA], [
Arb +
SA], [
Arb +
SA +
CHCl3], [
Arb +
SA+
ACN], [
Arb +
Mlc], [
Arb +
Gentisic acid]). However, in the [
Arb +
HCl +
H2O] and [
Arb +
Glutaric acid] crystals, the dimethylamino group approaches the ethyl ester group (|τ
3| < 90°) to form the N
2+–H
2···O
3 intramolecular contacts [
38].
The torsion angle τ4 (∠C3-C2-C11-O3) corresponds to the orientation of the ethyl ester group. In most structures, the C11=O3 bond of the ester groups is oriented towards the dimethylamino group, so that the τ4-angle alters in the relatively narrow range of −30° to +30°. However, in the zwitterionic form of Arb ([Arb + Suc]), the C11=O3 bond is pointed in the opposite direction, and the ester group conformation is characterized by a τ4 value of 164.0°.
The results of the above-mentioned analysis indicate that the conformation of the
Arb molecule in the crystals is greatly influenced by its supramolecular surroundings. On the other hand, different conformers are embedded in a crystal environment, so that a change in molecular conformation is inevitably accompanied by a change in packing arrangement and, as a consequence, packing efficiency of a crystal. In the case of the
Arb molecule, the most considerable conformational changes are observed for the torsion angle τ
1 (∠C
1-C
10-S
1-C
13), which is mainly responsible for the overall shape of the
Arb molecule in the crystal (“closed” or “open”). Thus, it is the most informative parameter to be analyzed.
Figure 5 illustrates the τ
1-values of
Arb in different crystal forms as a function of the calculated density of the corresponding crystals (D
c).
Figure 5.
Value of the torsion angle τ1 in the Arb molecule as a function of the calculated density of the corresponding crystals.
Figure 5.
Value of the torsion angle τ1 in the Arb molecule as a function of the calculated density of the corresponding crystals.
It is evident that the crystals containing an “open” Arb conformation have lower density compared to those that consist of molecules with a “closed” conformation. In addition, the decrease in the absolute values of the τ1 angle is accompanied by an almost linear increase in the packing density of the crystals. However, above a Dc of ca. 1.5 g·cm−3, the τ1 angle reaches a plateau value of ≈−55°, indicating that further increasing of the crystal packing density has no influence on the geometry of the τ1 angle. This fact suggests that the conformational energy penalty of the molecule associated with the plateau value of τ1 becomes far too high to be overcome by the packing forces. Increasing of the packing efficiency in those crystals is probably achieved on account of structural adjustment of the other flexible torsion angles, namely τ2, τ3 and τ4.
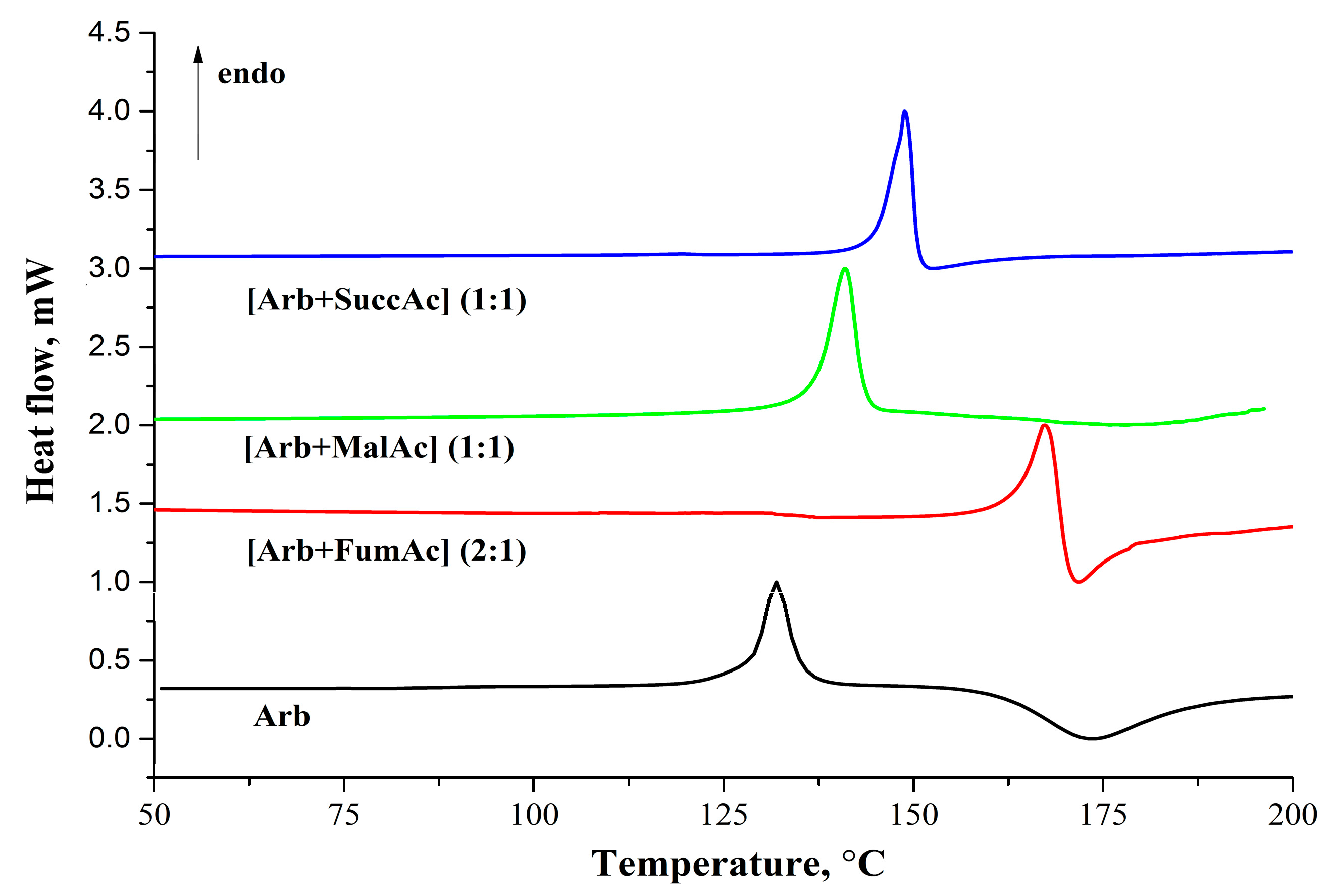
2.3. Thermal Analysis
DSC measurements were conducted to investigate the thermal properties of arbidol hemifumarate, maleate, and
Arb-succinic acid cocrystal. The DSC traces for arbidol base, [
Arb +
Fum], [
Arb +
Mlc], and [
Arb +
Suc] are shown in
Figure 6, and the thermal data are represented in
Table 3.
Figure 6.
DSC curves for arbidol base and its salts recorded at 10 °C·min–1 heating rate.
Figure 6.
DSC curves for arbidol base and its salts recorded at 10 °C·min–1 heating rate.
Table 3.
Thermophysical data for arbidol and its salts.
Table 3.
Thermophysical data for arbidol and its salts.
| -- | Arb | [Arb + Fum] (2:1) | [Arb + Mlc] (1:1) | [Arb + Suc] (1:1) |
|---|
| Tfus, K (onset) | 397.9 ± 1.0 | 432.9 ± 1.0 | 406.5 ± 1.4 | 415.8 ± 1.0 |
| , kJ∙mol−1 | 44.6 ± 2.8 | 47.9 ± 5.0 | 49.4 ± 4.0 | 59.3 ± 6.0 |
The melting temperatures of all new forms are higher than that of the arbidol base. The melting temperature growth for salts and cocrystal is similar to the changes in the melting temperatures of co-formers: [
Arb + Mlc] < [
Arb + Suc] < [
Arb + Fum]. The salts’ fusion enthalpy values are comparable and close to the
Arb fusion enthalpy value, while
for [
Arb + Suc] is found to be at least
ca. 10 kJ·mol
−1 higher than the others. It should be noted that the majority of the known cocrystals and salts (namely about 50% of all known cocrystals) belong to the melting temperature domain of 130–160 °C. [
39] Moreover, the difference between the melting temperatures of API and co-former is most often (in 26.8% of cocrystals) known to vary within the range of 0–30 °C. In the studied case, only the [
Arb + Mlc] salt has the difference in components melting temperatures within this temperature interval. For [
Arb + Suc] cocrystal, T
fus (API) − T
fus(CF) = 59.2 °C, which is characteristic of 23.5% of cocrystals (with melting temperatures difference from 45 °C to 75 °C). The [
Arb + Fum] salt is made up of components with the melting temperature difference equaling about 164 °C and is formed only in 9.1% of cases.
The distribution of cocrystals according to the difference between the melting temperatures of API and cocrystal shows that it is most typical of cocrystals to have 30–50 °C higher melting temperatures than the low-melting component does—API in this case (33.56% of all cocrystals) [
39]. Only the [
Arb + Fum] salt follows this trend. The melting temperature increase in [
Arb + Mlc] salt and [
Arb + Suc] cocrystal is not so significant, and equals 8.6 and 17.9 °C, respectively. Such systems are found 2.5 times less frequently (in 13.61% of cocrystals).
As shown by Perlovich [
39], the analysis of fusion temperatures of cocrystals with fumaric, maleic, and succinic acids make it possible to find correlations between cocrystal and API melting temperatures by the following equation (where A and B are correlation coefficients):
The correlation coefficients for cocrystals and salts of different API with fumaric, maleic, and succinic acids, to be used in Equation (1), are given in
Table 4.
Table 4.
Coefficients of the correlation equation (1) for multicomponent crystals with selected dicarboxylic acids, calculated values of melting temperatures () for the Arb salts/co-crystal, and difference (Δfus) between calculated and experimental melting temperatures for the Arb salts/co-crystal.
Table 4.
Coefficients of the correlation equation (1) for multicomponent crystals with selected dicarboxylic acids, calculated values of melting temperatures () for the Arb salts/co-crystal, and difference (Δfus) between calculated and experimental melting temperatures for the Arb salts/co-crystal.
| Compound | Aa | Ba | SDa,b | [Arb+acid], K | Δfus(), K |
|---|
| Fumaric acid | 309.83 ± 23.43 | 0.324 ± 0.053 | 8.78 | 438.7 | 5.8 |
| Maleic acid | 199.26 ± 45.76 | 0.482 ± 0.093 | 13.90 | 391.1 | −15.5 |
| Succinic acid | 263.76 ± 24.56 | 0.392 ± 0.055 | 15.79 | 419.8 | 3.9 |
As
Table 4 shows, the calculated values of melting temperatures for [
Arb + Fum] and [
Arb + Suc] are in good agreement with the experimental values. The difference between
and
of the systems under study is much smaller than the standard deviation of the correlation equation. The biggest deviation from the calculated value of the melting temperature compared to the experimental one is found in [
Arb +
Mlc] salt (−15.5 K), which is, however, comparable to the SD value of Equation (1) for maleic acid systems.
The melting temperature of
Arb is 124.8 °C, but after further heating to 150 °C and higher, an exoeffect is observed with a peak at 175 °C (
Figure 6). The exoeffect is also observed at melting of
Arb salts. This effect is usually caused by compound decomposition. Thermogravimetric (TG) and mass-spectrometry analyses of compounds have been carried out to interpret the observed process. TG thermograms have shown that there is no weight loss before melting (
Figure S2). All arbidol crystals have decomposition temperatures at which a weight loss is observed after melting, and the temperatures are different in every crystal.
The tallest peak in a mass spectrum (base peak) is observed at 40 m/z. This peak is characterized by the presence of argon in which the experiment took place. In addition, one of the most intense peaks is the peak at m/z = 18–20, indicating presence of water. No products of
Arb decomposition are observed at 175 °C. It is only after the temperature exceeds 200 °C that peaks characteristic of
Arb degradation products (for example, m/z = 110, which corresponds to thiophenol, see
Scheme S1) begin to appear on the mass-spectrum (
Figure S3). The presence of ethylformate (m/z = 76) can be determined on the mass spectrum. The peak near 140 indicates the presence of an
Arb fragment 1,2-dimethyl-1
H-indole among degradation products. Consequently, it should be assumed that the
Arb decomposition process must produce trimethylamine (m/z = 59) and HBr (m/z = 81) fragments. Indeed, their characteristic peaks are present in the mass spectrum, but their intensities are very low. The intensity of the peaks, which indicates the presence of CO
2 (m/z = 44) and N
2 or CO (m/z = 28), is greatly enhanced. No m/z value for
Arb (477.4) has been found on the mass-spectra. Note that there is no exothermic peak at 175 °C for the cocrystal of arbidol with succinic acid, but the mass-spectra are the same for salts and
Arb.
2.4. Aqueous Dissolution Study
As it has been mentioned,
Arb is a relatively strong base with pK
a value near 6.0 [
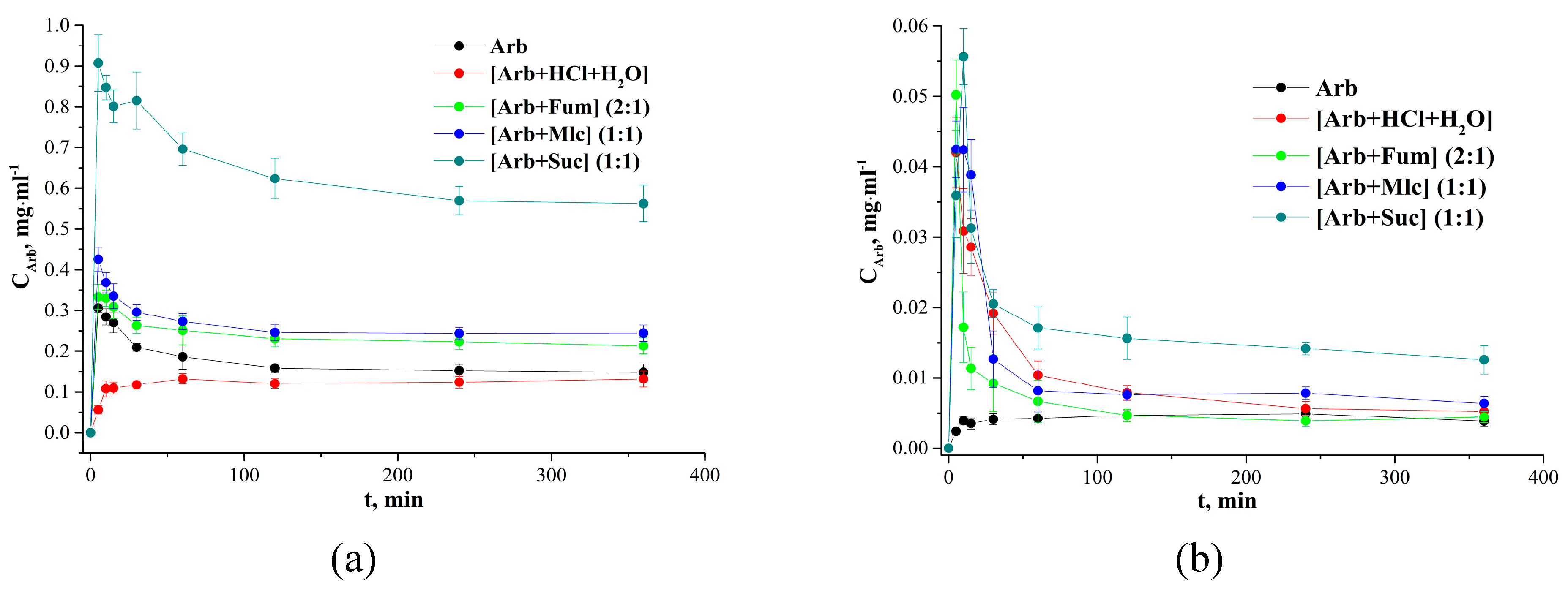
27]. Therefore, the solubility of the drug, as well as its salts, in aqueous solutions is expected to be pH-dependent. A dissolution study has been conducted to compare the dissolution profiles of arbidol freebase, arbidol hydrochloride monohydrate, hemifumarate, maleate, and succinic acid cocrystal with arbidol zwitterion at different pH values. The solubility experiments (at 25 °C) of the salts were examined in the hydrochloric buffer with pH 1.2 and the phosphate buffer with pH 6.8. The results of the dissolution experiments are summarized in
Table 5.
Figure 7 shows the dissolution profiles for the salts and
Arb at different pH values.
Table 5.
Aqueous solubilities at 25 °C for Arb salts and pure Arb in pH 1.2 and pH 6.8 media.
Table 5.
Aqueous solubilities at 25 °C for Arb salts and pure Arb in pH 1.2 and pH 6.8 media.
| -- | Сmax (mg·mL−1) a | Solubility (mg·mL−1) b | Solid Phase Recovered after Solubility Experiment c |
|---|
| pH 1.2 |
| Arb | 0.30 ± 0.01 | 0.15 ± 0.01 | [Arb + HCl + H2O] |
| [Arb + HCl + H2O] | 0.13 ± 0.01 | 0.13 ± 0.01 | [Arb + HCl + H2O] |
| [Arb + Fum] (2:1) | 0.33 ± 0.03 | 0.21 ± 0.02 | [Arb + HCl + H2O] |
| [Arb + Mlc] (1:1) | 0.42 ± 0.03 | 0.24 ± 0.02 | [Arb + HCl + H2O] |
| [Arb + Suc] (1:1) | 0.91 ± 0.07 | 0.56 ± 0.04 | [Arb + HCl + H2O] |
| pH 6.8 |
| Arb | 0.005 ± 0.001 | 0.004 ± 0.001 | Arb |
| [Arb + HCl + H2O] | 0.042 ± 0.005 | 0.005 ± 0.001 | Arb |
| [Arb + Fum] (2:1) | 0.050 ± 0.005 | 0.004 ± 0.001 | Arb |
| [Arb + Mlc] (1:1) | 0.042 ± 0.006 | 0.006 ± 0.001 | Arb |
| [Arb + Suc] (1:1) | 0.055 ± 0.006 | 0.012 ± 0.002 | Arb |
Figure 7.
Dissolution profiles at 25 °C for salts, cocrystal and pure Arb in pH 1.2 (a) and pH 6.8 (b).
Figure 7.
Dissolution profiles at 25 °C for salts, cocrystal and pure Arb in pH 1.2 (a) and pH 6.8 (b).
The maximum concentration (C
max) of
Arb in the pH 1.2 solution reaches 0.91 mg·mL
−1 (for [
Arb + Suc]), which is about three times higher than that of
Arb pure base, and seven times higher than that of [
Arb + HCl + H2O]. The maximum concentration values for
Arb salts considerably exceed the solubility value of [
Arb + HCl + H2O], and equal 0.33 mg·mL
−1 for [
Arb + Fum] and 0.42 mg·mL
−1 for [
Arb + Mlc]. The
Arb concentration gradually decreases both in the salts and the cocrystal with time. This behavior of API alternative crystalline form in solution is known as the “spring and parachute” effect, which is normally used to describe the cocrystal solubility curves [
3,
40]. The decline in the drug concentration can be attributed to a solution-mediated transformation of the bottom phase during the experiment. An X-ray powder diffraction (XRPD) analysis of the bottom phase shows that all the studied systems totally transform into [
Arb + HCl + H2O], which is the most thermodynamically stable form of the drug under the current conditions (
Figure S4). The final concentration levels for salts and
Arb are achieved in less than 4 h and they are close to the solubility value of [
Arb + HCl + H2O], which is equal to
ca. 0.13 ± 0.02 mg·mL
−1. The [
Arb + Suc] cocrystal dissolution process is a little different. The transformation process of the cocrystal to [
Arb + HCl + H2O] takes more time than that for
Arb salts. The concentration level of
Arb for the cocrystal after 6 h is about three and a half times higher than that for the solubility value of [
Arb + HCl + H2O], and is about two times higher than the maximal concentration of
Arb free base. This behavior can probably be explained by the fact that the
Arb molecule in cocrystal is in the zwitterion form. Kumar and Nangia have found that the solubility of the zwitterionic form is higher, even when it is a more stable modification than the neutral form [
41]. The high polarity and ionic nature of acidic/basic groups promote hydrogen bonding with water (for higher solubility) as well as a tighter crystal lattice of ionized molecules (polymorph stability). The cocrystal with
Arb in the zwitterionic form can improve both the solubility and the stability of API when compared to its ionic form (as salt) and the neutral form (as
Arb pure base or as cocrystal). Therefore, the
Arb cocrystal in the zwitterionic form is a desirable optimization in pharmaceutical development of high soluble arbidol with prolonged action.
There are considerable decreases in solubilities observed in all the systems under investigation at pH 6.8 as compared to the solubility in water with pH 1.2. The decrease in the maximum concentration values varies from 3 times (for [
Arb + HCl + H2O]), to 60 times (for
Arb). The maximum concentration of
Arb salts and cocrystal with dicarboxylic acids fall in the following order: 6.6 times (
[Arb + Fum]) < 10 times (
[Arb + Mlc]) < 16.5 times ([
Arb + Suc]). Nevertheless, salts and cocrystal demonstrate elevated concentration levels during the first 60 min of dissolution. However, it is obvious that the
Arb concentration value in cocrystal (as for
Arb salts) decreases to
Arb free base solubility value, which is equal to
ca. 0.004 ± 0.001 mg·mL
−1. XRPD analysis of the solid phase recovered after the experiment reveals no transformation of
Arb during the solubility study. All the salts, however, were seen to undergo a solution-mediated transformation to
Arb during the solubility process. Thus, it can be assumed that dissolution in a pH 6.8 buffer leads to
Arb salts transformation into pure
Arb in the bottom phase. This assumption has been confirmed by the XRPD analysis of solid phases recovered after the experiment (
Figure S5). As in buffer with pH 1.2, the cocrystal dissolution process in buffer pH 6.8 differs from salt dissolution. The
Arb concentration level for cocrystal [
Arb + Suc] is still two times higher than the solubility values for the other systems after 6 h of the experiment. However, the
Arb concentration values decrease faster in the phosphate buffer with pH 6.8 than in the hydrochloric buffer with pH 1.2.
The melting temperatures of single-component molecular crystals are normally used to describe/evaluate/imitate the energy of crystal lattice, as in the general solubility equation of Yalkowsky and Valvani [
42]. In addition, it has been shown previously that there is a linear correlation between the sublimation Gibbs energy and the melting temperatures of structurally similar compounds [
39,
43]. An increase in the melting temperature leads to the sublimation Gibbs energy growth, which must reduce the solubility values. Thus, it can be assumed that in multicomponent crystals (cocrystals and salts) a melting temperature increase lowers the solubility values. To prove this assumption, we analyzed the known cocrystals and salts of different API with dicarboxylic acids.
Figure 8 shows the analysis results as a diagram in coordinates of the melting temperature ratio (T
fus(CC)/T
fus(API)) versus their maximal solubility ratio (C
max(CC)/C
max(API))).
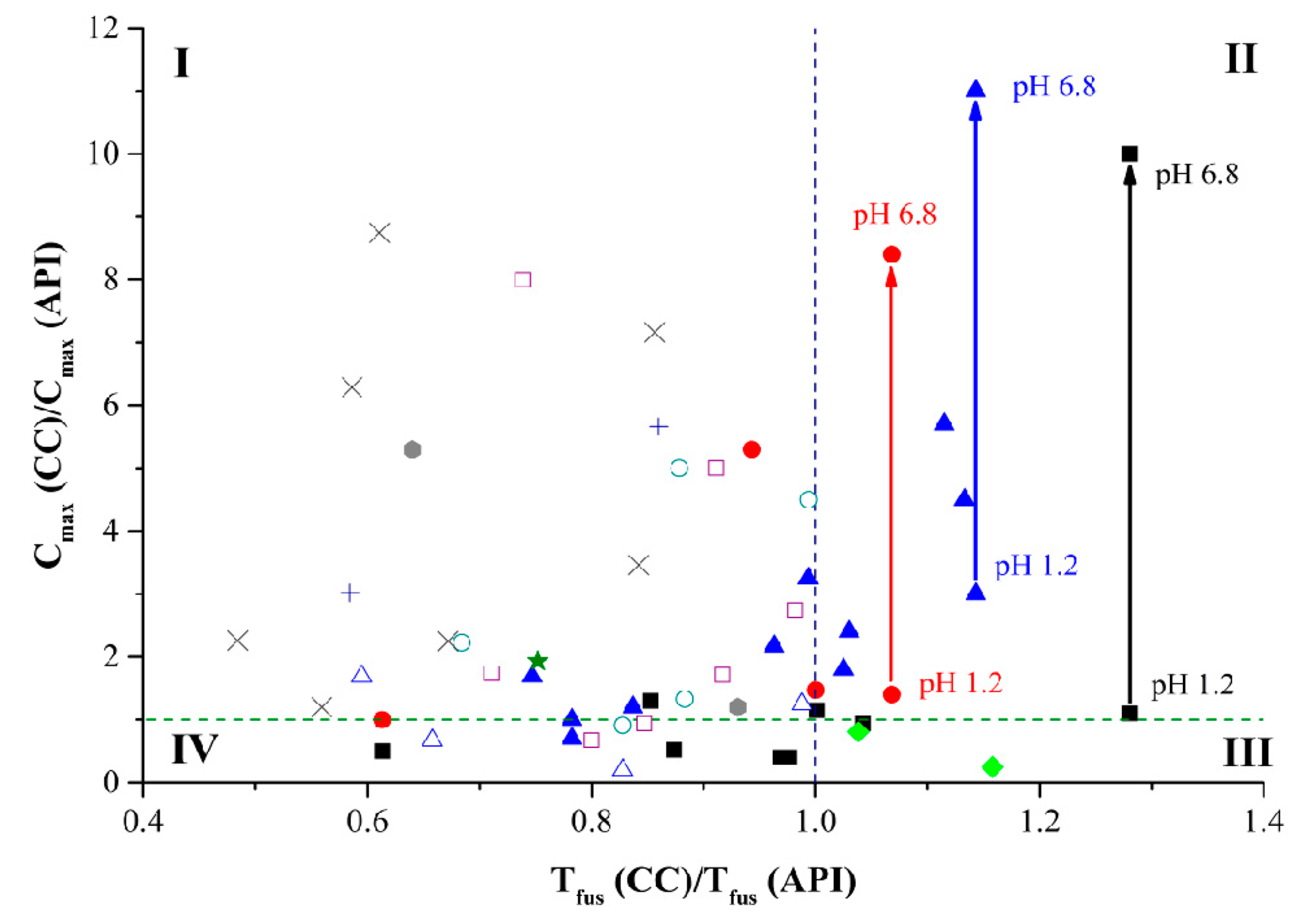
Figure 8.
Diagram of the influence of API melting temperature change in cocrystals and salts with dicarboxylic acids on their solubility. The cocrystals and salts with different acids as co-formers are represented by the following symbols: with oxalic (♦); with malonic (+); with fumaric (■); with maleic (●); with succinic (▲); with tartaric (
![Crystals 05 00650 i001]()
); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.
Figure 8.
Diagram of the influence of API melting temperature change in cocrystals and salts with dicarboxylic acids on their solubility. The cocrystals and salts with different acids as co-formers are represented by the following symbols: with oxalic (♦); with malonic (+); with fumaric (■); with maleic (●); with succinic (▲); with tartaric (
![Crystals 05 00650 i001]()
); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.
The diagram is divided into four zones. Zone I contains compounds in which cocrystal formation results in a melting temperature decrease and a solubility increase. As the diagram shows, most of the compounds under study belong to this zone. Zone II contains compounds in which cocrystal/salt formation leads to API solubility increase and a simultaneous growth of
compared to the API value. It should be noted that six out of ten points in zone II represent solubility values of the arbidol salts and arbidol cocrystal in its zwitterionic form in media with different acidity values. It is known that it is more difficult to predict behavior of salts than that of cocrystals [
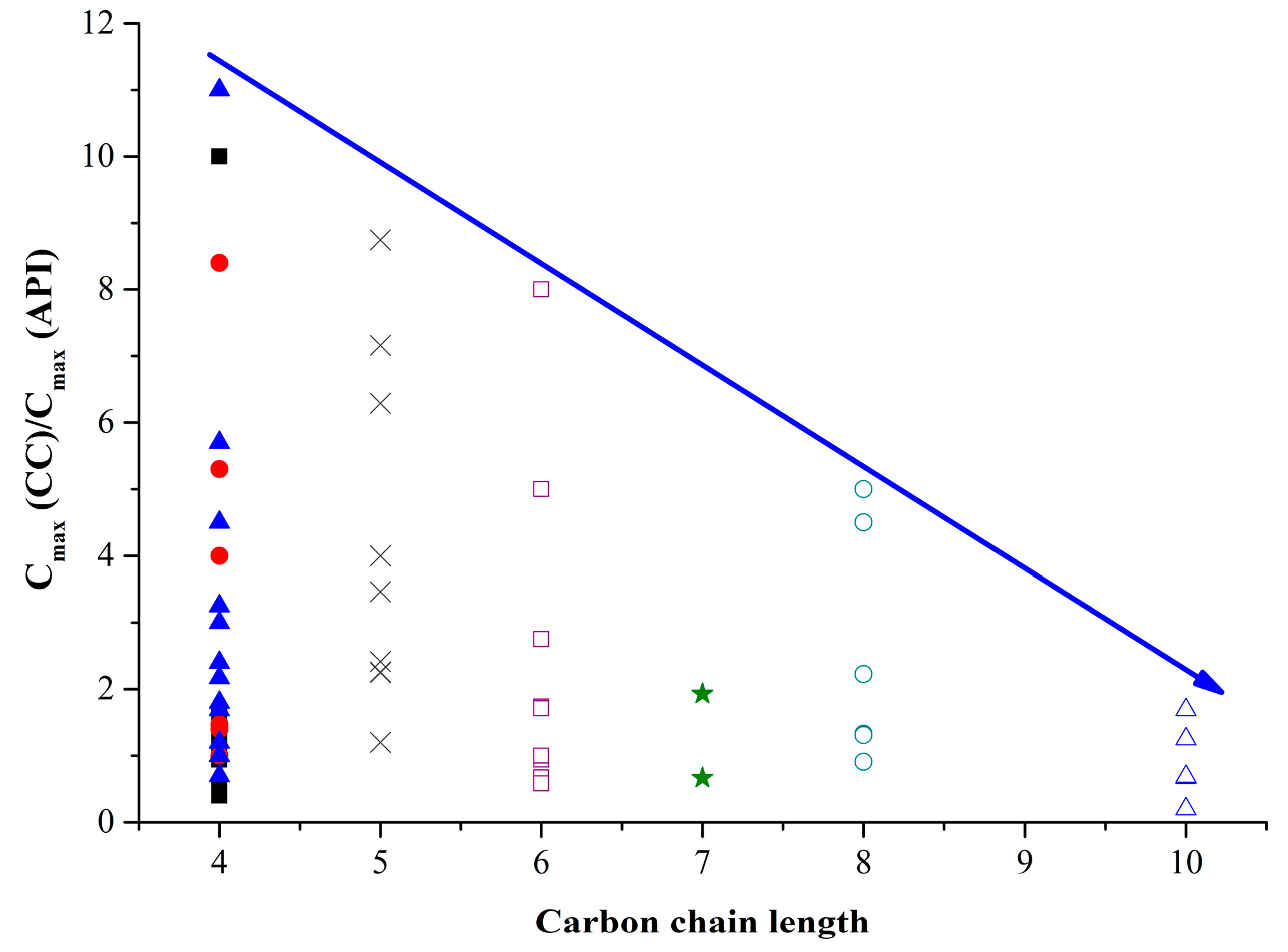
44]. Most likely, it is the ion form of co-formers of these multicomponent systems that causes the significant deviation from the general trend. Zone III includes cocrystals with melting temperatures higher than in pure API, which, as expected, reduces their solubility. This group includes salts with oxalic acid, sildenafil, and sulfamethizole. The melting temperatures of dicarboxylic acids rarely exceed 200 °C. That is why the melting temperatures of the shown cocrystals and salts are unlikely to exceed that of API. The highest melting temperature is registered in fumaric (287 °С) and succinic (185 °С) acids, which affects their cocrystals/salts as well. For example, cocrystals/salts with fumaric acid often have lower solubility than API, while cocrystal formation with succinic acid often leads to a melting temperature increase accompanied by a solubility growth. Zone IV includes cases when a decrease in the melting temperature of a cocrystal/salt does not result in their solubility growth. This zone mostly contains cocrystals with sebacic, suberic, and adipic acids. Carbon aliphatic chain lengthening enhances the molecule hydrophobicity and reduces its acidity, which negatively affects the ability of dicarboxylic acids to increase API solubility. Indeed, analysis of the influence of the carbon chain length of the salts and cocrystals under study on their solubility parameters indicates that the increased number of carbons in chain narrows the interval of potential API solubility growth (
Figure 9).
Figure 9.
Cocrystal-API solubility ratio as a function of carbon chain length of dicarboxylic acids. The cocrystals and salts with different acids as co-formers are represented by the following symbols: with fumaric (■); with maleic (●); with succinic (▲); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); and sebacic (△) acids.
Figure 9.
Cocrystal-API solubility ratio as a function of carbon chain length of dicarboxylic acids. The cocrystals and salts with different acids as co-formers are represented by the following symbols: with fumaric (■); with maleic (●); with succinic (▲); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); and sebacic (△) acids.
 ); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.
); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.
 ); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.
); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.