1. Introduction
Crystallization of active pharmaceutical ingredients (APIs) is the process that determines the physical properties of the API solid phases. It is especially important for the orally delivered drugs, where the API crystals are directly used in pharmaceutical dosage forms, such as tablets and capsules. The properties affected by the crystal structures include solubility, stability, and mechanical properties [
1,
2,
3].
Cocrystallization is a relatively recent tool to control the properties of the API crystals, compared to, for example, salt formation and polymorph selection [
4,
5]. In general, the basic building block of a pharmaceutical cocrystal is the strongly interacting molecules of an API and a cocrystal former (coformer), although cocrystals of two different APIs and multicomponent systems have been also studied [
6,
7,
8]. A large degree of freedom to select the coformers and the subsequent diversity of the cocrystals have made the cocrystallization an attractive strategy to design the structures and properties of API crystals, even when the coformers are limited as those approved by the regulatory authorities [
9].
Some of the processes to prepare pharmaceutical cocrystals are solution crystallization, grinding, sublimation, and so on [
5,
10]. Among these, the traditional solution crystallization appears the industrially preferable method because the process is well established, easily scalable, and less prone to contamination [
11,
12]. However, there were cases where the solution method was not successful in yielding cocrystals, such as caffeine/glutaric acid, paracetamol/oxalic acid, and itraconazole/malonic acid, in their pure forms, which could be obtained through liquid-assisted grinding [
13,
14,
15].
We have studied the cocrystallization of adefovir dipivoxil (AD,
Figure 1), a prodrug of a broad-spectrum antiviral known as adefovir, mainly through the solution process [
16]. Cocrystals of AD and suberic acid (SUB) formed from both methanol and ethanol solutions, and those of AD and succinic acid formed from ethanol solutions. However, AD cocrystals with glutaric acid (GLU) could not be obtained in their pure form through the solution process. In the present study, liquid-assisted grinding was utilized to prepare the AD/GLU cocrystals, and their thermal stability and release behavior were compared with those of AD/SUB cocrystals as well as neat AD crystals.
Figure 1.
Chemical structures: (a) adefovir dipivoxil (AD); (b) glutaric acid (GLU); (c) suberic acid (SUB).
Figure 1.
Chemical structures: (a) adefovir dipivoxil (AD); (b) glutaric acid (GLU); (c) suberic acid (SUB).
2. Results and Discussion
2.1. Cocrystal Formation
AD cocrystals with GLU and SUB were produced by liquid-assisted grinding, and their morphologies were summarized in
Figure 2. As a comparison, the neat AD crystals before grinding were also shown in
Figure 2a,b. Morphologies of GLU and SUB raw materials were displayed in Figure S1 (Supplementary Materials). OM and SEM micrographs were shown to exhibit overall configurations and individual shapes of crystals, respectively. AD/GLU cocrystals were largely aggregated (
Figure 2c) in
ca. 100–200 μm clusters. Closer observation with SEM revealed that the individual crystals were of a platelet shape (
Figure 2d). Their overall size was diverse with the long axes
ca. 2–20 μm, but their thickness was relatively uniform at about 1–2 μm. While the overall shapes of AD raw material (
Figure 2a,b) somewhat resembled the AD/GLU crystals, its individual crystals appeared thinner (0.5–1 μm) and more loosely attached to each other with visible gaps between large surfaces. In contrast, AD/SUB appeared better dispersed with many crystals of sub-micron size, although some bigger crystals were also noticeable (
Figure 2e,f). (Note that the shapes of SUB and GLU crystals (Figure S1, Supplementary Materials) did not show clear correlations with their respective cocrystals.) In general, the breakage of particles during milling is related to the maximum fracture energy, Young’s modulus, Poisson ratio, hardness as well as initial particle size [
17,
18]. Fundamentally, these materials properties originate from the intermolecular interactions that govern the crystal structures and determine responses when subject to mechanical stress [
14,
19]. In the present study, further analysis on the different morphologies of AD cocrystals formed by grinding was hindered by the lack of AD/GLU crystal structure. Also, more defined milling process needs to be employed to further investigate the correlation between the structure and morphology of the cocrystals.
Figure 2.
Optical micrographs of AD (a), AD/GLU (b), and AD/SUB (c); scanning electron micrographs of AD (d), AD/GLU (e), and AD/SUB (f).
Figure 2.
Optical micrographs of AD (a), AD/GLU (b), and AD/SUB (c); scanning electron micrographs of AD (d), AD/GLU (e), and AD/SUB (f).
The XRD pattern of the AD/GLU cocrystal was compared with those of neat AD and GLU in
Figure 3. The diffraction pattern of AD/GLU was distinctively different from those of AD and GLU. Some of the most characteristic diffraction peaks were at 10.54°, 17.16°, 17.76°, 20.70° and 25.94° (marked by asterisks). We note here that the diffraction pattern of AD/GLU is also different from AD methanol solvate. For example, the AD/GLU peaks at 10.54°, 20.70° and 25.94° and the methanol solvate peaks at 16.5°, 19.4° and 25.4° were mutually exclusive [
20]. Overall, the XRD analysis confirmed that a new crystal phase was obtained through the liquid-assisted grinding. Since the diffraction pattern does not match any of the known polymorphs of AD and GLU, the obtained phase is mostly likely the AD/GLU cocrystal, which could not be successfully acquired from solution crystallization. This was also in part supported by the sharp melting point of the crystal as shown in the next section, which suggested the formation of a pure phase.
Figure 3.
X-ray diffraction (XRD) patterns of the AD/GLU cocrystal and its raw materials (AD and GLU): 2θ regions of 6°–30° (a) and 6°–18° (b). Some characteristic peaks of the AD/GLU cocrystal were marked by asterisks.
Figure 3.
X-ray diffraction (XRD) patterns of the AD/GLU cocrystal and its raw materials (AD and GLU): 2θ regions of 6°–30° (a) and 6°–18° (b). Some characteristic peaks of the AD/GLU cocrystal were marked by asterisks.
Figure 4 was shown to verify the validity of the liquid-assisted grinding in the formation of AD cocrystals. The XRD patterns of the AD/SUB crystals grown through the solution crystallization and liquid-assisted grinding matched nearly perfectly in their peak positions as previously reported [
21]. Also shown is the calculated pattern from the single crystal X-ray data [
16].
Figure 4.
XRD patterns of neat AD and AD/SUB cocrystals. The pattern of the cocrystal calculated from the known crystal structure was compared with those of the crystals prepared by the grinding method and solution growth as previously reported [
21].
Figure 4.
XRD patterns of neat AD and AD/SUB cocrystals. The pattern of the cocrystal calculated from the known crystal structure was compared with those of the crystals prepared by the grinding method and solution growth as previously reported [
21].
2.2. Thermal Stability and Release Behavior
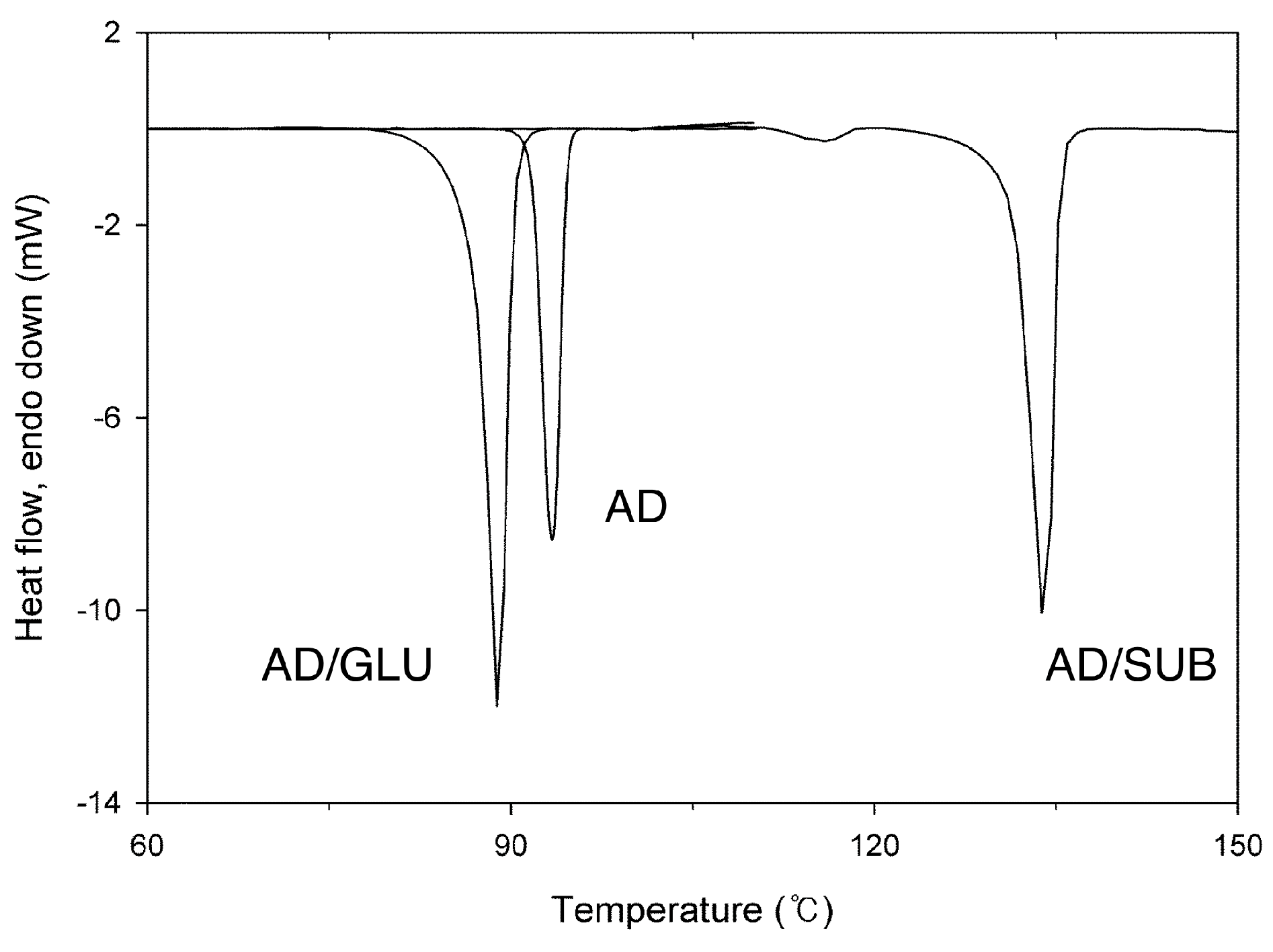
Thermal stability of the prepared crystals could be studied through their melting, since there was no premature thermal degradation of the constituent molecules or loss of small molecules (such as water and solvents). Melting behavior of AD/GLU observed with DSC (
Figure 5) was compared with those of AD and AD/SUB, which had been previously reported [
16,
21]. The melting points were in the order: AD/GLU (88.9 °C) < AD (93.3 °C) < AD/SUB (133.9 °C), and the melting enthalpy was also in the same order: AD/GLU (60.8 J/g) < AD (75.4 J/g) < AD/SUB (107.4 J/g). The melting point of AD/GLU cocrystal was lower than those of its pure individual components (T
m and enthalpy of GLU: 99.8 °C and 161.0 J/g, see Figure S2 in Supplementary Materials), whereas AD/SUB had a melting point between those of its components (T
m and enthalpy of SUB: 143.8 °C and 162.6 J/g, see Figure S2 in Supplementary Materials). In fact, other previously reported AD cocrystals with saccharin or succinic acid had their melting points between their respective cocrystal components [
22,
23]. The unique melting behavior of AD/GLU suggested that its crystal structure could possess a dramatically different structure (weaker intermolecular interactions) from other AD cocrystals, although the unsolved crystal structure of AD/GLU made the conclusion speculative at this moment. Similar phenomenon was seen in other API cocrystals using 4,4’-bipyridine as a coformer [
24]. The cocrystal with asprin possessed a crystal structure of a channel inclusion, and it had a lower melting point than its individual components. Other cocrystals with ibuprofen and flurbiprofen had herringbone packing structures, and their melting points were higher than those of their respective cocrystal components.
Figure 5.
Differential Scanning Calorimetry (DSC) thermograms of AD and its cocrystals. The melting point of AD/GLU was compared with those of AD and AD/SUB [
16,
21].
Figure 5.
Differential Scanning Calorimetry (DSC) thermograms of AD and its cocrystals. The melting point of AD/GLU was compared with those of AD and AD/SUB [
16,
21].
We note here that a previously unnoticed small melting peak was found for the AD/SUB cocrystal at 115.9 °C (6.6 J/g), which was still at significantly higher temperature than the melting point of AD. This was possibly related to the crystals with structural defects caused during the milling process, because a similar endothermic peak was not observed with the crystals prepared through solution crystallization [
16]. The portion of the crystals was estimated
ca. 6% when only enthalpy values were roughly considered. No such melting behavior related to the probable structural defects was observed for AD/GLU cocrystals. Further detailed studies with controlled processing parameters would be necessary to confirm the correlation between the stability and structural defects.
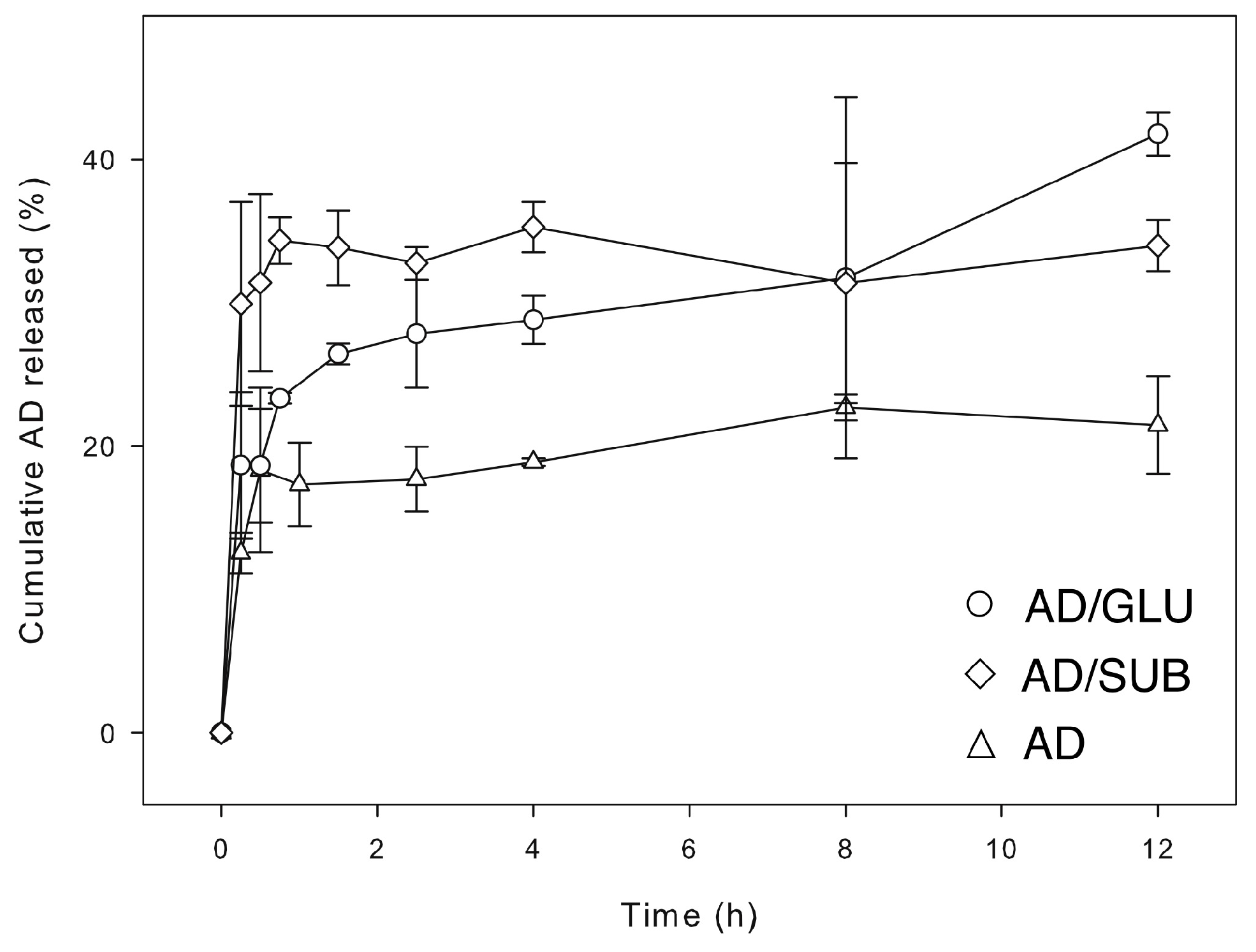
Release behaviors of AD cocrystals and AD raw material were compared in
Figure 6. In general, AD cocrystals showed better release behaviors than AD raw material. This is consistent with the previous solubility study, and it is probably because hydrogen bonding is the major interaction between AD and coformers that contribute to the structural integrity of the cocrystals [
16]. Also, AD/SUB cocrystal showed initially very fast release rate, which could be related to the combination of smaller crystal size (
Figure 2) and the portion of defected crystal structure as discussed with melting (
Figure 5). Dissolution rate of particles is generally regarded as a function of surface area, diffusion constant, boundary layer thickness as well as solubility [
25,
26]. The current method of liquid-assisted grinding was not controlled in terms of the particle size and shape that could affect the surface area and boundary layer thickness, and the particle-aggregated structures could also modify the diffusion of AD molecules by affecting the hydrodynamic properties. Therefore, the current release results should be considered only preliminary. Nonetheless, they suggest that the cocrystal formation based on grinding method is a potentially useful method to regulate API release behavior.
Figure 6.
Release behaviors of AD and its cocrystals.
Figure 6.
Release behaviors of AD and its cocrystals.
3. Experimental Section
AD (l-form, >99%) was obtained from Amore Pacific Co. (Yongin, Korea). GLU (99%) and SUB (98%) were purchased from Sigma-Aldrich (Milwaukee, WI, USA). Methanol (HPLC grade) was from J.T.Baker (Center Valley, PA, USA).
Cocrystallization of AD and coformers (GLU or SUB) was performed through liquid-assisted grinding. AD and coformer was mixed in 1:1 mole ratio, and the mixture was ground using an agate mortar and pestle for 20–30 min. AD/GLU and AD/SUB cocrystals were prepared in 0.20 and 0.40 mmol scale, respectively. Methanol was added dropwise over the course of grinding (0.5 mL/mmol cocrystal). After grinding, the cocrystals were dried in a vacuum oven (J-DV01; JISICO, Seoul, Korea) at 40 °C for 24 h before further characterization.
Morphologies of AD and its cocrystals were microscopically observed. Gross morphologies were surveyed with OM using a BX51 microscope (Olympus, Tokyo, Japan) in the reflectance mode with cross polarization. Detailed morphologies were observed with FE-SEM using an Auriga microscope (Carl Zeiss, Oberkochen, Germany) after thin platinum coating to minimize charging.
XRD was performed to identify the crystal phases. A D8 Discover diffractometer (Bruker AXS, Billerica, MA, USA) was used in the 2θ–θ mode to scan the 2θ region of 6°–40° with a scanning rate of 1°/min. CuKα radiation (λ = 1.5406 Å) at 40 kV and 40 mA was employed.
DSC was utilized to assess the melting properties of the AD and its cocrystals. A DSC821e (Mettler-Toledo, Columbus, OH, USA) was employed under N2 with a scanning rate of 10 °C/min. The instrument was pre-calibrated using indium for enthalpy and temperature.
The in vitro release behaviors of the AD and its cocrystals were measured using a dissolution tester (USP type 2 paddle apparatus). A KDT-600 (Kukje Engineering Co., Seoul, Korea) was used under a sink condition. A typical experiment consisted of 750 mg crystal powders in a 300 mL aqueous solution (pH 6, 37 °C) stirred at 100 rpm. Sampling (5 mL) was done until 12 h at pre-determined time points, and a fresh 5 mL solution was added to the system after each sampling. Each sampled solution was filtered through a syringe filter of 0.45 μm pore size (MFS-13; Avantec, Dublin, CA, USA), and its UV absorbance was measured at 260 nm using a JASCO (Tokyo, Japan) V-550 UV-Vis spectrophotometer. AD concentration was calculated using a pre-constructed calibration curve.
4. Conclusions
In summary, liquid-assisted grinding was utilized to obtain the AD/GLU cocrystal that was difficult to isolate in its pure form through solution crystallization. It had lower thermal stability than neat AD as well as other AD cocrystals, which had been successfully prepared through solution crystallization. The low enthalpy of fusion of AD/GLU suggests that the intermolecular bonding that holds its structure could be much weaker than those in neat AD and the other AD cocrystals, although further investigation would be needed to clarify the exact nature of the intermolecular interactions. The release behavior of the AD/GLU was comparable with AD/SUB, and some improvement appears possible if its particle processing is optimized. The current study demonstrates the usefulness of the grinding method to prepare a thermodynamically less stable AD cocrystal phase, and similar findings are expected in the future for other APIs.