Synthesis, Crystal Structure and DFT Studies of 8-chloro-3-((3-chlorobenzyl)thio)-[1,2,4]triazolo[4,3-a]pyridine
Abstract
:1. Introduction
2. Results and Discussion
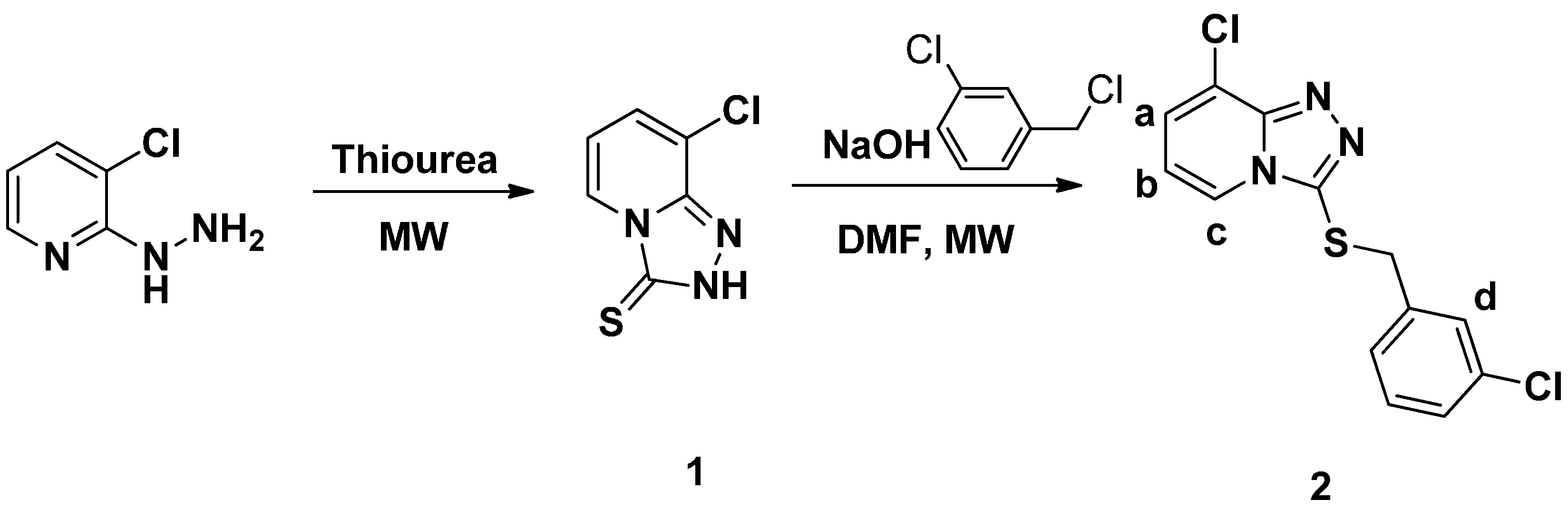
2.1. Synthesis and Spectra Analysis
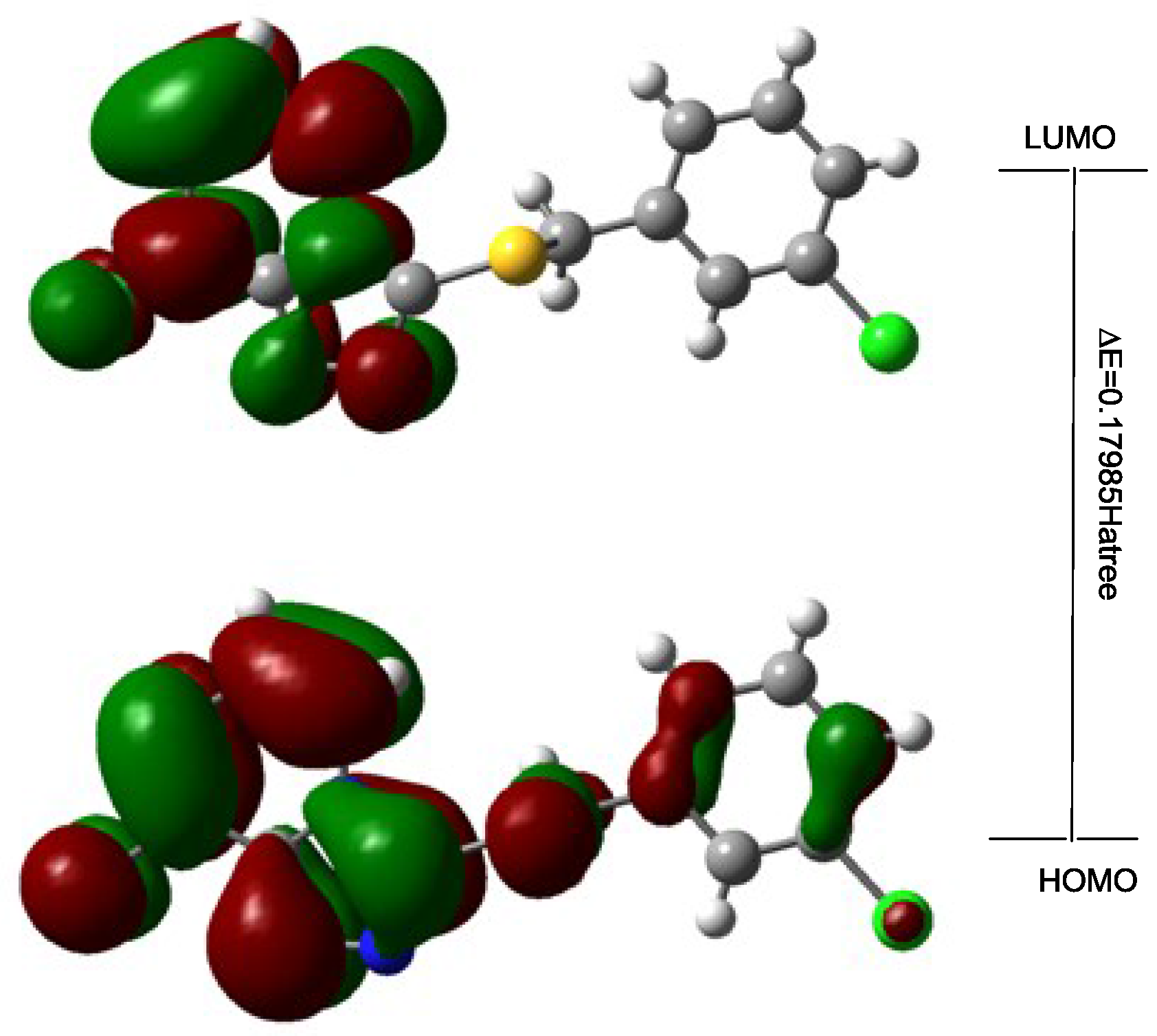
2.2. Frontier Orbital Energy Analysis and Molecular Total Energies
| -- | DFT |
|---|---|
| Etotal/Hartree a | −1983.14769316 |
| EHOMO/Hartree | −0.24453 |
| ELUMO/Hartree | −0.06468 |
| ΔE b/Hartree | 0.1229 |
2.3. Crystal Structure

| Bond | X-ray Crystal | DFT | Angle | X-ray Crystal | DFT |
|---|---|---|---|---|---|
| S1-C6 | 1.743(3) | 1.74330 | C6-S1-C7 | 98.57(11) | 98.55541 |
| S1-C7 | 1.837(3) | 1.83750 | C5-N1-C1 | 123.3(2) | 123.28802 |
| Cl1-C2 | 1.718(3) | 1.71782 | C5-N1-C6 | 132.2(2) | 132.15746 |
| Cl2-C10 | 1.742(2) | 1.74147 | C1-N2-N3 | 106.84(19) | 106.81997 |
| N1-C5 | 1.377(3) | 1.37688 | N2-C1-N1 | 110.3(2) | 110.31368 |
| N1-C1 | 1.378(3) | 1.37831 | N1 C1 C2 | 117.5(2) | 117.48357 |
| N1-C6 | 1.384(3) | 1.38396 | N3-C6-S1 | 127.63(18) | 127.62512 |
| N2-C1 | 1.323(3) | 1.32294 | N1-C6-S1 | 122.75(18) | 122.74936 |
| N2-N3 | 1.382(3) | 1.38169 | C8-C7-S1 | 108.99(17) | 108.96455 |
| N3-C6 | 1.310(3) | 1.30950 | C13-C8-C9 | 119.0(2) | 118.99908 |
| C1-C2 | 1.417(3) | 1.41733 | C11-C10-Cl2 | 119.06(19) | 119.07111 |
| C7-C8 | 1.502(3) | 1.50164 | C3-C2-Cl1 | 123.4(2) | 123.37263 |
| C8-C13 | 1.383(3) | 1.38280 | C2-C3-C4 | 119.9(2) | 119.90145 |
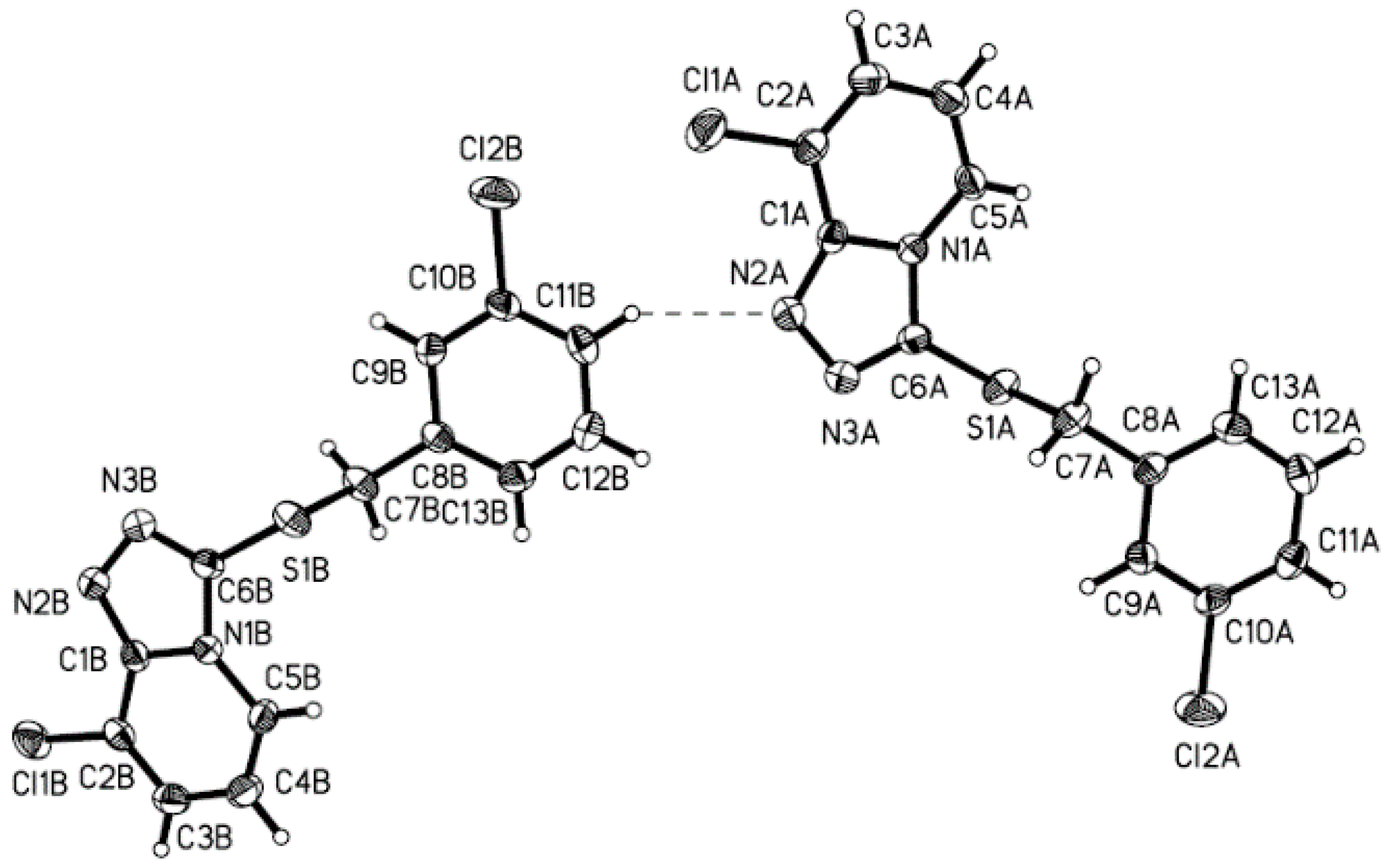
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) |
|---|
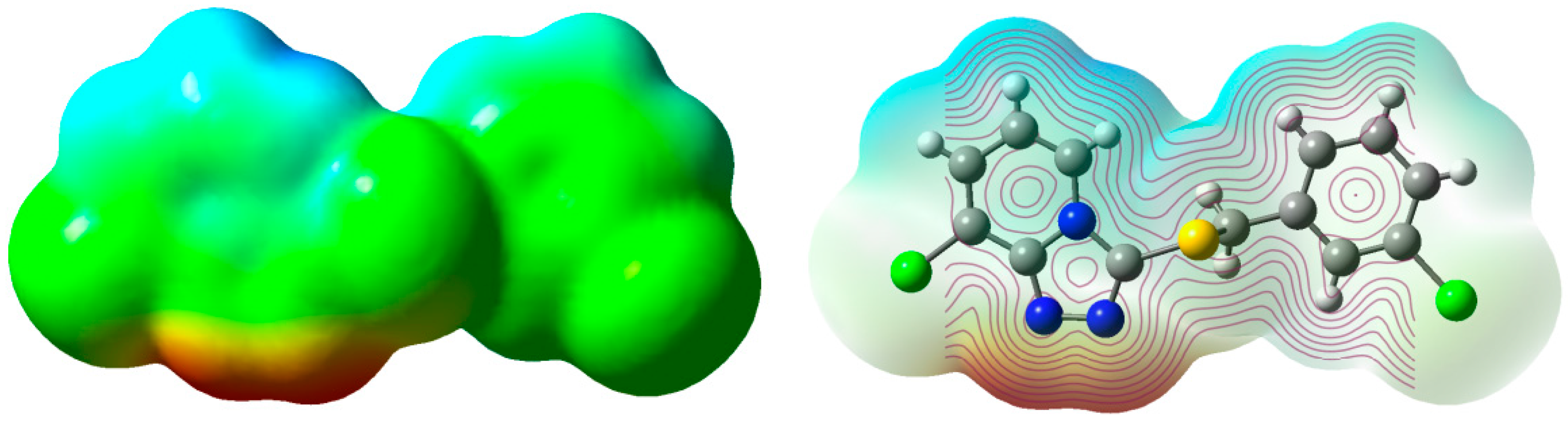
2.4. Mulliken Atomic Charges and ESP
| Atom | DFT |
|---|---|
| S1 | 0.413 |
| N1 | −0.694 |
| N2 | −0.292 |
| N3 | −0.243 |
| Cl1 | 0.222 |
| Cl2 | 0.128 |
| C1 | 0.456 |
| C2 | −0.272 |
| C3 | −0.037 |
| C4 | −0.105 |
| C5 | 0.222 |
| C6 | 0.052 |
| C7 | 0.565 |
| C8 | 0.102 |
| C9 | −0.045 |
| C10 | −0.290 |
| C11 | −0.029 |
| C12 | −0.052 |
| C13 | −0.088 |
3. Experimental Section
3.1. Instruments
3.2. Therotical Calculations
3.3. General Procedure
3.4. Structure Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plech, T.; Kaproń, B.; Paneth, A.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Stączek, P.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M. Determination of the primary molecular target of 1,2,4-triazole-ciprofloxacin hybrids. Molecules 2015, 20, 6254–6272. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, A.; Saeed, A.; Malik, I. Synthesis, characterization and antimicrobial activities of mercaptoxadiazoles, mercaptotriazoles and their derivatives. J. Chem. Soc. Pak. 2014, 36, 852–857. [Google Scholar]
- Tan, C.X.; Shi, Y.X.; Weng, J.Q.; Liu, X.H.; Zhao, W.G.; Li, B.J. Synthesis and antifungal activity of novel 1,2,4-triazole derivatives containing 1,2,3-thiadiazole moiety. J. Heterocycl. Chem. 2014, 51, 690–694. [Google Scholar] [CrossRef]
- Sun, N.-B.; Fu, J.-Q.; Weng, J.-Q.; Jin, J.-Z.; Tan, C.-X.; Liu, X.-H. Microwave assisted synthesis, antifungal activity and DFT theoretical study of some novel 1,2,4-triazole derivatives containing the 1,2,3-thiadiazole moiety. Molecules 2013, 18, 12725–12739. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-X.; Yang, M.-Y.; Shi, Y.-X.; Sun, Z.-H.; Liu, X.-H.; Wu, H.-K.; Li, B.-J.; Zhang, Y.-G. Microwave assistant synthesis, antifungal activity and DFT theoretical study of some novel 1,2,4-triazole derivatives containing pyridine moiety. Int. J. Mol. Sci. 2014, 15, 8075–8090. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Pan, L.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Wang, B.L.; Li, Z.M. Synthesis, structure, and biological activity of novel (oxdi/tri)azoles derivatives containing 1,2,3-thiadiazole or methyl moiety. Mol. Divers. 2012, 16, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Weng, J.Q.; Wang, B.L.; Li, Y.H.; Tan, C.X.; Li, Z.M. Microwave-assisted synthesis of novel fluorinated 1,2,4-triazole derivatives, and study of their biological activity. Res. Chem. Intermed. 2014, 40, 2605–2612. [Google Scholar] [CrossRef]
- Tehrani, K.H.M.E.; Mashayekhi, V.; Azerang, P.; Minaei, S.; Sardari, S.; Kobarfard, F. Synthesis and antimycobacterial activity of some triazole derivatives—New route to functionalized triazolopyridazines. Iran. J. Pharm. Res. 2015, 14, 59–68. [Google Scholar] [PubMed]
- Al-Omair, M.A.; Sayed, A.R.; Youssef, M.M. Synthesis of novel triazoles, tetrazine, thiadiazoles and their biological activities. Molecules 2015, 20, 2591–2610. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Sun, Z.H.; Yang, M.Y.; Tan, C.X.; Weng, J.Q.; Zhang, Y.G.; Ma, Y. Microwave assistant one pot synthesis, crystal structure, antifungal activities and 3D-QSAR of novel 1,2,4-triazolo[4,3-a]pyridines. Chem. Biol. Drug Des. 2014, 84, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Shi, Y.X.; Sun, Z.H.; Yang, M.Y.; Wu, H.K.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhang, Y.G. Synthesis and bioactivities of novel 1,3,4-oxadiazole derivatives containing pyridine moiety. Lett. Drug Des. Discov. 2014, 11, 1119–1123. [Google Scholar] [CrossRef]
- Liu, X.H.; Tan, C.X.; Weng, J.Q. Phase transfer-catalyzed, one-potsynthesis of some novel N-pyrimidinyl-N′-nicotinyl thioureaderivatives. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 552–557. [Google Scholar] [CrossRef]
- Sun, Z.H.; Zhai, Z.W.; Yang, M.Y.; Liu, X.H.; Tan, C.X.; Weng, J.Q. Microwave assistant synthesis and dimeric crystal structure of 2-(((6-chloropyridin-3-yl)methyl)thio)-5-(pyridin-4-yl)-1,3,4-thiadiazole. Chin. J. Struct. Chem. 2014, 33, 1779–1783. [Google Scholar]
- Zhang, L.J.; Yang, M.Y.; Hu, B.Z.; Sun, Z.H.; Liu, X.H.; Weng, J.Q.; Tan, C.X. Microwave-assisted synthesis of novel 8-chloro-[1,2,4]triazolo[4,3-a]pyridinederivatives. Turk. J. Chem. 2015, 39, 867–873. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, X.Y.; Tan, C.X.; Weng, J.Q.; Xin, J.H.; Chen, J. Synthesis, crystal structure, herbicidal activities and 3D-QSAR study of some novel 1,2,4-triazolo[4,3-a]pyridine derivatives. Pest. Manag. Sci. 2015, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.O.; Abdelall, E.K.A.; Abdel-Riheem, N.A.; Ahmed, S.A. Synthesis and antimicrobial activity of some new 5-arylazothiazole, Pyrazolo[1,5-a] pyrimidine, [1,2,4]triazolo[4,3-a]pyrimidine, and pyrimido[1,2-a]benzimidazole derivatives containing the thiazole moiety. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 709–718. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhai, Z.W.; Sun, Z.H.; Yu, S.J.; Liu, X.H.; Weng, J.Q.; Tan, C.X.; Zhao, W.G. A facile one pot synthesis of novel 1,2,4-triazolo[4,3-a]pyridine derivatives containing the trifluoromethyl moiety via microwave irradiation. J. Chem. Res. 2015, 39, 395–397. [Google Scholar] [CrossRef]
- Sadana, A.K.; Mirza, Y.; Aneja, K.R.; Prakash, O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003, 38, 533–536. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, M.Y.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhao, W.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Liu, X.H. Synthesis and biological activity of acylthiourea derivatives contain 1,2,3-thiadiazole and 1,3,4-thiadiazole. Lett. Drug Des. Discov. 2015, 12, 314–318. [Google Scholar] [CrossRef]
- Liu, X.H.; Pan, L.; Ma, Y.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Shi, Y.X.; Li, B.J.; Li, Z.M.; Zhang, Y.G. Design, synthesis, biological activities, and 3D-QSAR of new N,N′-diacylhydrazines containing 2-(2,4-dichlorophenoxy)propane moiety. Chem. Biol. Drug Des. 2011, 78, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Weng, J.Q.; Liu, Z.X.; Liu, X.H.; Zhao, W.G. Synthesis, crystal structure and fungicidal activity of a novel 1,2,3-thiadiazole compound. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 990–996. [Google Scholar] [CrossRef]
- Yan, S.L.; Yang, M.Y.; Sun, Z.H.; Min, L.J.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole Moiety. Lett. Drug Des. Discov. 2014, 11, 940–943. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhao, W.G.; Wang, B.L.; Li, Z.M. Synthesis, bioactivity and DFT structure-activity relationship study of novel 1,2,3-thiadiazole derivatives. Res. Chem. Intermed. 2012, 38, 1999–2008. [Google Scholar] [CrossRef]
- Chen, G.L.; Tan, D.S.; Xu, L.Y.; Dong, J.H. Synthesis of trazodone hydrochloride. Chin. J. Med. Chem. 2002, 46, 92–93. (In Chinese) [Google Scholar]
- Liu, X.F. Substitution reactions of diiron dithiolate complexes with phosphine or isocyanide ligands. J. Organomet. Chem. 2014, 750, 117–124. [Google Scholar] [CrossRef]
- Liu, X.H.; Pan, L.; Tan, C.X.; Weng, J.Q.; Wang, B.L.; Li, Z.M. Synthesis, crystal structure, bioactivity and DFT calculation of new oxime ester derivatives containing cyclopropane moiety. Pestic. Biochem. Phys. 2011, 101, 143–147. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhao, W.; Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, crystal structure and antifungal activity of 4-(5-((2,4-Dichlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine. Chin. J. Struct. Chem. 2015, 34, 203–207. [Google Scholar]
- Weng, J.Q.; Wang, L.; Liu, X.H. Synthesis, Crystal structure and herbicidal activity of a 1, 2, 4-triazol-5(4H)-one derivative. J. Chem. Soc. Pak. 2012, 34, 1248–1252. [Google Scholar]
- Wang, Z.X.; Jian, F.F.; Duan, C.Y.; Bai, Z.P.; You, X.Z. 2-(2-Hydroxybenzylidene)-1-(2-picoloyl)hydrazine Hemihydrate. Acta. Cryst. 1998, C54, 1927–1929. [Google Scholar] [CrossRef]
- Frisch, M.-J.; Trucks, G.-W.; Schlegel, H.-B.; Scuseria, G.-E.; Robb, M.-A.; Cheeseman, J.-R.; Montgomery, J.-A., Jr.; Vreven, T.; Kudin, K.-N.; Burant, J.-C.; et al. Gaussian 03, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXS97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, J.-X.; Yang, M.-Y.; Sun, Z.-H.; Tan, C.-X.; Weng, J.-Q.; Wu, H.-K.; Liu, X.-H. Synthesis, Crystal Structure and DFT Studies of 8-chloro-3-((3-chlorobenzyl)thio)-[1,2,4]triazolo[4,3-a]pyridine. Crystals 2015, 5, 491-500. https://doi.org/10.3390/cryst5040491
Mu J-X, Yang M-Y, Sun Z-H, Tan C-X, Weng J-Q, Wu H-K, Liu X-H. Synthesis, Crystal Structure and DFT Studies of 8-chloro-3-((3-chlorobenzyl)thio)-[1,2,4]triazolo[4,3-a]pyridine. Crystals. 2015; 5(4):491-500. https://doi.org/10.3390/cryst5040491
Chicago/Turabian StyleMu, Jin-Xia, Ming-Yan Yang, Zhao-Hui Sun, Cheng-Xia Tan, Jian-Quan Weng, Hong-Ke Wu, and Xing-Hai Liu. 2015. "Synthesis, Crystal Structure and DFT Studies of 8-chloro-3-((3-chlorobenzyl)thio)-[1,2,4]triazolo[4,3-a]pyridine" Crystals 5, no. 4: 491-500. https://doi.org/10.3390/cryst5040491






