One-Pot Synthesis and Crystal Structure of Methyl 5-Hydroxy-1-phenyl-1H-pyrazole-3-carboxylate
Abstract
:1. Introduction
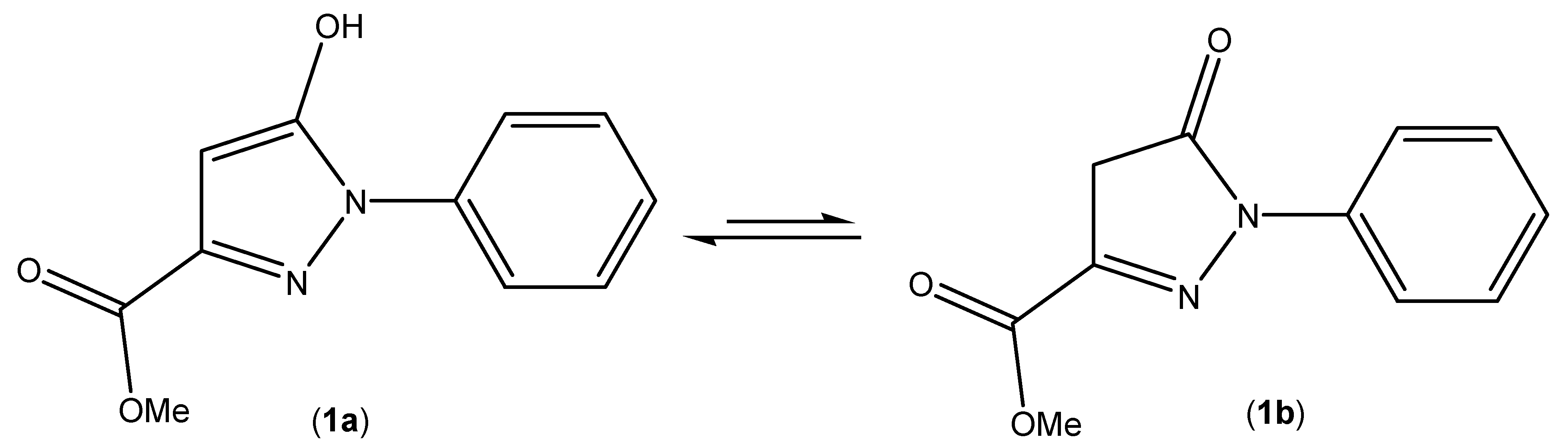
2. Results and Discussion
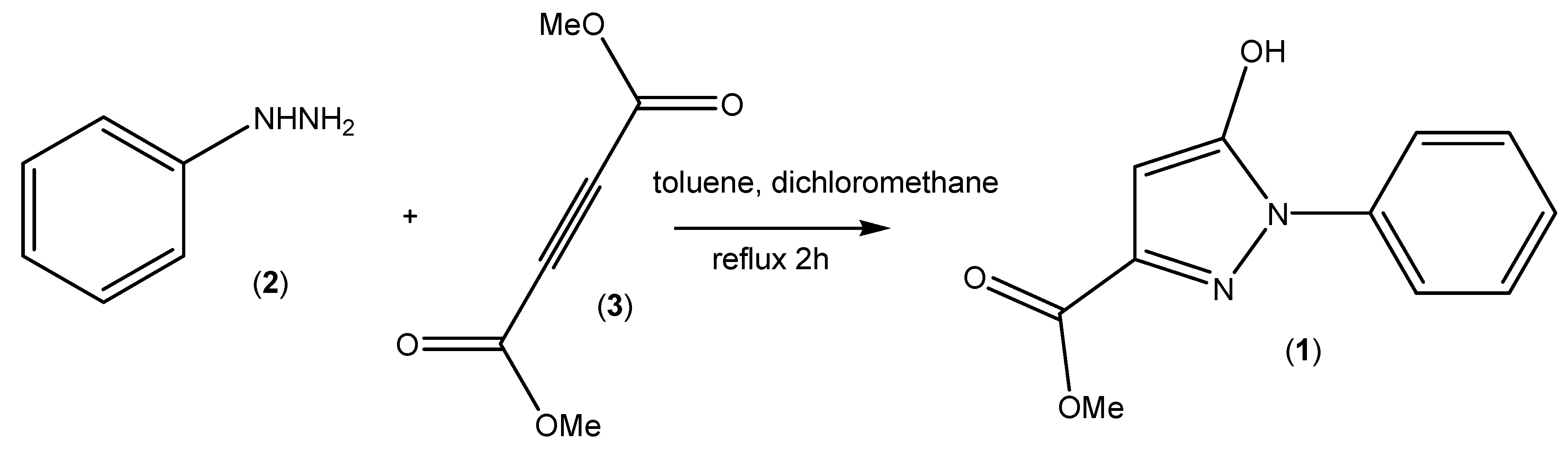

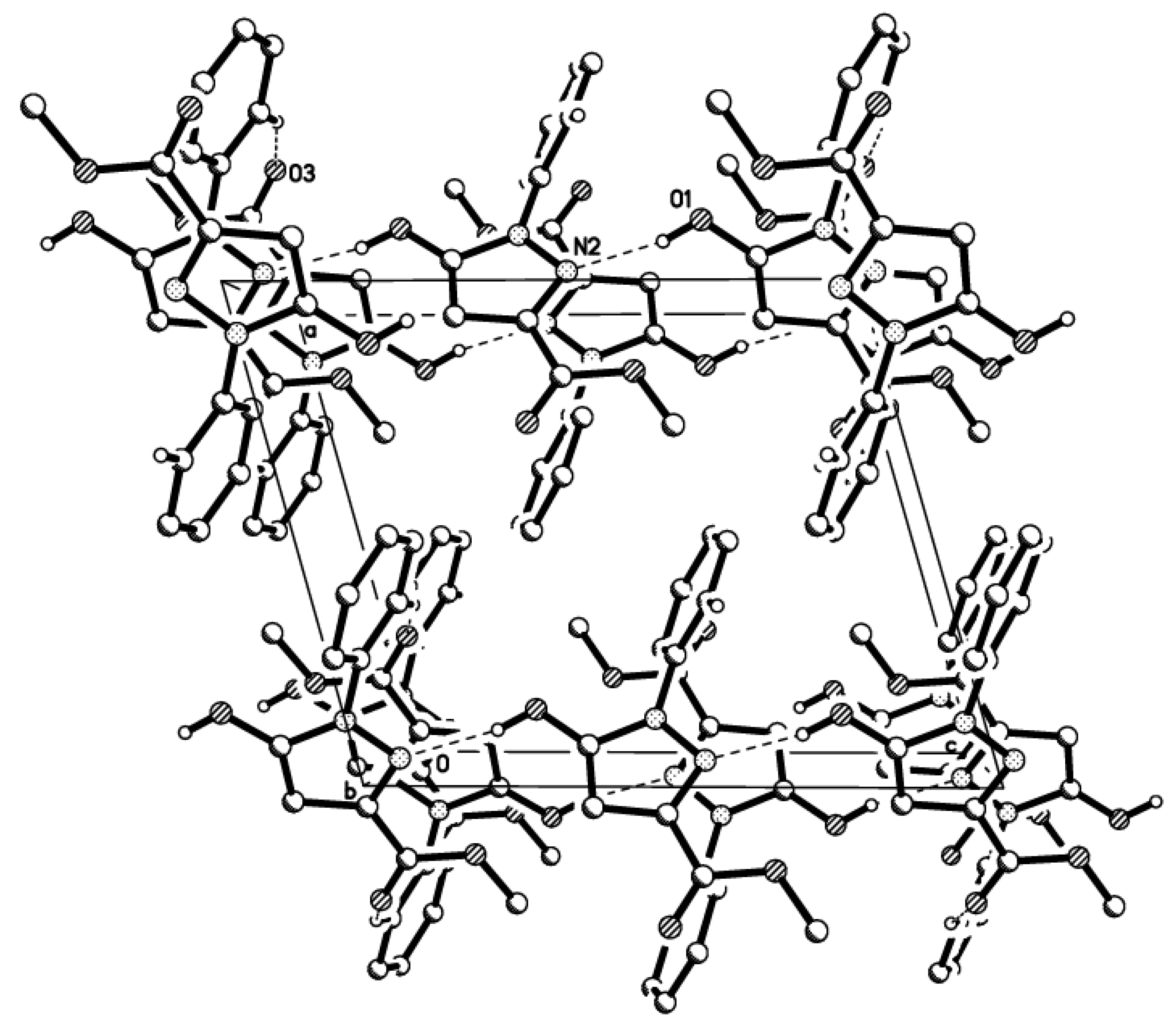
3. Experimental Section
3.1. General
3.2. Synthesis of Methyl 5-Hydroxy-1-phenyl-1H-pyrazole-3-carboxylate (1)
3.3. X-ray Structure Determination
4. Conclusions
References
- Noga, E.J.; Barthalmus, G.T.; Mitchell, M.K. Cyclic amines are selective cytotoxic agents for pigmented cells. Cell Biol. Int. 1986, 10, 239–247. [Google Scholar] [CrossRef]
- Subas, M.S.; Kristin, M.L.D.; Martha, L.M.; Bryson, R.; Andrei, S.; Robert, J.R.; David, A.K.; Hengmiao, C.; Jin, L.; Burton, H.J.; et al. 5-Heteroatom substituted pyrazoles as canine COX-2 inhibitors. Part 1: Structure–Activity relationship studies of 5-alkylamino pyrazoles and discovery of a potent, selective, and orally active analog. Bioorg. Med. Chem. Lett. 2006, 16, 288–292. [Google Scholar]
- Katritzky, A.R.; Wang, M.; Zhang, S.; Voronkov, M.V. Regioselective synthesis of polysubstituted pyrazoles and isoxazoles. J. Org. Chem. 2001, 66, 6787–6791. [Google Scholar]
- Serena, T. Rimonabant: A cannabinoid receptor blocker for the treatment of metabolic and cardiovascular risk factors. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 156–162. [Google Scholar] [CrossRef]
- David, J.W.; Thomas, C.; Ronald, R.; James, A.K.; Hyacinth, A.; Duff, D.; Robert, M.; Thomas, A.P.; Kim, T.Z.; Thomas, G.H.; Lawrence, D.W. Pyrazolo[1,5-a]pyrimidine CRF-1 receptor antagonists. Bioorg. Med. Chem. Lett. 1998, 8, 2067–2070. [Google Scholar]
- Genin, M.J.; Biles, C.; Keiser, B.J.; Poppe, S.M.; Swaney, S.M.; Tarpley, W.G.; Romero, D.L. Novel 1,5-diphenylpyrazole non nucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: Lead identification and SAR of 3- and 4-substituted derivatives. J. Med. Chem. 2000, 43, 1034–1040. [Google Scholar] [CrossRef]
- Cottineau, P.; Toto, C.; Marot, A.; Pipaud, J.C. Synthesis and hypoglycemic evaluation of substituted pyrazole-4-carboxylic acids. Bioorg. Med. Chem. Lett. 2002, 12, 2105–2108. [Google Scholar]
- Lee, K.Y.; Kim, J.M. Regioselective synthesis of 1,3,4,5-tetrasubstituted pyrazoles from Baylis–Hillman adducts. Tetrahedron Lett. 2003, 44, 6737–6740. [Google Scholar] [CrossRef]
- Tzeng, Z.H.; Yang, S.W.; Liu, P.M.; Chen, K.M. Optical resolution of 5-Oxo-1-phenyl-pyrazolidine-3-carboxylic acid as a new organocatalyst for organic reactions. J. Chin. Chem. Soc. (Taipei) 2008, 55, 626–631. [Google Scholar]
- Zheng, P.W.; Fan, M.L.; Duan, X.M.; Li, J.S.; Huang, P.M. 3-Ethoxycarbonyl-1-phenyl-1H-pyrazol-5-yl 4-chlorobenzoate. Acta Cryst. 2006, E62, o311–o312. [Google Scholar]
- Bruker AXS Inc. SMART (Version 5.63),SAINT (Version 6.02); Bruker AXS Inc.: Madison, WI, USA, 2002.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saeed, A.; Arshad, I.; Flörke, U. One-Pot Synthesis and Crystal Structure of Methyl 5-Hydroxy-1-phenyl-1H-pyrazole-3-carboxylate. Crystals 2012, 2, 1248-1252. https://doi.org/10.3390/cryst2031248
Saeed A, Arshad I, Flörke U. One-Pot Synthesis and Crystal Structure of Methyl 5-Hydroxy-1-phenyl-1H-pyrazole-3-carboxylate. Crystals. 2012; 2(3):1248-1252. https://doi.org/10.3390/cryst2031248
Chicago/Turabian StyleSaeed, Aamer, Ifzan Arshad, and Ulrich Flörke. 2012. "One-Pot Synthesis and Crystal Structure of Methyl 5-Hydroxy-1-phenyl-1H-pyrazole-3-carboxylate" Crystals 2, no. 3: 1248-1252. https://doi.org/10.3390/cryst2031248




