New Fulvalenium Salts of Cobalt Bis(dicarbollide): Crystal Structures and Electrical Conductivities
Abstract
:1. Introduction
2. Results and Discussion
2.1. (BEDT-TTF)[8,8',(7)-Cl2(Cl0.09)-3,3'-Co(1,2-C2B9H9.91)(1',2'-C2B9H10)]
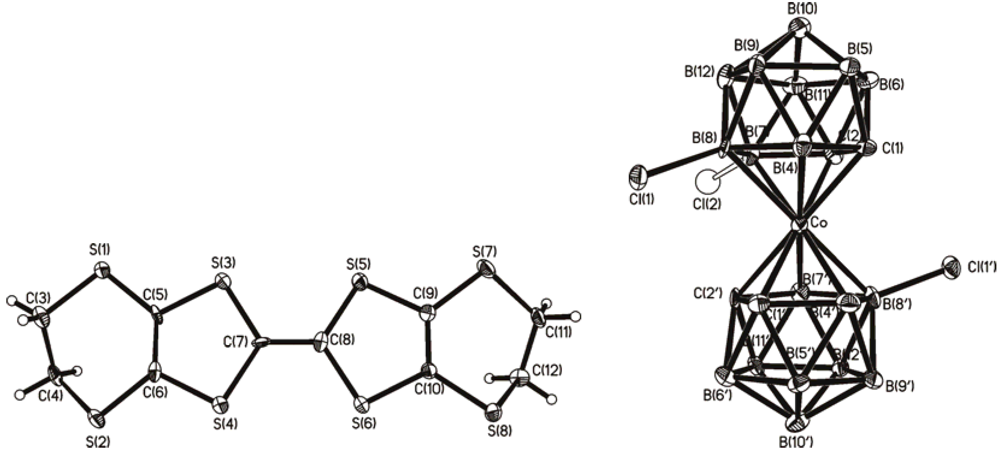

2.2. (BEDT-TTF)[8,8'-Br0.75Cl1.25-3,3'-Co(1,2-C2B9H10)2]
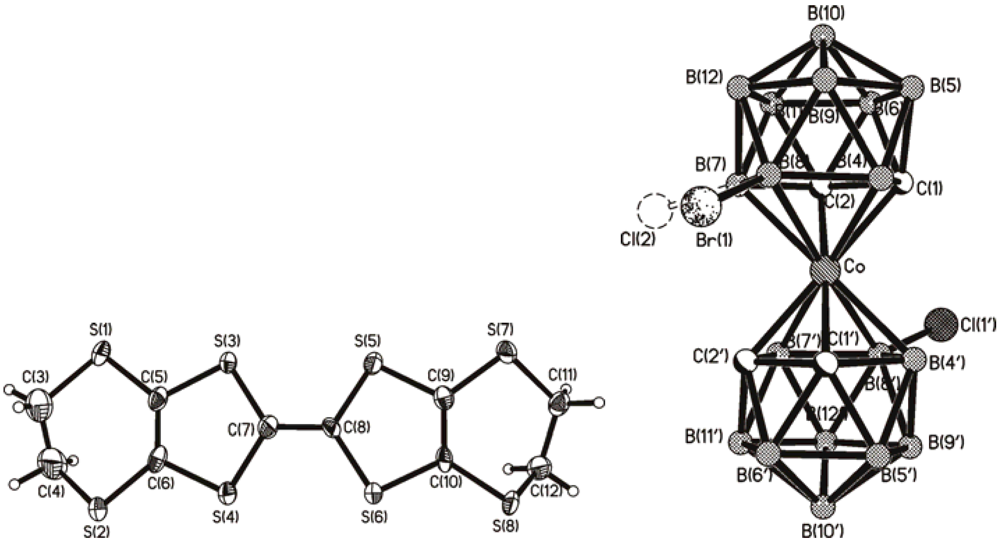

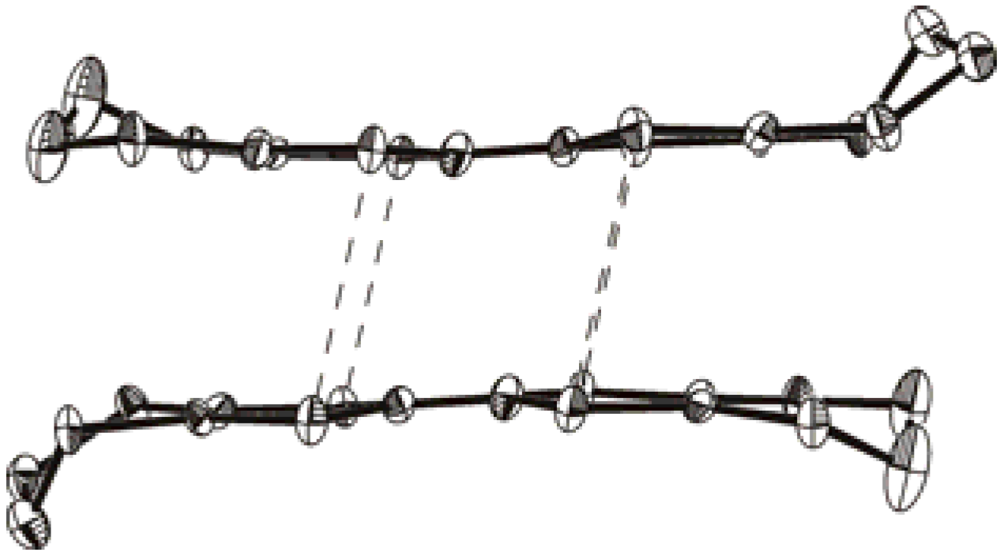

2.3. (BMDT-TTF)4[8,8'-Br1.16(OH)0.72-3,3'-Co(1,2-C2B9H10.06)2]

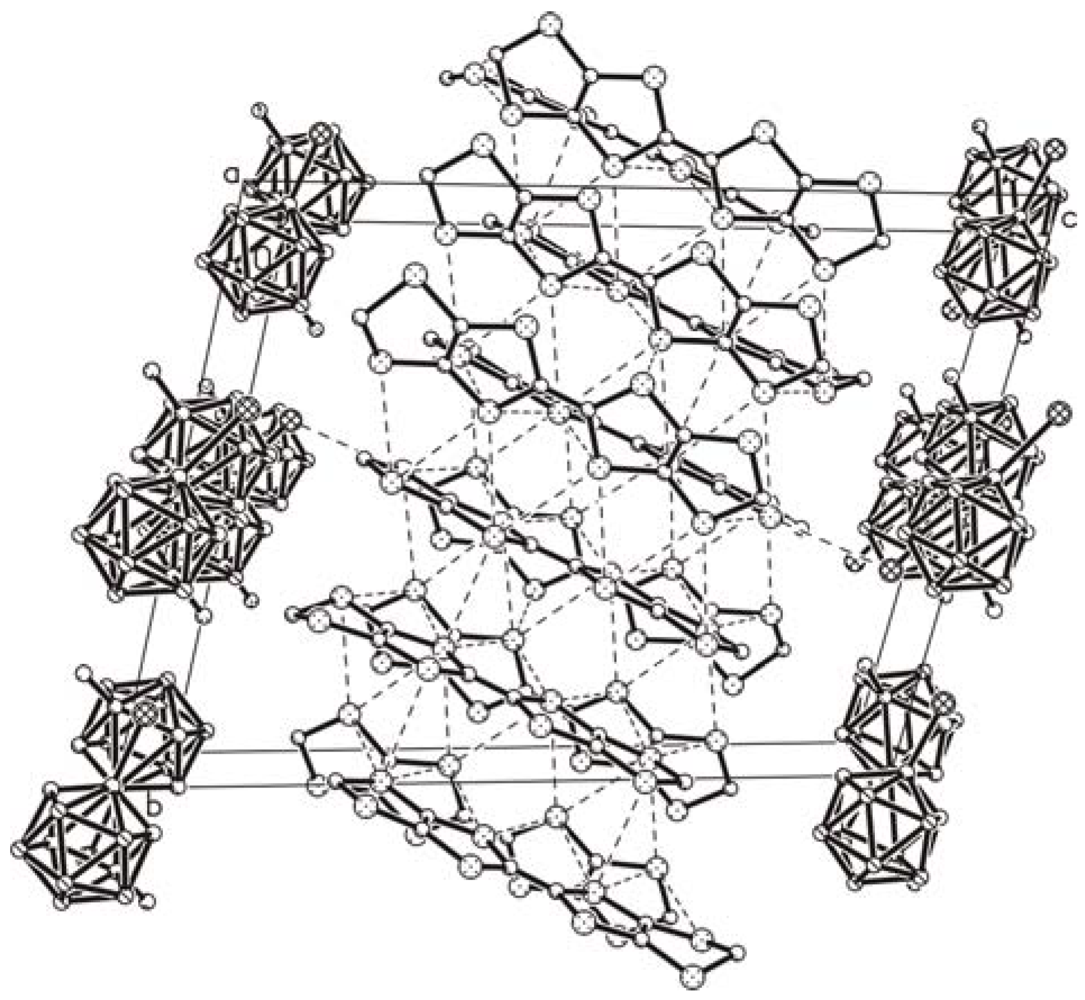

3. Experimental Section
3.1. Synthesis
3.2. Data Collection and Refinements
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C14H27.91B18Cl2.09CoS8 | C14H27.75B18Br0.75Cl1.25CoS8 | C36H36.84B18Br1.16CoO0.72S32 |
| Formula weight | 780.54 | 810.51 | 1853.14 |
| Crystal system | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | P-1 | P-1 |
| a (Å) | 8.8565(7) | 8.711(2) | 10.809(1) |
| b (Å) | 13.349(1) | 13.441(2) | 16.187(1) |
| c (Å) | 14.654(1) | 14.742(3) | 20.412(1) |
| α (°) | 106.617(2) | 106.991(3) | 100.22(1) |
| β (°) | 102.684(2) | 101.784(3) | 100.43(1) |
| γ (°) | 98.963(2) | 99.504(3) | 107.21(1) |
| U (Å3) | 1574.7(2) | 1568.6(5) | 3252.1(4) |
| Z | 2 | 2 | 2 |
| λ (Å) | 0.71073 | 0.71073 | 0.71073 |
| Dcalc (mg m−3) | 1.64 | 1.72 | 1.89 |
| μ (mm−1) | 1.268 | 2.159 | 2.043 |
| Number of reflections collected | 10902 | 13848 | 11525 |
| Number of independent reflections | 6081 | 6324 | 11525 |
| Number of reflections with [ F0 > 4σ(F0)] | 3391 | 2424 | 6107 |
| Number of parameters refined | 395 | 393 | 865 |
| (2θ)max, (°) | 53.68 | 52.98 | 61.22 |
| R | 0.059 | 0.076 | 0.051 |
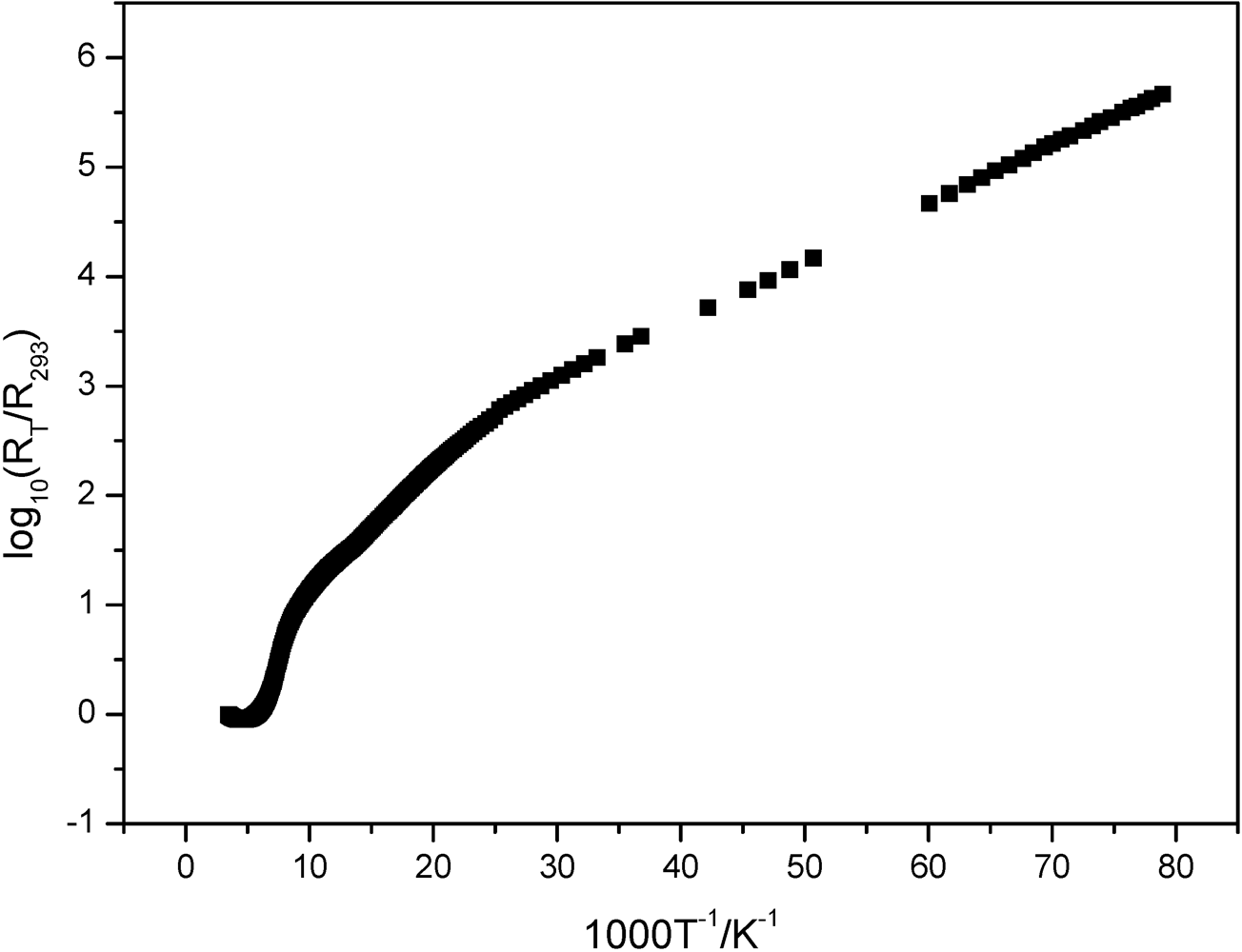
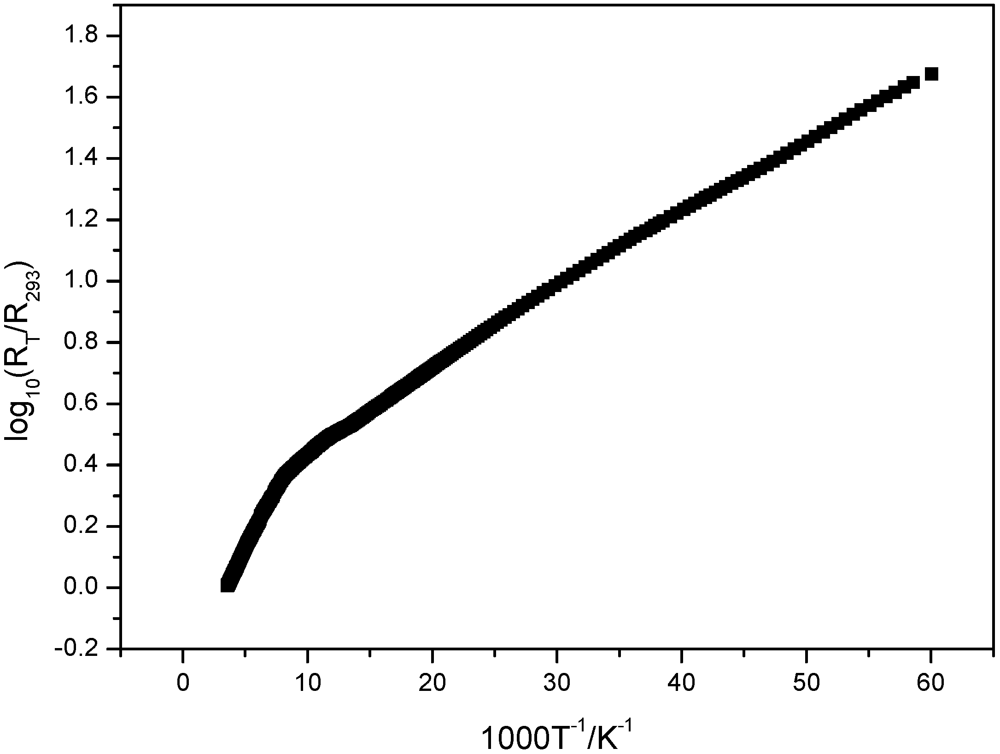
3.3. Electric Conductivity Measurements
4. Conclusions
Acknowledgments
References
- Shibaeva, R.P.; Yagubskii, E.B. Molecular conductors and superconductors based on trihalides of BEDT-TTF and some of its analogues. Chem. Rev. 2004, 105, 5347–5378. [Google Scholar]
- Saito, G.; Yoshida, Y. Organic superconductors. Chem. Rec. 2011, 11, 124–145. [Google Scholar]
- Schlueter, J.A. Tetrathiafulvalene-based conductors containing organometallic compounds. In Conducting and Magnetic Organometallic Molecular Materials. (Topics in Organometallic Chemistry, V.27); Fourmigue, M., Ouahab, L., Eds.; Springer-Verlag: Berlin, Germany, 2009; pp. 1–33. [Google Scholar]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of cobalt bis(dicarbollides). A review. Collect. Czech. Chem. Commun. 1999, 64, 783–805. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of nickel and iron bis(dicarbollides). A review. J. Organomet. Chem. 2000, 614-615, 27–36. [Google Scholar]
- Yan, Y.-K.; Mingos, D.M. Structural, magnetic, and conductivity properties of charge-transfer salts derived from metallacarboranes. Chem. Soc. Rev. 1995, 203–213. [Google Scholar]
- Bregadze, V.; Sivaev, I.; Lobanova, I.; Kazheva, O.; Alexandrov, G.; Kravchenko, A.; Starodub, V.; Buravov, L.; Titov, L.; Dyachenko, O. Synthesis, structure and electrical conductivity of fulvalenium salts of cobalt bis(dicarbollide) anion and its derivatives. J. Chem. Sci. 2010, 122, 37–41. [Google Scholar]
- Bregadze, V.I.; Timofeev, S.V.; Sivaev, I.B.; Lobanova, I.A. Substitution reactions at the boron atoms in metallacarboranes. Russ. Chem. Rev. 2004, 73, 470–491. [Google Scholar]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Starodub, V.A.; Sivaev, I.B.; Lobanova, I.A.; Bregadze, V.I.; Buravov, L.I.; Dyachenko, O.A. New fulvalenium salts of bis(dicarbollide) cobalt and iron: Synthesis, crystal structure and electrical conductivity. J. Organomet. Chem. 2007, 692, 5033–5043. [Google Scholar]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Starodub, V.A.; Lobanova, I.A.; Sivaev, I.B.; Bregadze, V.I.; Titov, L.V.; Buravov, L.I.; Dyachenko, O.A. Molecular conductors with 8,8'-diiodo cobalt bis(dicarbollide) anion. J. Organomet. Chem. 2009, 694, 2336–2342. [Google Scholar]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Starodub, V.A.; Zhigareva, G.G.; Sivaev, I.B.; Bregadze, V.I.; Buravov, L.I.; Titov, L.V.; Dyachenko, O.A. Synthesis, structures and conductivities of salts (BEDT-TTF)[9,9'(12')-I2-3,3'-Co(1,2-C2B9H10)2] and (TTF)[9,9',12,12'-I4-3,3'-Co(1,2-C2B9H9)2]. Russ. Chem. Bull. 2010, 59, 1137–1144. [Google Scholar]
- Kazheva, O.; Alexandrov, G.; Kravchenko, A.; Starodub, V.; Lobanova, I.; Sivaev, I.; Bregadze, V.; Buravov, L.; Dyachenko, O. First molecular conductors with 8,8'-dibromo cobalt bis(dicarbollide) anion. Solid State Sci. 2008, 10, 1734–1739. [Google Scholar]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Kosenko, I.D.; Lobanova, I.A.; Sivaev, I.B.; Filippov, O.A.; Shubina, E.S.; Bregadze, V.I.; Starodub, V.A.; et al. Molecular conductors with 8-hydroxy cobalt bis(dicarbollide) anion. Inorg. Chem. 2011, 50, 444–450. [Google Scholar]
- Rudakov, D.A.; Shirokii, V.L.; Knizhnikov, V.A.; Bazhanov, A.V.; Vecher, E.I.; Maier, N.A.; Potkin, V.I.; Ryabtsev, A.N.; Petrovskii, P.V.; Sivaev, I.B.; et al. Electrochemical synthesis of halogen derivatives of bis(dicarbollyl)cobalt(III). Russ. Chem. Bull. 2004, 53, 2554–2557. [Google Scholar]
- Zefirov, Y.V. Reduced intermolecular contacts and specific interactions in molecular crystals. Crystallogr. Rep. 1997, 42, 865–886. [Google Scholar]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the charge distribution in BEDT-TTF salts. Synth. Met. 1997, 86, 1973–1974. [Google Scholar]
- Kirillova, N.I.; Zhdanov, A.S; Gusev, A.I.; Kirin, V.N.; Knyazev, S.P.; Sokolova, T.V. The molecular structures of the dicarbaboryl derivatives [3,3'-M(8,1,2-ClC2B9H10)2]K. Organomet. Chem. USSR 1989, 2, 448–450. [Google Scholar]
- Hurlburt, P.K.; Miller, R.L.; Abney, K.D.; Foreman, T.M.; Butcher, R.J.; Kinkead, S.A. New synthetic routes to B-halogenated derivatives of cobalt dicarbollide. Inorg. Chem. 1995, 34, 5215–5219. [Google Scholar]
- Kosenko, I.D.; Lobanova, I.A.; Sivaev, I.B.; Petrovskii, P.V.; Bregadze, V.I. Synthesis of heterosubstituted derivatives of cobalt bis(1,2-dicarbollide). Izv. Akad. Nauk, Ser. Khim. 2011, 60, 2308–2311, Russ. Chem. Bull. 2011, 60, in press. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- APEX2 Softwarwe Package, Bruker AXS: Madison, WI, USA, 2005.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kazheva, O.N.; Aleksandrov, G.G.; Kravchenko, A.V.; Starodub, V.A.; Lobanova, I.A.; Kosenko, I.D.; Sivaev, I.B.; Bregadze, V.I.; Buravov, L.I.; Dyachenko, O.A. New Fulvalenium Salts of Cobalt Bis(dicarbollide): Crystal Structures and Electrical Conductivities. Crystals 2012, 2, 43-55. https://doi.org/10.3390/cryst2010043
Kazheva ON, Aleksandrov GG, Kravchenko AV, Starodub VA, Lobanova IA, Kosenko ID, Sivaev IB, Bregadze VI, Buravov LI, Dyachenko OA. New Fulvalenium Salts of Cobalt Bis(dicarbollide): Crystal Structures and Electrical Conductivities. Crystals. 2012; 2(1):43-55. https://doi.org/10.3390/cryst2010043
Chicago/Turabian StyleKazheva, Olga N., Grigory G. Aleksandrov, Andrey V. Kravchenko, Vladimir A. Starodub, Irina A. Lobanova, Irina D. Kosenko, Igor B. Sivaev, Vladimir I. Bregadze, Lev I. Buravov, and Oleg A. Dyachenko. 2012. "New Fulvalenium Salts of Cobalt Bis(dicarbollide): Crystal Structures and Electrical Conductivities" Crystals 2, no. 1: 43-55. https://doi.org/10.3390/cryst2010043






