(B15c5)BiI3(I2): Molecular Benzo-15-Crown-5―BiI3 Complexes Bridged by Iodine Molecules to Chains
Abstract
: The reaction of bismuth triiodide with iodine and benzo-15-crown-5 in ethanol/dichloromethane yielded red single crystals of (b15c5)BiI3(I2) (monoclinic, P21/c (no. 14), a = 1376.9(1), b = 1172.7(1), c = 1700.2(2) pm, β = 115.197(6), V = 2484.1(4)·106 pm3, Z = 4). Neutral pseudo-octahedral complexes (b15c5)BiI3 are connected by secondary bonding interactions via iodine molecules to chains. Electronic structure calculations of the neutral complex (b15c5)BiI3 reveal that the compound can indeed be described as b15c5 interacting with a molecular BiI3 unit. However, bonding has to be mainly electrostatic as the interactions of the bismuth 6s lone pair with the 2p orbitals of the oxygen atoms of the crown ether are clearly antibonding.1. Introduction
Transition metals form numerous iodido-complexes which are normally anionic, as for example in (NH4)7[HgI4]2[Hg2I7](H2O) [1] or (Bu4N)2[Hg4I10] [2]. In iodine-rich systems, such anions may be bridged by iodine molecules as in [Me3S]2[Hg2I6(I2)6] [3]. Neutral complexes such as [Cd(NH3)4I2] are also known to be incorporated in chains by bridging iodine molecules as in [Cd(NH3)4I2(I2)] [4]. For further examples see [5].
In an attempt to include Pd(II) into crown-ether ligands, we have recently found another example of the first kind of iodine-molecule bridging in [H5O2(db24c8)]2[Pd2I6(I2)] [6]. In the anionic chains, I−—I–I—I− distances are 332.4(2) and 276.5(2) pm, respectively. Thus, the iodine molecule is only slightly elongated when compared with the I–I distance in solid iodine, 271.5(6) pm at 110 K [7,8]. The crown ether dibenzo-24-crown-8 even includes an iodine molecule, in (db24c8)I2 [9], with an I–I distance of only 268.39(7) pm, even closer to gaseous iodine, 267 pm at 360 K [10].
Although not with palladium, crown ethers have been found to be excellent ligands for a large number of mono- to trivalent metal ions, functioning as large templating cations for polyiodide architectures [11,12]. Post-transition metal iodides such as bismuth(III) iodide, BiI3, have also been tested, and one of these reactions yielded red crystalline (b15c5)BiI3(I2).
2. Results and Discussion
2.1. Crystal Structure of (b15c5)BiI3(I2)
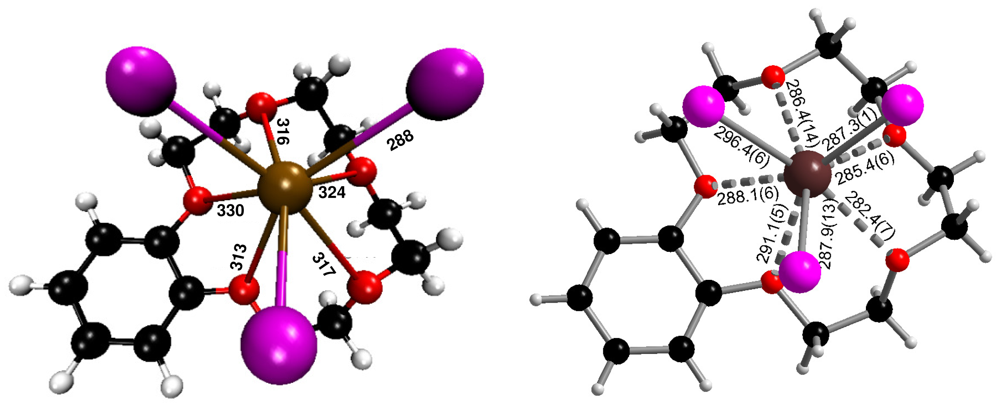
In the crystal structure of (b15c5)BiI3(I2), a bismuth(III) iodide molecule of ψ3-octahedral structure is attached to a benzo-15-crown-5 ligand to form a neutral (b15c5)BiI3 complex. Bi3+−I− distances are 287.9(1) (to I1), 296.4(6) (I2) and 287.3(1) pm (I3), see Figure 1. These distances with an average of 290.5 pm correspond very well to the Bi–I(terminal) distances of 292.0(2) pm in Cs3Bi2I9 [13], which contains confacial bioctahedra [Bi2I9]3−. The distance from the central Bi3+ to the bridging I− in this compound is 324.4(2) pm; for one [BiI6] octahedron they average 308.2 pm, a distance that matches very well the 309.0 pm average of the individual Bi–I distances of 305.41(3) and 312.53(3) pm (three times each) in solid BiI3 [14]. In (b15c5)BiI3(I2), I–Bi–I angles are 88.62(2) (I1–Bi–I2), 92.90(2) (I1–Bi–I3), and 91.45(2) pm (I2–Bi–I3), hence there is a meridional configuration with respect to an octahedron. The five oxygen atoms of benzo-15-crown-5 are situated opposite to the I1–I2–I3 triangle with Bi–O distances ranging from 282.4(7) to 291.1(5) pm, with an average of 286.7 pm.
Isostructural complexes (b15c5)BiX3 with X = Cl, Br have already been characterized [15]. In these complexes the Bi–O distances are 283 (X = Cl) and 285 (Br) pm; hence the Bi–O distances are very similar, as expected, although there seems to be a slight trend from chloride to iodide. The Bi–X distances follow, with averages of 253 (X = Cl), 268 (Br) and 291 (I) pm, the increase of ionic radii from Cl− via Br− to I−. When these (167, 182, and 206 pm) [16] are subtracted from the mentioned averaged Bi–X distances, a reasonable ionic radius for Bi3+ of 85–86 pm is obtained.
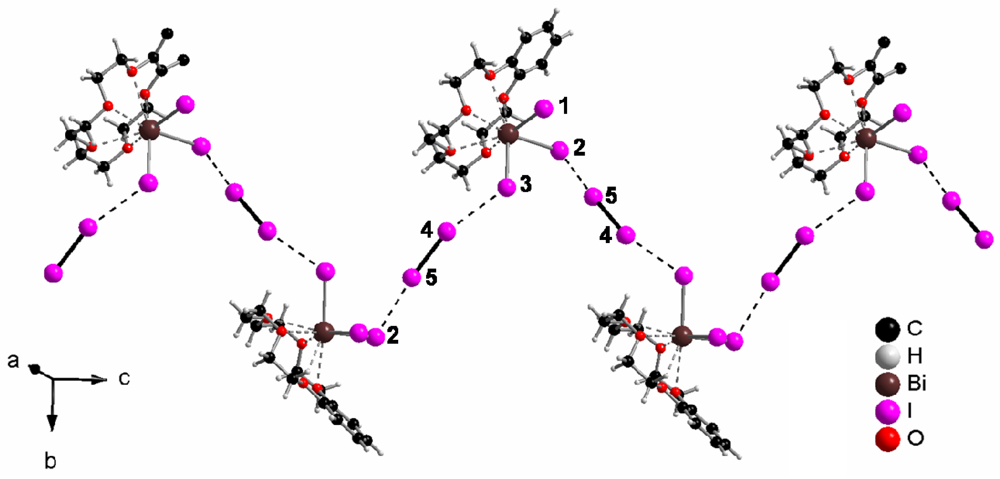
In (b15c5)BiI3(I2), the discrete complexes (b15c5)BiI3 are connected via iodine molecules to zig-zag chains, as Figure 1 shows. The I–I distance in the I2 molecule is, with 273.9(3) pm, only slightly longer than in solid iodine, 271.5(6) pm at 110 K [7,8]. The distances I−–I (from (b15c5) (I-)2Bi3+–I− to the I–I molecule) are 335.0(5) (I2-I5) and 353.7(2) pm (I3-I4), respectively. Both distances are close to the sum of the ionic radius of iodide (206 pm) and the atomic radius of iodine (140 pm) [17], 346 pm. The latter distance, 354 pm, is close to I–I distances between neighboring iodine molecules in solid iodine (350 pm). Both distances are considerably shorter than twice the van der Waals radius [18] of iodine, 2 × 198 = 396 pm. In summary, iodide–iodine interactions that form the zig-zag chains, qualify as “secondary bonding” [19].
Topologically, the zig-zag chains are arranged in layers, as Figure 2 shows, and the layers are stacked parallel to [100], as Figure 3 exhibits.
Trivalent bismuth has an electronic configuration of [Xe]6s25d104f14, hence filled 4f and 5d subshells and a lone 6s2 electron pair. One could, therefore, believe that the special architecture of the (b15c5)BiI3 complex were due to the 6s2 pair being stereochemically active, as was suggested previously for (b15c5)BiX3 (X = Cl, Br) [15]. If this were the case, a ψ1-tetrahedral (with I–Bi–I angles around 109°) rather than a ψ3-octahedral structure (with I–Bi–I angles around 90°) would be expected. Indeed, it has been shown recently that lone-pair ions such as Tl(I)—isoelectronic with Bi(III)—suffer from 6s2-lone-pair–oxygen-2p orbital antibonding interactions leading to structural distortions [20].
2.2. Electronic Structure of (b15c5)BiI3
As the interactions between the molecular complex (b15c5)BiI3 and the iodine molecules in (b15c5)BiI3(I2) qualify as “secondary bonding”, calculations of the electronic structure have only been carried out for the (b15c5)BiI3 complex. This subunit can be structurally described as a BiI3 molecule interacting with the crown ether b15c5. Albeit BiI3 itself forms an extended structure [14], it appears that the interaction with the crown ether favors the formation of a molecular unit.
Ab initio calculations were carried out with the aid of density functional theory (DFT); for technical details see Experimental Section and Supporting Information. Figure 4 shows the optimized ground state geometry of (b15c5)BiI3 in comparison with the corresponding structural motif as observed in the crystal structure of (b15c5)BiI3(I2). No geometric or symmetry restraints were applied during the optimization process.
The theoretically calculated gas phase ground state geometry compares quite well with the experimentally observed structure of the complex. With 288 pm, the calculated interatomic Bi–I distances of the complex (b15c5)BiI3 are slightly shorter than the average of the experimentally observed distances, 291 pm. However, as the calculations are done for T = 0 K and, furthermore, two of the iodide ions are connected to iodine molecules in (b15c5)BiI3(I2), these small deviations are no surprise. The mean interatomic Bi–O distances for the calculated (b15c5)BiI3 unit are somewhat larger (324 pm) than experimentally observed distances, 291 pm, which is perhaps the result of packing effects in solid (b15c5)BiI3(I2).
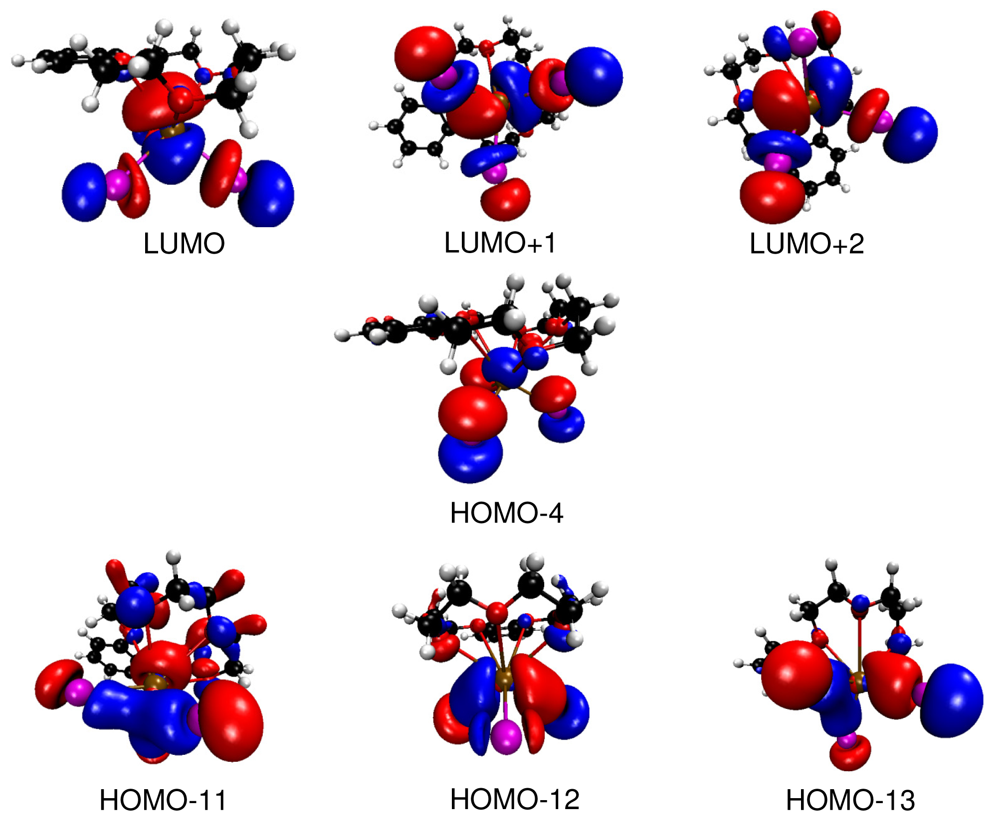
Bi—I bonding interactions as represented by the HOMOs -11, -12 and -13 (Figure 5) are found at comparatively low energies. They are formed by interaction of iodine 6p orbitals with bismuth 6p orbitals. These molecular orbitals show only small contributions from the crown ether. The empty LUMOs (LUMO, LUMO+1 and LUMO+2) correspond to the anti-bonding set of the bismuth 6p and iodine 6p interactions. Again, only a small contribution of the crown ether moiety to these orbitals is observed. Thus, it is indeed justified to speak of a molecular BiI3 unit interacting with the crown ether b15c5. However, the attractive BiI3—crown-ether interactions have to be mainly electrostatic as HOMO-4, which is basically Bi—I nonbonding, shows a clearly antibonding interaction of the 6s orbital of bismuth with the 2p orbitals of the oxygen atoms of the crown ether. This is a similar situation as found for the interaction of isolated Tl+ cations with organic macrocycles [20]. In this case the combination of the occupied 6s orbital of Tl+ with the oxygen 2p orbitals of the macrocycle lead to fully occupied bonding and anti-bonding levels, and a subtle counterplay between repulsive covalent interactions and attractive electrostatic forces is observed. In the case of (b15c5)BiI3 where BiI3 interacts with the organic macrocycle b15c5 a similar bonding scenario is found. However, here it is not an isolated cation but a molecular BiI3 unit which interacts. Thus, the anti-bonding interaction forms by a Bi-I group orbital with strong Bi-6s contribution.
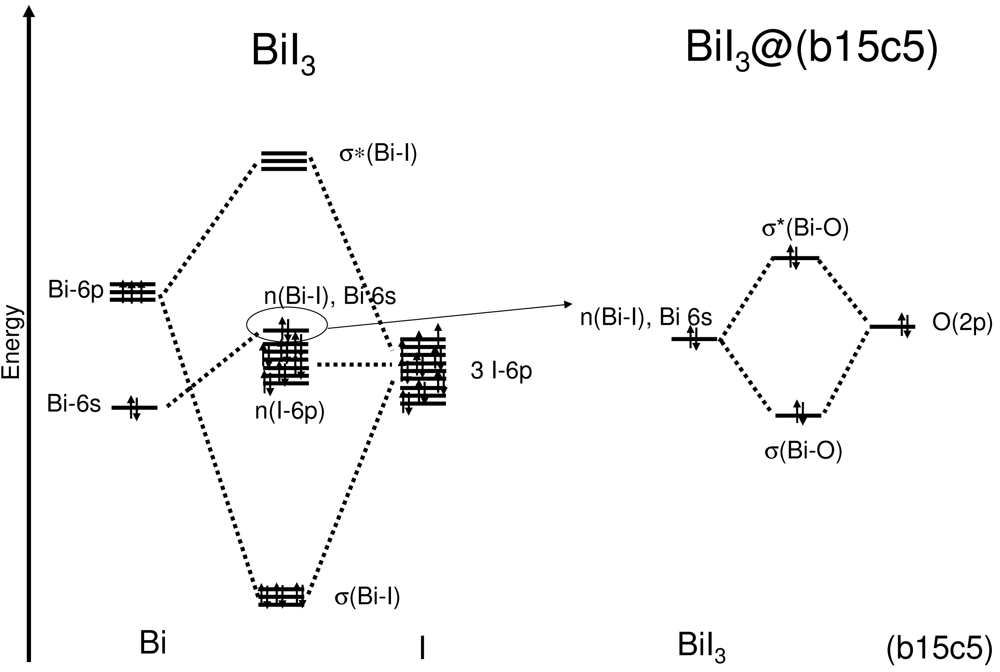
A simplified, schematic energy diagram describing the interaction between bismuth and iodine to form BiI3 and then between the BiI3 fragment and the crown ether yielding (b15c5)BiI3 is depicted in Figure 6.
3. Experimental Section
Anhydrous bismuth triiodide, BiI3 (Alfa Aesar, 99.999%, 0.059 g, 0.1 mmol), benzo-15-crown-5, b15c5 (Alfa Aesar, 98%, 0.025 g, 0.1 mmol) and iodine, I2 (Merck, 0.888 g, 3.5 mmol) were dissolved in 40 mL of a 1:1 mixture of ethanol and dichloromethane. Slow evaporation of the solvent yielded red polyhedral single crystals of (b15c5)BiI3(I2).
Single crystals were selected under a microscope and mounted in thin-walled glass capillaries. Their quality was checked on a single-crystal X-ray diffractometer (Stoe Image Plate Diffraction System, IPDS II) and a complete intensity data set was collected at ambient temperature using graphite-monochromated Mo-Kα radiation. The data were corrected for Lorentz and polarization effects. A numerical absorption correction based on crystal-shape optimization was applied for all data; the programs used in this work are Stoe's X-Area, including X-RED and X-SHAPE for data reduction and absorption correction [21,22]. The WinGX suite of programs [23], including SIR-92 [24] and the SHELX programs [25-27] were used for structure solution and refinement. Hydrogen atoms were placed in idealized positions and constrained to ride on their respective parent atom. The last refinement cycles included atomic positions for all atoms, anisotropic thermal parameters for all non-hydrogen atoms and isotropic thermal parameters for all hydrogen atoms. Crystallographic data for the structure have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-838985. Copies of the data can be obtained, free of charge, on application to CHGC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223 336033 or e-mail: [email protected]).
Crystal data for (b15c5)BiI3(I2). C14H20O5I5Bi, 1111.78 g mol−1. Monoclinic, P21/c (no. 14), a = 1376.9(1), b = 1172.7(1), c = 1700.2(2) pm, β = 115.197(6)°, V = 2484.1(4)·106 pm3, Z = 4. Diffractometer IPDS-II, Stoe Darmstadt; Mo-Kα (graphite monochromator, λ = 71.073 pm); T = 293(2) K; 4.36° ≤ 2θmax ≤ 54.62°; 0° ≤ ω ≤ 180°, φ = 0°; 0° ≤ ω < 88°, φ = 90°; Δω = 2°; 134 images; −17 ≤ h ≤ 15, −15 ≤ k ≤ 15, −21 ≤ l ≤ 21; ρcalc = 2.973 g cm−3; 17644 reflections measured of which 5371 were symmetrically independent; Rint = 0.0470; F(000) = 1968; μ = 13.330 mm−1. 229 refined parameters; R values: R1/wR2 for 4389 reflections with [I0 > 2σ(I0)]: 0.0290/0.0637, for all data: 0.0395/0.0713; Sall = 1.047; Δρ(min/max): −1.236·× 10−6 pm−3 / +1.515·× 10−6 pm−3.
For the calculations of the electronic structure of (b15c5)BiI3, the BP86 hybrid functional [28] was used to describe exchange and correlation in combination with a doubled polarized triplet-ζ-valence basis set (def2-TZVPP) [29,30] within the framework of the RI-approximation [31,32]. This method is well known for obtaining accurate results concerning geometries and vibrational spectra especially for metal complexes [33]. The quantum chemistry program package TURBOMOLE (V. 6.1) [34] was used for all calculations.


Acknowledgments
This work was generously supported by the State of Nordrhein-Westfalen through the Universität zu Köln and the Ruhr-Universität Bochum.
References
- Nockemann, P.; Pantenburg, I.; Meyer, G. Tetrahedra and Vertex-Sharing Double Tetrahedra in the Ammonium Iodomercurates(II) (NH4)7[HgI4]2[Hg2I7](H2O) and (NH4)3[Hg2I7]. Z. Anorg. Allg. Chem. 2006, 632, 1972–1974. [Google Scholar]
- Nockemann, P.; Meyer, G. Bis(tetrabutylammonium)dodecaiodotetra-mercurate(II), (Bu4N)2[Hg4I10]. Acta Crystallogr. 2003, E59, m236–m238. [Google Scholar]
- Stegemann, H.; Tebbe, K.-F.; Bengtsson, L.A. Der erste Polyiodokomplex—Triethylsulfoniumtriiodomercurat(II)-tris(diiod), (Et3S)[Hg2I6]1/2·3I2. Z. Anorg. Allg. Chem. 1995, 621, 165–170. [Google Scholar]
- Tebbe, K.-F.; Plewa, M. Untersuchungen an Polyhalogeniden. IV. Tetrammin-cadmium-tetraiodid, [Cd(NH3)4I2·I2], und Tetrammin-cadmium-hexaiodid, [Cd(NH3)4(I·I2)2]. Z. Anorg. Allg. Chem. 1982, 489, 111–125. [Google Scholar]
- Svensson, P.H.; Kloo, L. Synthesis, Structure, and Bonding in Polyiodide and Metal Iodide–Iodine Systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar]
- Walbaum, C.; Pantenburg, I.; Meyer, G. Molecular iodine catenation in the anionic chains [Pd2I6(I2)]2−. Cryst. Res. Technol. 2008, 43, 1183–1186. [Google Scholar]
- Harris, P.M.; Mack, E.; Blake, F.C. The atomic arrangement in the crystal of orthorhombic iodine. J. Am. Chem. Soc. 1928, 50, 1583–1600. [Google Scholar]
- Van Bolhuis, F.; Koster, P.B.; Migchelsen, T. Refinement of the crystal structure of iodine at 110° K. Acta Crystallogr. 1967, 23, 90–91. [Google Scholar]
- Walbaum, C.; Pantenburg, I.; Meyer, G. Iodine Molecules Included in the Structure of Dibenzo-24-Crown-8, (I2)@(db24c8). Crystals 2011, 1, 215–219. [Google Scholar]
- Karle, I.L. Anomalous Electron Scattering from Iodine Vapor. J. Chem. Phys. 1955, 23, 1739. [Google Scholar]
- Walbaum, C. Neue Poly(inter)halogenide mit Kronenether-stabilisierten Kationen. PhD Dissertation, Universität zu Köln, Köln, Germany, 2009. [Google Scholar]
- Fiolka, C. Polyiodide komplexer Übergangsmetalle. PhD Dissertation, Universität zu Köln, Köln, Germany, 2010. [Google Scholar]
- Chabot, B.; Parthé, E. Cs3Sb2I9 and Cs3Bi2I9 with the Hexagonal Cs3Cr2Cl9 Structure Type. Acta Cryst. 1978, B34, 645–648. [Google Scholar]
- Ruck, M. Darstellung und Kristallstruktur von fehlordnungsfreiem Bismuttriodid. Z. Kristallogr. 1995, 210, 650–655. [Google Scholar]
- Rogers, R.D.; Bond, A.H.; Aguinaga, S.; Reves, A. Complexation Chemistry of Bismuth(III) Halides with Crown Ethers and Polyethelyne Glycols. Structural Manifestations of a Stereochemically Active Lone Pair. J. Am. Chem. Soc. 1992, 114, 2967–2977. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar]
- Slater, J.C. Atomic Radii in Crystals. J. Chem. Phys. 1964, 41, 3199–3205. [Google Scholar]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar]
- Alcock, N.W. Secondary Bonding to Nonmetallic Elements. Adv. Inorg. Chem. Radiochem. 1972, 15, 1–58. [Google Scholar]
- Mudring, A.-V.; Rieger, F. Lone Pair Effect in Thallium(I) Macrocyclic Compounds. Inorg. Chem. 2005, 44, 6240–6243. [Google Scholar]
- X-RED 1.22, Stoe Data Reduction Program (C); Stoe & Cie GmbH: Darmstadt, Germany, 1999.
- X-Shape 1.06, Crystal Optimisation for Numerical Absorption Correction (C); Stoe & Cie GmbH: Darmstadt, Germany, 1999.
- Farrugia, L.J. WinGX, A MS-Windows System of Programs for Solving, Refining and Analysing Single X-ray Diffraction Data for Small Molecules; University of Glasgow: Glasgow, Scotland, 2005. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Gualardi, A. SIR92, A Program for Automatic Solution of Crystal Structures by Direct Methods. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for Structure Analysis; University of Göttingen: Göttingen, Germany, 1998. [Google Scholar]
- Sheldrick, G.M. SHELXL-93, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1993. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys.Rev. 1988, A38, 3098–3100. [Google Scholar]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets of Triple Zeta Valence Quality for Atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design an assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized accurate auxiliary basis sets for RI-MP2 and RI-CC2 calculations for the atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar]
- Ahlrichs, R. Efficient evaluation of three-center two-electron integrals over Gaussian functions. Phys. Chem. Chem. Phys. 2004, 6, 5119–5121. [Google Scholar]
- Bühl, M.; Kabrede, H. Geometries of Transition-Metal Complexes from Density Functional Theory. J. Chem. Theory Comput. 2006, 2, 1282–1290. [Google Scholar]
- TURBOMOLE V6.1 2009—A development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, TURBOMOLE GmbH, since 2007
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fiolka, C.; Richter, M.; Pantenburg, I.; Mudring, A.-V.; Meyer, G. (B15c5)BiI3(I2): Molecular Benzo-15-Crown-5―BiI3 Complexes Bridged by Iodine Molecules to Chains. Crystals 2011, 1, 220-228. https://doi.org/10.3390/cryst1040220
Fiolka C, Richter M, Pantenburg I, Mudring A-V, Meyer G. (B15c5)BiI3(I2): Molecular Benzo-15-Crown-5―BiI3 Complexes Bridged by Iodine Molecules to Chains. Crystals. 2011; 1(4):220-228. https://doi.org/10.3390/cryst1040220
Chicago/Turabian StyleFiolka, Christoph, Mark Richter, Ingo Pantenburg, Anja-Verena Mudring, and Gerd Meyer. 2011. "(B15c5)BiI3(I2): Molecular Benzo-15-Crown-5―BiI3 Complexes Bridged by Iodine Molecules to Chains" Crystals 1, no. 4: 220-228. https://doi.org/10.3390/cryst1040220



