Hydrogen Production from Chemical Looping Steam Reforming of Ethanol over Perovskite-Type Oxygen Carriers with Bimetallic Co and Ni B-Site Substitution
Abstract
:1. Introduction
2. Results and Discussion
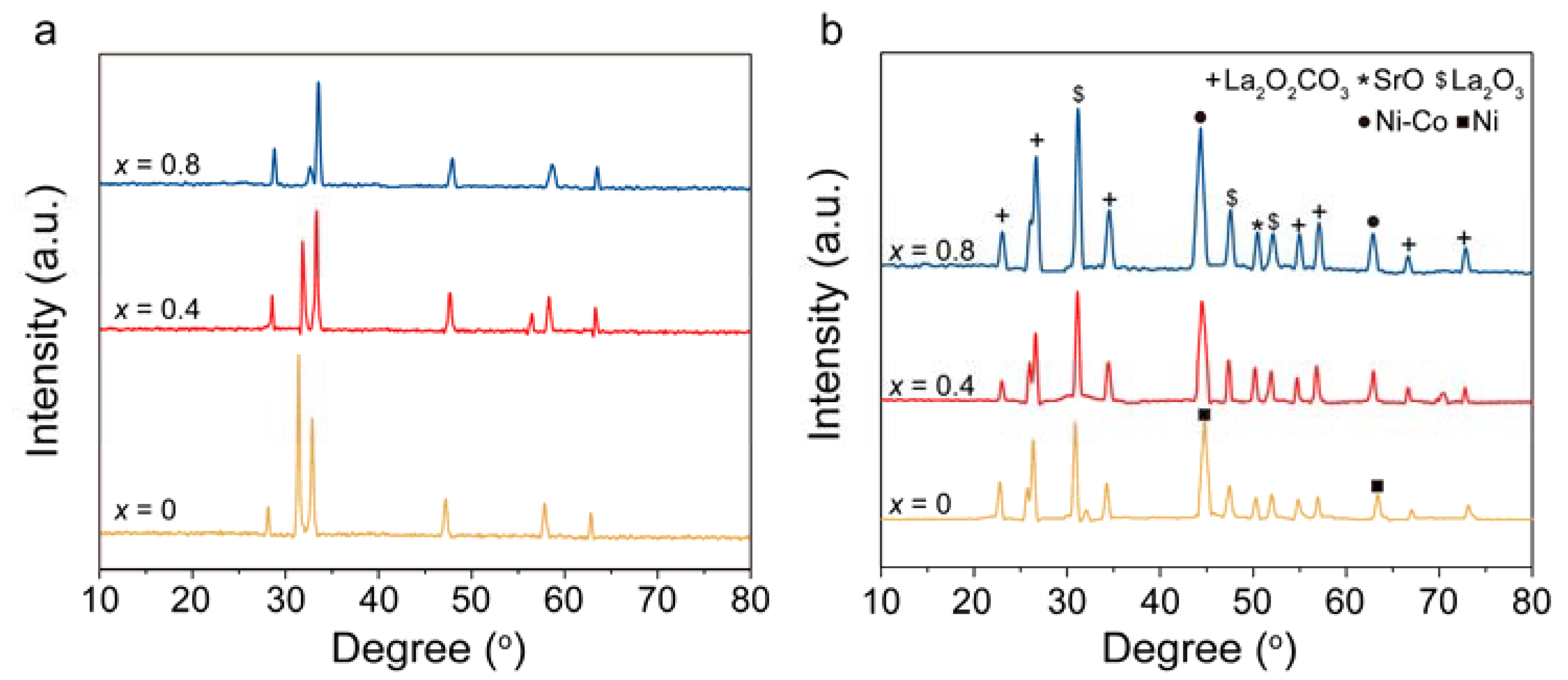
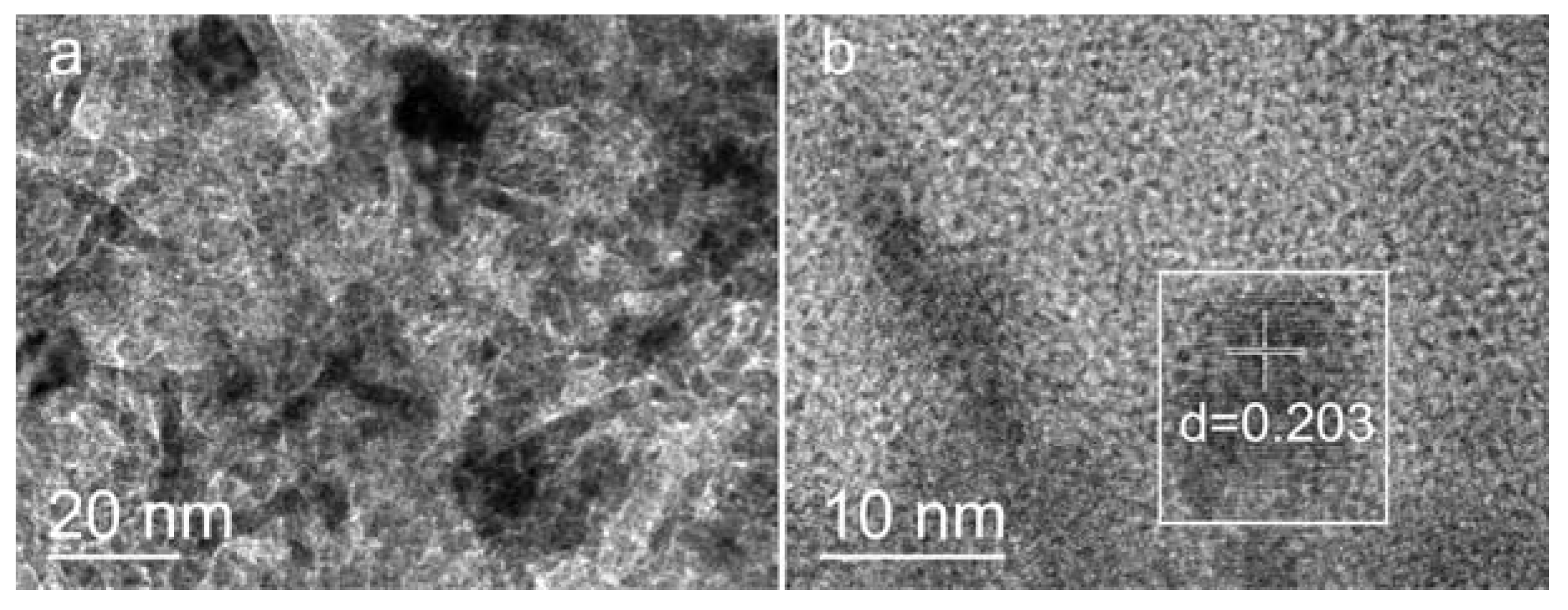
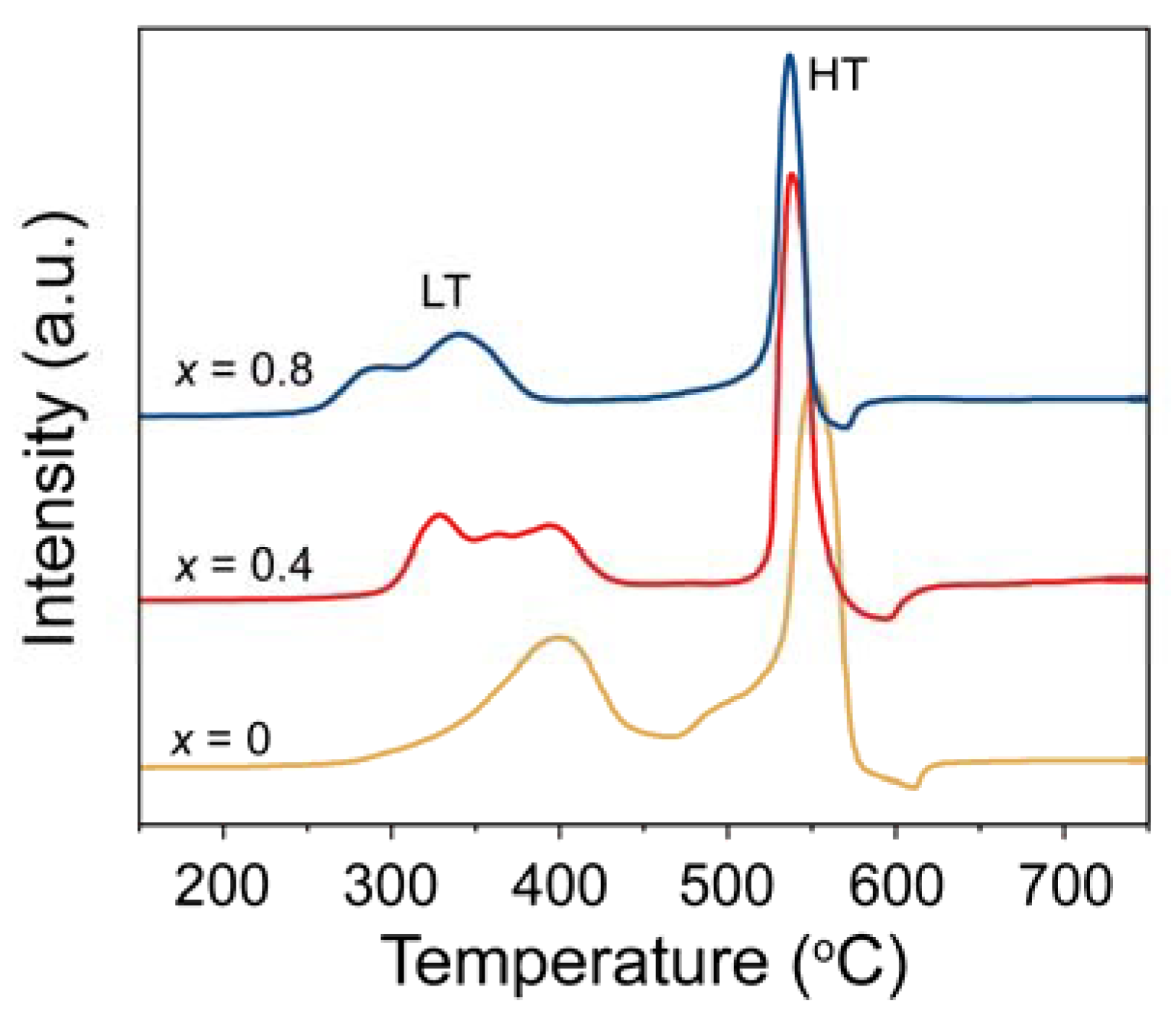
2.1. Characterization of OCs
2.2. Activity Tests of OCs
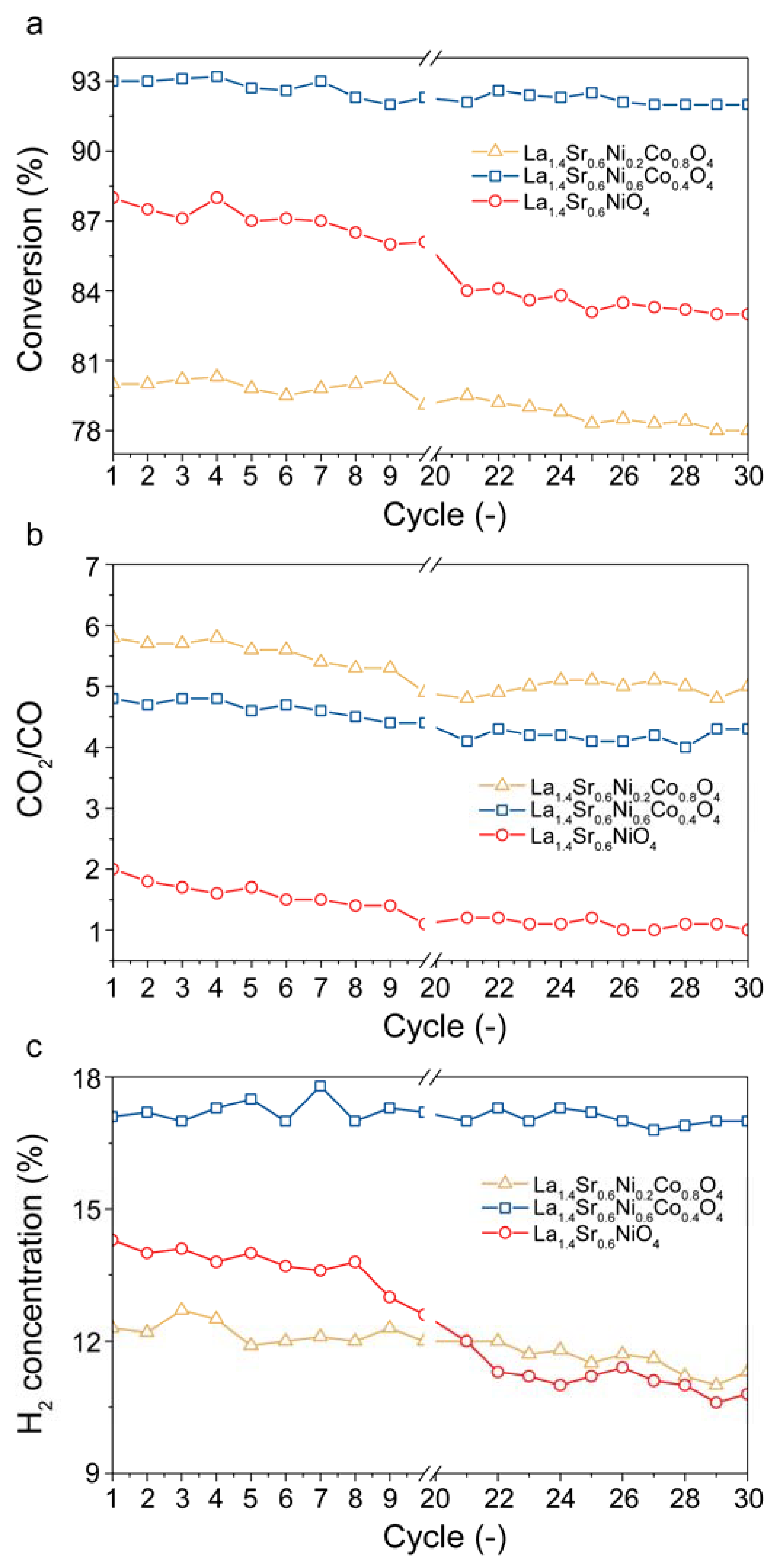
2.3. Stability Tests
3. Materials and Methods
3.1. Preparation of OCs
3.2. Characterization of OCs
3.3. Activity and Stability Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Zhang, C.; Li, S.; Huang, Z.; Yan, S.; Wang, S.; Ma, X.; Gong, J. Sorption enhanced steam reforming of ethanol on Ni-CaO-Al2O3 multifunctional catalysts derived from hydrotalcite-like compounds. Energy Environ. Sci. 2012, 5, 8942–8949. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Cheng, Z.; Luo, X.; Jia, L.; Song, M.; Jiang, B.; Dou, B. Sorption-enhanced steam reforming of glycerol for hydrogen production over a NiO/NiAl2O4 Catalyst and Li2ZrO3-Based Sorbent. Energy Fuel 2015, 29, 7408–7418. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K.; Chen, H.; Xu, Y. Hydrogen production by sorption-enhanced chemical looping steam reforming of ethanol in an alternating fixed-bed reactor: Sorbent to catalyst ratio dependencies. Energy Convers. Manag. 2018, 155, 243–252. [Google Scholar] [CrossRef]
- Li, L.; Song, Y.; Jiang, B.; Wang, K.; Zhang, Q. A novel oxygen carrier for chemical looping reforming: LaNiO3 perovskite supported on montmorillonite. Energy 2017, 131, 58–66. [Google Scholar] [CrossRef]
- Wang, K.; Dou, B.; Jiang, B.; Song, Y.; Zhang, C.; Zhang, Q.; Chen, H.; Xu, Y. Renewable hydrogen production from chemical looping steam reforming of ethanol using xCeNi/SBA-15 oxygen carriers in a fixed-bed reactor. Int. J. Hydrog. Energy 2016, 41, 12899–12909. [Google Scholar] [CrossRef]
- Jiang, B.; Li, L.; Bian, Z.; Li, Z.; Othman, M.; Sun, Z.; Tang, D.; Kawi, S.; Dou, B. Hydrogen generation from chemical looping reforming of glycerol by Ce-doped nickel phyllosilicate nanotube oxygen carriers. Fuel 2018, 222, 185–192. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Tang, D.; Song, Y.; Jiang, B.; Zhang, Q. Hydrogen production from ethanol steam reforming on Ni-Ce/MMT catalysts. Energy 2018, 149, 937–943. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, C.; Wang, K.; Dou, B.; Song, Y.; Chen, H.; Xu, Y. Highly dispersed Ni/montmorillonite catalyst for glycerol steam reforming: Effect of Ni loading and calcination temperature. Appl. Therm. Eng. 2016, 109, 99–108. [Google Scholar] [CrossRef]
- Li, L.; Jiang, B.; Tang, D.; Zhang, Q.; Zheng, Z. Hydrogen generation by acetic acid steam reforming over Ni-based catalysts derived from La1−xCexNiO3 perovskite. Int. J. Hydrog. Energy 2018, 43, 6795–6803. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Jiang, B.; Wang, K.; Tang, D.; Dou, B. An intelligent oxygen carrier of La2−xSrxNiO4−λ for hydrogen production by chemical looping reforming of ethanol. Int. J. Hydrog. Energy 2017, 42, 17102–17111. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Barbero, B.P.; Gamboa, J.A.; Cadús, L.E. Synthesis and characterisation of La1−xCaxFeO3 perovskite-type oxide catalysts for total oxidation of volatile organic compounds. Appl. Catal. B 2006, 65, 21–30. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. LaNiO3 perovskite catalyst precursor for rapid decomposition of methane: Influence of temperature and presence of H2 in feed stream. Catal. Today 2011, 171, 24–35. [Google Scholar] [CrossRef]
- Morales, M.; Segarra, M. Steam reforming and oxidative steam reforming of ethanol over La0.6Sr0.4CoO3−δ perovskite as catalyst precursor for hydrogen production. Appl. Catal. A 2015, 502, 305–311. [Google Scholar] [CrossRef]
- Wu, G.; Li, S.; Zhang, C.; Wang, T.; Gong, J. Glycerol steam reforming over perovskite-derived nickel-based catalysts. Appl. Catal. B 2014, 144, 277–285. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Cui, G.; Wang, Z.; Jiang, B.; Wang, K.; Chen, H.; Xu, Y. Hydrogen production and reduction of Ni-based oxygen carriers during chemical looping steam reforming of ethanol in a fixed-bed reactor. Int. J. Hydrog. Energy 2017, 42, 26217–26230. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Wang, K.; Zhang, C.; Li, M.; Chen, H.; Xu, Y. Sorption enhanced steam reforming of biodiesel by-product glycerol on Ni-CaO-MMT multifunctional catalysts. Chem. Eng. J. 2017, 313, 207–216. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, C.; Chen, Y.; Cai, X.; Dou, B.; Chen, H.; Xu, Y.; Jiang, B.; Wang, K. High purity hydrogen production from sorption enhanced chemical looping glycerol reforming: Application of NiO-based oxygen transfer materials and potassium promoted Li2ZrO3 as CO2 sorbent. Appl. Therm. Eng. 2017, 124, 454–465. [Google Scholar] [CrossRef]
- Lim, H.S.; Kang, D.; Lee, J.W. Phase transition of Fe2O3–NiO to NiFe2O4 in perovskite catalytic particles for enhanced methane chemical looping reforming-decomposition with CO2 conversion. Appl. Catal. B 2017, 202, 175–183. [Google Scholar] [CrossRef]
- Zhao, L.; Han, T.; Wang, H.; Zhang, L.; Liu, Y. Ni-Co alloy catalyst from LaNi1−xCoxO3 perovskite supported on zirconia for steam reforming of ethanol. Appl. Catal. B 2016, 187, 19–29. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, L.; Tian, H.; Li, D.; Wang, X.; Li, X.; Gong, J. Efficient hydrogen production from ethanol steam reforming over La-modified ordered mesoporous Ni-based catalysts. Appl. Catal. B 2016, 181, 321–331. [Google Scholar] [CrossRef]
- Taihei, N.; Motohiko, M.; Makoto, M. The Valence Control and Catalytic Properties of La2−xSrxNiO4. Bull. Chem. Soc. Jpn. 1988, 61, 3831–3837. [Google Scholar]
- Nakamura, T.; Misono, M.; Yoneda, Y. Reduction-oxidation and catalytic properties of La1−xSrxCoO3. J. Catal. 1983, 83, 151–159. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K.-I. Titania-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2005, 232, 268–275. [Google Scholar] [CrossRef]
- Valderrama, G.; Goldwasser, M.R.; de Navarro, C.U.; Tatibouët, J.M.; Barrault, J.; Batiot-Dupeyrat, C.; Martinez, F. Dry reforming of methane over Ni perovskite type oxides. Catal. Today 2005, 107–108, 785–791. [Google Scholar] [CrossRef]
- Müller, C.A.; Maciejewski, M.; Koeppel, R.A.; Baiker, A. Combustion of Methane over Palladium/Zirconia Derived from a Glassy Pd–Zr Alloy: Effect of Pd Particle Size on Catalytic Behavior. J. Catal. 1997, 166, 36–43. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; Goldwasser, M.R. La-Sr-Ni-Co-O based perovskite-type solid solutions as catalyst precursors in the CO2 reforming of methane. J. Power Sources 2010, 195, 1765–1771. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Song, Y.; Zhang, C.; Du, B.; Chen, H.; Wang, C.; Xu, Y. Hydrogen production from chemical looping steam reforming of glycerol by Ni-based oxygen carrier in a fixed-bed reactor. Chem. Eng. J. 2015, 280, 459–467. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, B.; Wang, K.; Zhang, C.; Song, Y.; Chen, H.; Xu, Y. Hydrogen production by chemical looping steam reforming of ethanol using NiO/montmorillonite oxygen carriers in a fixed-bed reactor. Chem. Eng. J. 2016, 298, 96–106. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H.I. Reactivity and stability of Co-Ni/Al2O3 oxygen carrier in multicycle CLC. AIChE J. 2007, 53, 1817–1829. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Gong, J. Catalytic reforming of oxygenates: State of the art and future prospects. Chem. Rev. 2016, 116, 11529–11653. [Google Scholar] [CrossRef] [PubMed]
- Sinfelt, J.H.; Yates, D.J.C. Catalytic hydrogenolysis of ethane over the noble metals of Group VIII. J. Catal. 1967, 8, 82–90. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. Investigation of steam reforming of acetic acid to hydrogen over Ni–Co metal catalyst. J. Mol. Catal. A Chem. 2007, 261, 43–48. [Google Scholar] [CrossRef]
- Shafiefarhood, A.; Galinsky, N.; Huang, Y.; Chen, Y.; Li, F. Fe2O3@LaxSr1−xFeO3 core–shell redox catalyst for methane partial oxidation. ChemCatChem 2014, 6, 790–799. [Google Scholar] [CrossRef]
- Chen, D.; Christensen, K.O.; Ochoa-Fernández, E.; Yu, Z.; Tøtdal, B.; Latorre, N.; Monzón, A.; Holmen, A. Synthesis of carbon nanofibers: Effects of Ni crystal size during methane decomposition. J. Catal. 2005, 229, 82–96. [Google Scholar] [CrossRef]
- Mei, D.; Lebarbier Dagle, V.; Xing, R.; Albrecht, K.O.; Dagle, R.A. Steam reforming of ethylene glycol over MgAl2O4 supported Rh, Ni, and Co catalysts. ACS Catal. 2016, 6, 315–325. [Google Scholar] [CrossRef]
- Deng, J.; Cai, M.; Sun, W.; Liao, X.; Chu, W.; Zhao, X.S. Oxidative methane reforming with an intelligent catalyst: Sintering-tolerant supported nickel nanoparticles. ChemSusChem 2013, 6, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Mawdsley, J.R.; Vaughey, J.T.; Krause, T.R. Neutron diffraction studies of nickel-containing perovskite oxide catalysts exposed to autothermal reforming environments. Chem. Mater. 2009, 21, 4830–4838. [Google Scholar] [CrossRef]
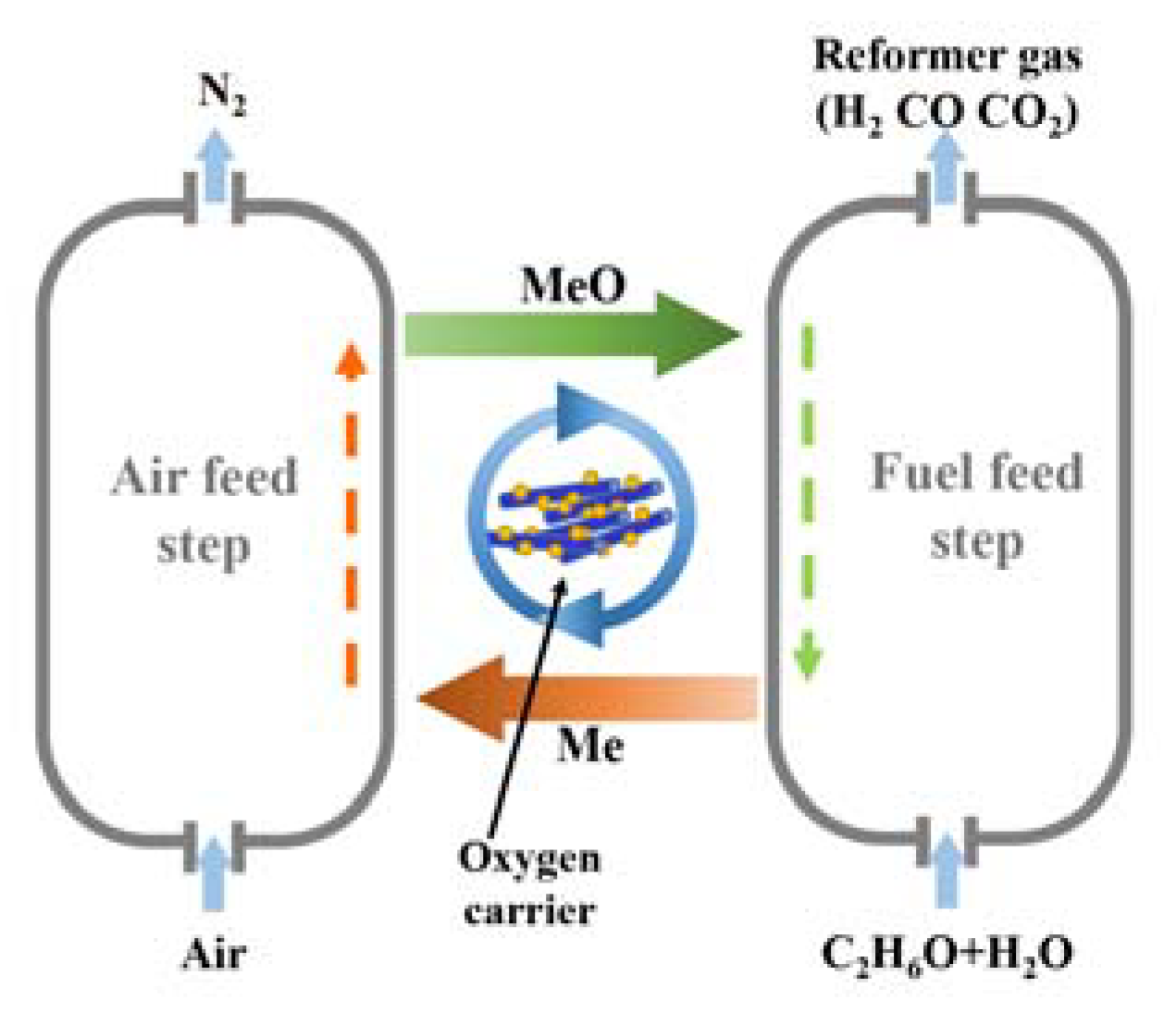


| Step | Reactions |
|---|---|
| Fuel Feed Step | |
| Air Feed Step |
| Sample | Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) | Ni Content a (wt.%) | Co Content a (wt.%) | Ni crystal Size after Reduction b (nm) |
|---|---|---|---|---|---|---|
| La1.4Sr0.6NiO4 | 5.3 | 40.3 | 0.02 | 16.87 | 0 | 14.5 |
| La1.4Sr0.6Ni0.6Co0.4O4 | 6.1 | 44.1 | 0.03 | 10.32 | 5.99 | 13.7 |
| La1.4Sr0.6Ni0.2Co0.8O4 | 6.7 | 41.9 | 0.03 | 4.26 | 11.14 | 12.8 |
| Metal Particle | Ni (111) | Co0.4-Ni | Co0.8-Ni | Co (111) |
|---|---|---|---|---|
| d spacing/Å | 2.026 | 2.031 | 2.050 | 2.052 |
| 2θ/° | 44.7 | 44.6 | 44.5 | 44.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jiang, B.; Sun, Z.; Zhang, Q.; Li, D.; Tang, D. Hydrogen Production from Chemical Looping Steam Reforming of Ethanol over Perovskite-Type Oxygen Carriers with Bimetallic Co and Ni B-Site Substitution. Catalysts 2018, 8, 372. https://doi.org/10.3390/catal8090372
Li L, Jiang B, Sun Z, Zhang Q, Li D, Tang D. Hydrogen Production from Chemical Looping Steam Reforming of Ethanol over Perovskite-Type Oxygen Carriers with Bimetallic Co and Ni B-Site Substitution. Catalysts. 2018; 8(9):372. https://doi.org/10.3390/catal8090372
Chicago/Turabian StyleLi, Lin, Bo Jiang, Zhehao Sun, Qian Zhang, Duyu Li, and Dawei Tang. 2018. "Hydrogen Production from Chemical Looping Steam Reforming of Ethanol over Perovskite-Type Oxygen Carriers with Bimetallic Co and Ni B-Site Substitution" Catalysts 8, no. 9: 372. https://doi.org/10.3390/catal8090372





