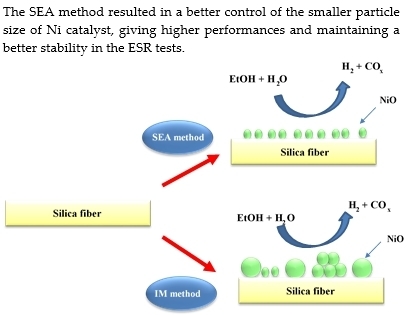
Investigation of Ni/SiO2 Fiber Catalysts Prepared by Different Methods on Hydrogen production from Ethanol Steam Reforming
Abstract
:1. Introduction
2. Results and Discussion
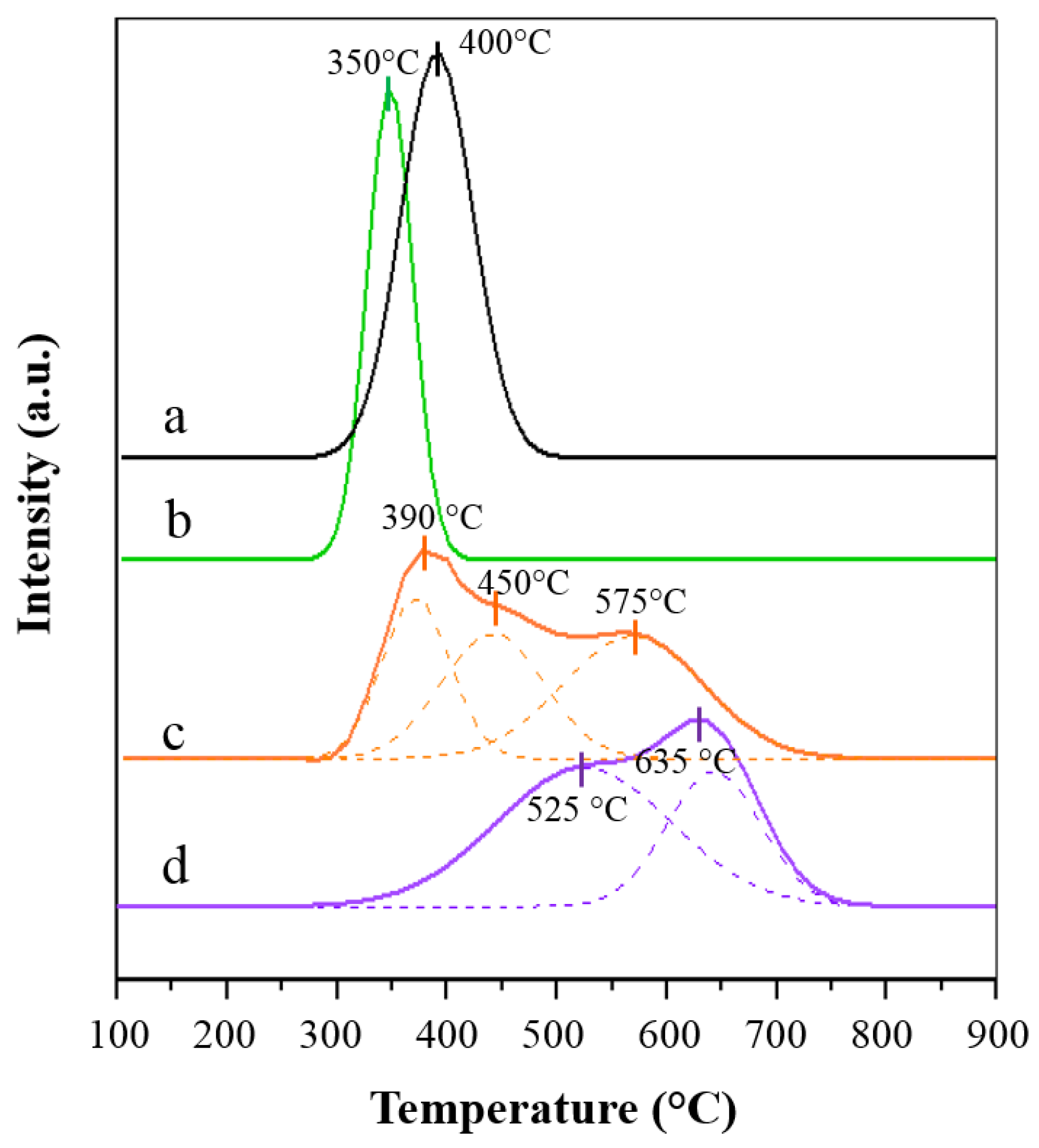
2.1. Characterization of the Catalysts
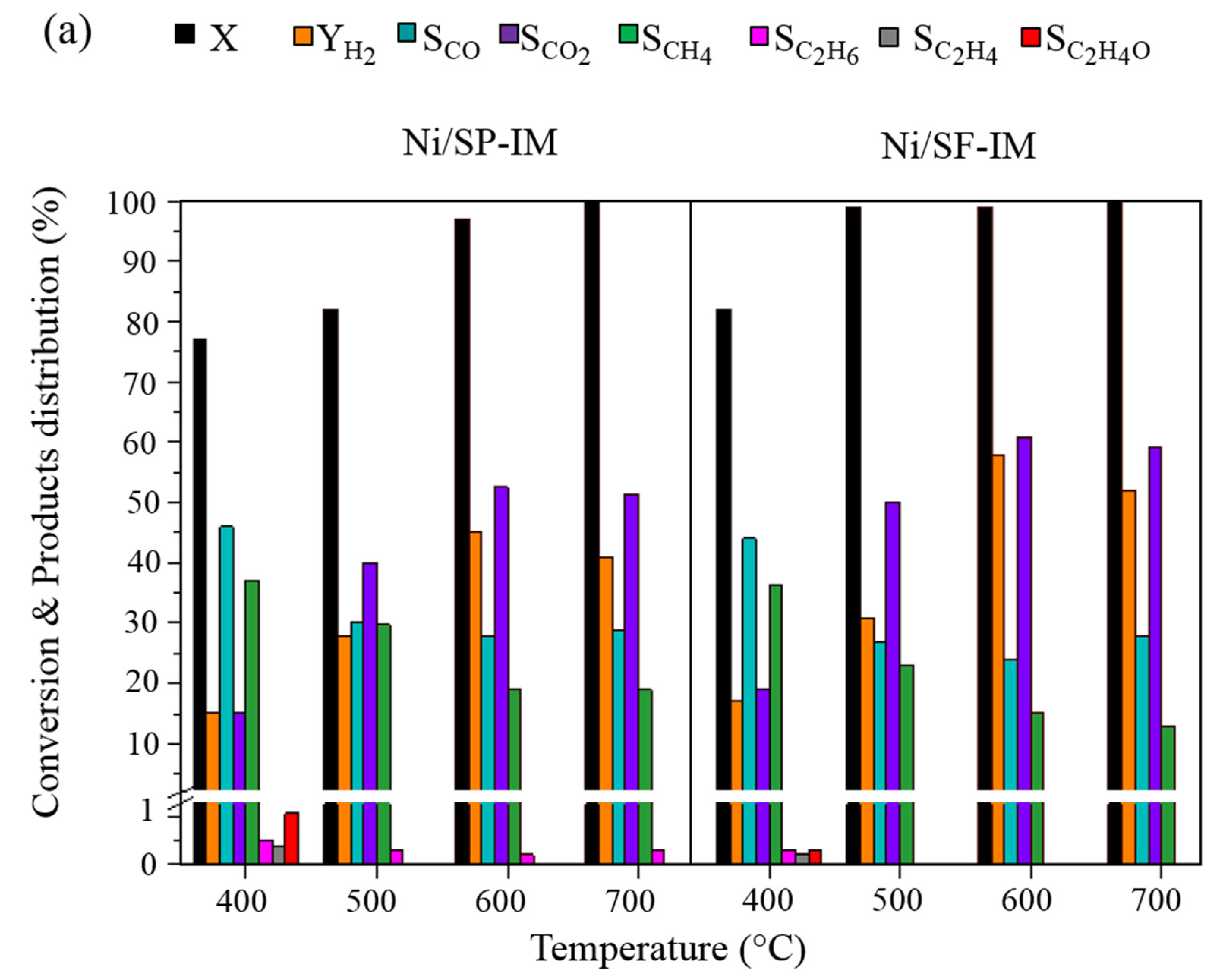
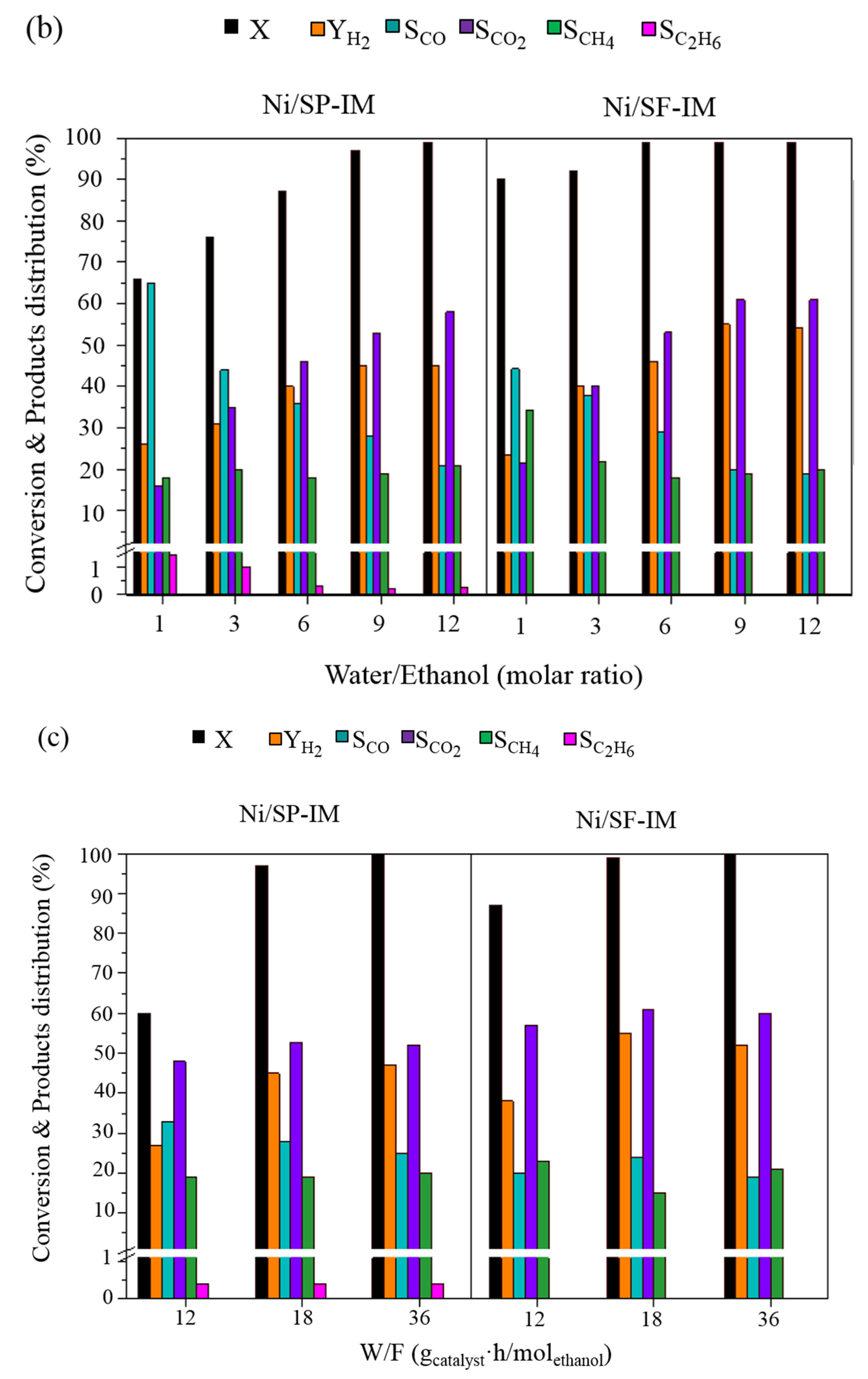
2.2. Catalytic Performance
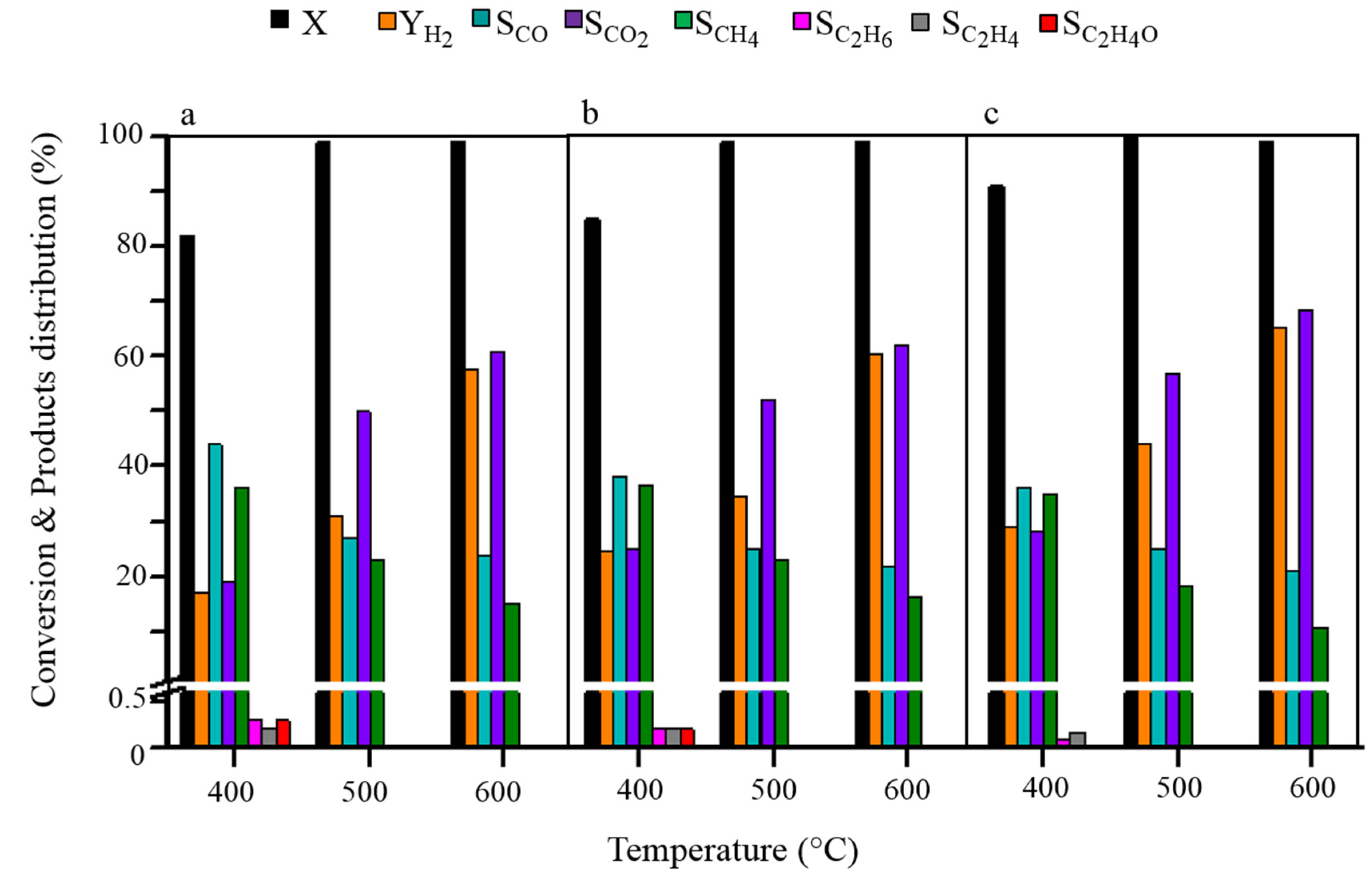
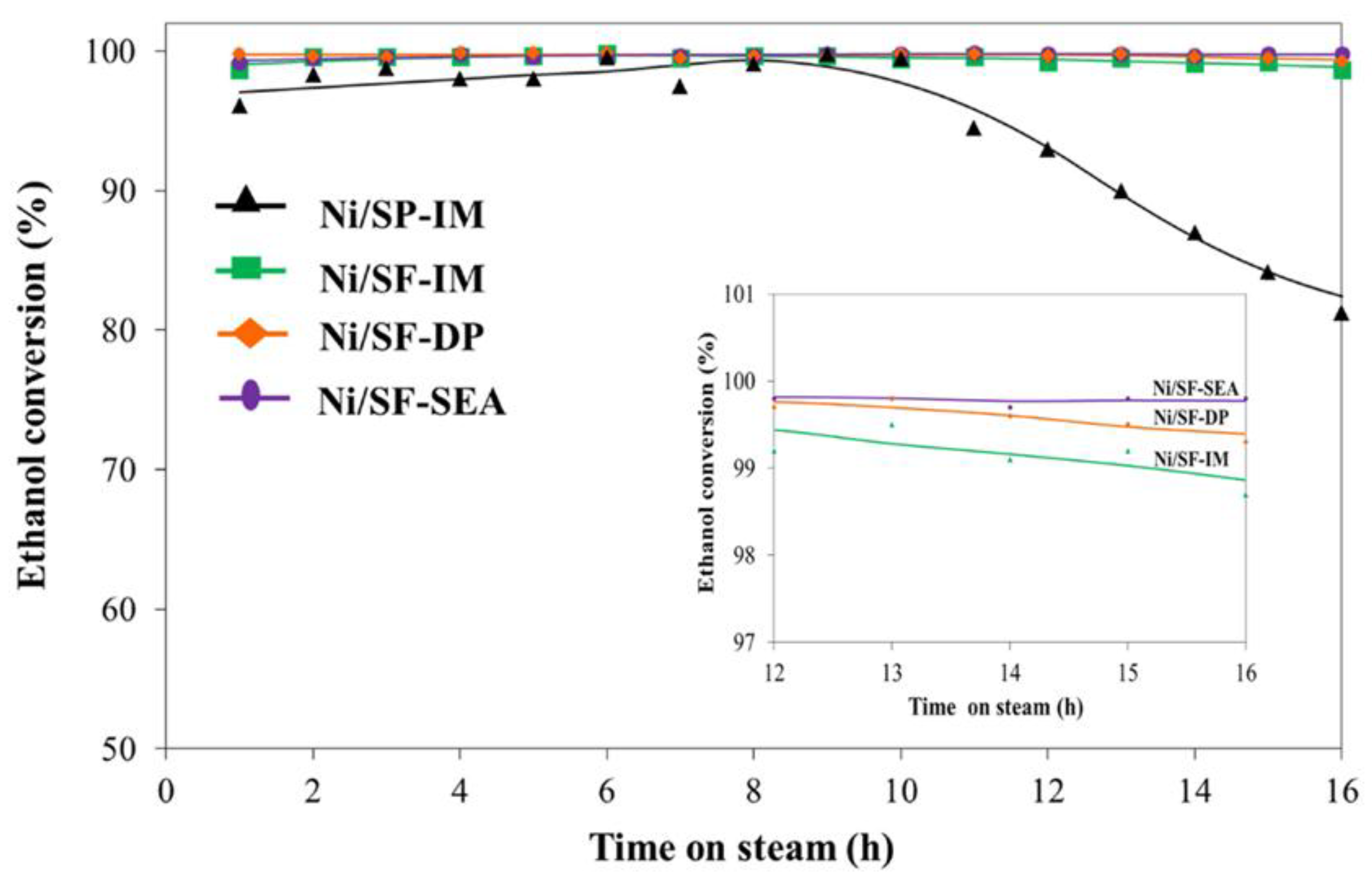
2.3. Stability Test
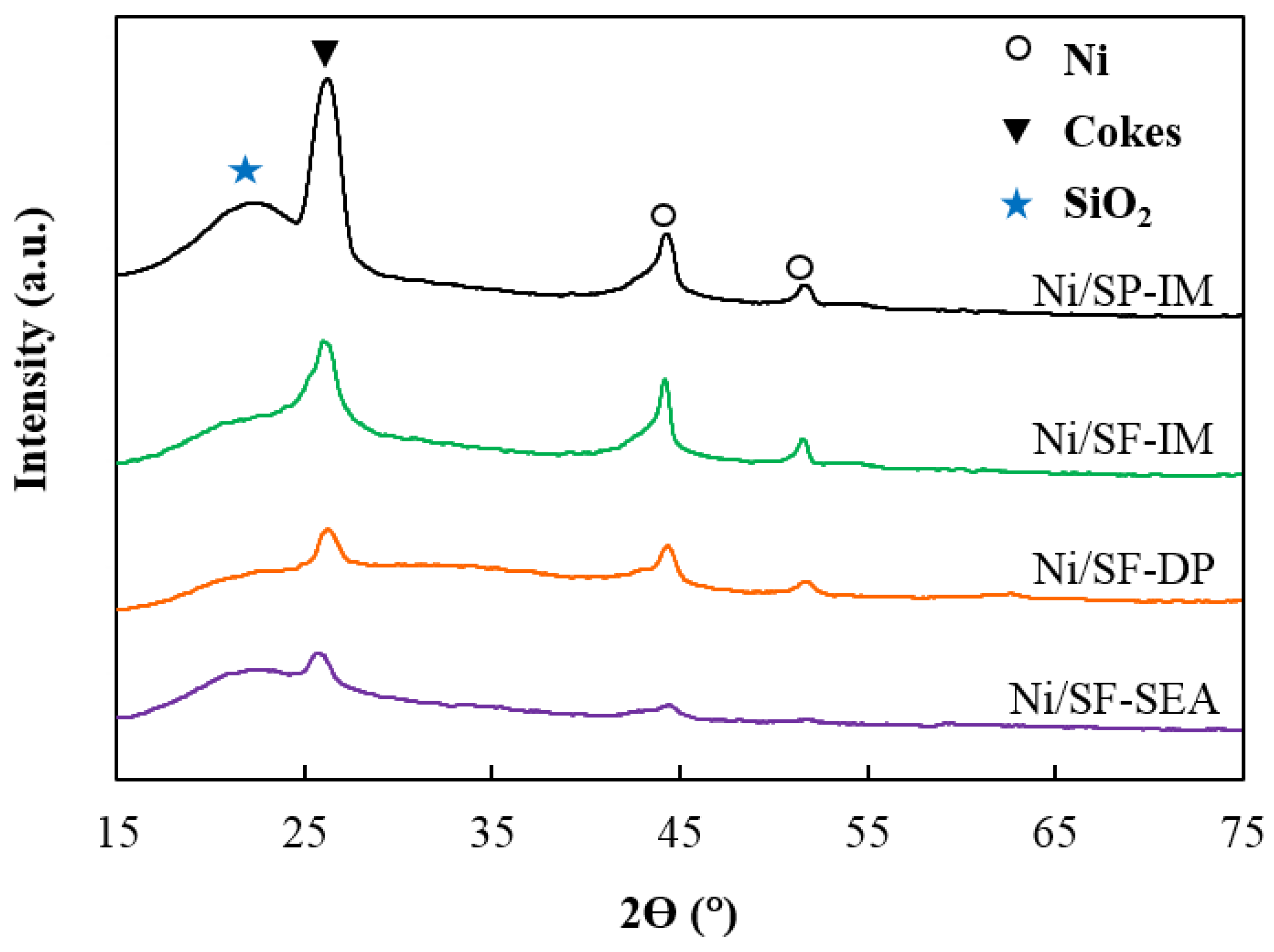
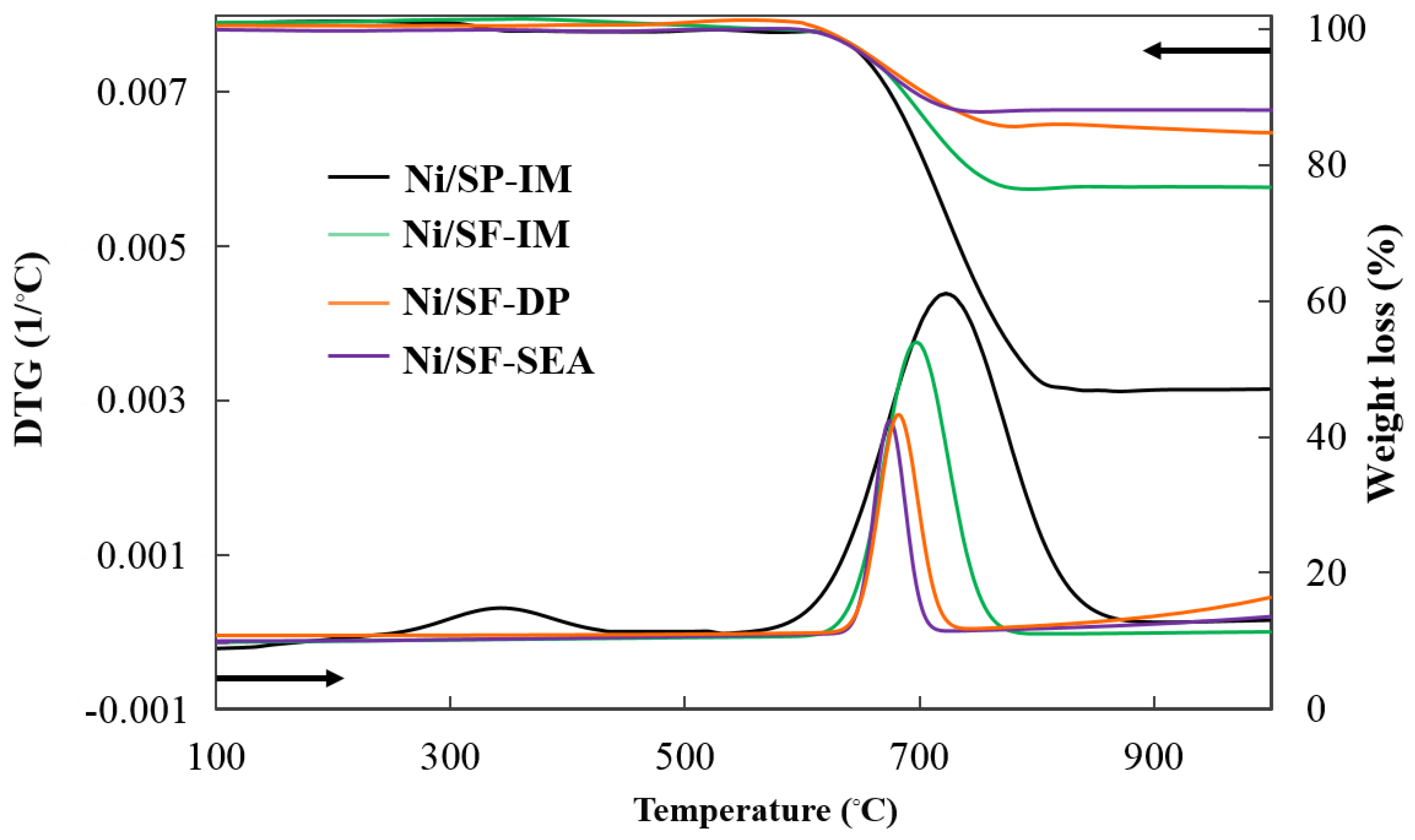
Characterization of the Spent Catalysts
3. Experimental
3.1. Catalyst Preparation
3.2. Characterization of the Catalysts
3.3. Catalyst Performance in the ESR
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Benito, M.; Sanz, J.L.; Isabel, R.; Padilla, R.; Arjona, R.; Daza, L. Bio-Ethanol Steam Reforming: Insights on the Mechanism for Hydrogen Production. J. Power Sources 2005, 151, 11–17. [Google Scholar] [CrossRef]
- Contrerasa, J.L.; Salmones, J.; Colín-Luna, J.A.; Nuño, L.; Quintana, B.; Córdova, I.; Zeifert, B.; Tapia, C.; Fuentes, G.A. Catalysts for H2 production using the ethanol steam reforming (a review). Int. J. Hydrogen Energy 2014, 39, 18835–18853. [Google Scholar] [CrossRef]
- Carvalho, F.L.S.; Asencios, Y.J.O.; Bellido, J.D.A.; Assaf, E.M. Bio-ethanol steam reforming for hydrogen production over Co3O4/CeO2 catalysts synthesized by one-step polymerization method. Fuel Process. Technol. 2016, 142, 182–191. [Google Scholar] [CrossRef]
- Bej, B.; Pradhan, N.C.; Neogi, S. Production of hydrogen by steam reforming of ethanol over alumina supported nano-NiO/SiO2 catalyst. Catal. Today 2014, 237, 80–88. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of hydrogen from ethanol: Review of reaction mechanism and catalyst deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.; Liu, J.; Guo, C.; Wang, Y.; Zhang, J. Ethanol steam reforming reactions over Al2O3.SiO2-supported Ni–La catalysts. Fuel 2009, 88, 511–518. [Google Scholar] [CrossRef]
- Vincenzo, P.; Concetta, R.; Eugenio, M.; Antonio, R. Renewable Hydrogen from Ethanol Reforming over CeO2-SiO2 Based Catalysts. Catalysts 2017, 7, 226. [Google Scholar] [CrossRef]
- Vicente, J.; Ereña, J.; Montero, C.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Reaction pathway for ethanol steam reforming on a Ni/SiO2 catalyst including coke formation. Int. J. Hydrogen Energy 2014, 39, 18820–18834. [Google Scholar] [CrossRef]
- Cerritos, R.C.; Ramírez, R.F.; Alvarado, A.F.A.; Rosales, J.M.M.; García, T.V.; Esquivel, I.R.G. Steam reforming of ethanol over Ni/Al2O3-La2O3 catalysts synthesized by sol-gel. Ind. Eng. Chem. Res. 2011, 50, 2576–2584. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.M. Ionic liquid immobilized onto fibrous nano-silica: A highly active and reusable catalyst for the synthesis of quinazoline-2,4(1 H,3 H)-diones. Catal. Commun. 2015, 72, 91–96. [Google Scholar] [CrossRef]
- Chinthaginjala, J.K.; Seshan, K.; Lefferts, L. Preparation and application of carbon-nanofiber based microstructured materials as catalyst supports. Ind. Eng. Chem. Res. 2007, 46, 3968–3978. [Google Scholar] [CrossRef]
- Hassan, M.A.; Sudarsanam, P.; Field, M.R.; Patel, J.; Bhargava, S.K. Effect of a Swelling Agent on the Performance of Ni/Porous Silica Catalyst for CH4−CO2 Reforming. Langmuir 2017, 33, 10632–10644. [Google Scholar]
- Reubroycharoen, P.; Tangkanaporn, N.; Chaiya, C. Ni/SiO2 fiber catalyst prepared by electrospinning technique for glycerol reforming to synthesis gas. Stud. Surf. Sci. Catal. 2010, 175, 689–693. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, M.; Zhang, T.; Wang, Y.; Tang, X.; Ning, P. A stable Ni/SBA-15 catalyst prepared by the ammonia evaporation method for dry reforming of methane. RSC Adv. 2015, 5, 94016–94024. [Google Scholar] [CrossRef]
- Backman, L.B.; Rautiainen, A.; Lindblad, M.; Krause, A.O.I. The interaction of cobalt species with alumina on Co/Al2O3 catalysts prepared by atomic layer deposition. Appl. Catal. A Gen. 2009, 360, 183–191. [Google Scholar] [CrossRef]
- López, E.; Kim, J.; Shanmugharaj, A.M.; Ryu, S.H. Multiwalled carbon nanotubes-supported nickel catalysts for the steam reforming of propane. J. Mater. Sci. 2011, 47, 2985–2994. [Google Scholar] [CrossRef]
- Naeem, M.A.; Al-Fatesh, A.S.; Abasaeed, A.E.; Fakeeha, A.H. Activities of Ni-based nano catalysts for CO2–CH4 reforming prepared by polyol process. Fuel Process. Technol. 2014, 122, 141–152. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for Preparation of Catalytic Materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Jiao, L.; Regalbuto, J.R. The synthesis of highly dispersed noble and base metals on silica via strong electrostatic adsorption: I. Amorphous silica. J. Catal. 2008, 260, 329–341. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Klaigaew, K.; Samart, C.; Chaiya, C.; Yoneyama, Y.; Tsubaki, N.; Reubroycharoen, P. Effect of preparation methods on activation of cobalt catalyst supported on silica fiber for Fischer–Tropsch synthesis. Chem. Eng. J. 2015, 278, 166–173. [Google Scholar] [CrossRef]
- Jose, A.C.; Alicia, C.; Arturo, J.V.; Montaña, L. Effect of Ce and Zr Addition to Ni/SiO2 Catalysts for Hydrogen Production through Ethanol Steam Reforming. Catalysts 2015, 5, 58–76. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, L.; Tian, H.; Li, D.; Wang, X.; Li, X.; Gong, J. Efficient hydrogen production from ethanol steam reforming over La-modified ordered mesoporous Ni-based catalysts. Appl. Catal. B Environ. 2016, 181, 321–331. [Google Scholar] [CrossRef]
- He, L.; Lin, Q.; Liu, Y.; Huang, Y. Unique catalysis of Ni-Al hydrotalcite derived catalyst in CO2 methanation: Cooperative effect between Ni nanoparticles and a basic support. J. Energy Chem. 2014, 23, 587–592. [Google Scholar] [CrossRef]
- Nakanishi, M.; Uddin, Md. A.; Kato, Y.; Nishina, Y.; Hapipi, A.M. Effects of preparation method on the properties of cobalt supported β-zeolite catalysts for Fischer-Tropsch synthesis. Catal. Today 2017, 291, 124–132. [Google Scholar] [CrossRef]
- Yang, M.; Jin, P.; Fan, Y.; Huang, C.; Zhang, N.; Weng, W.; Chen, M.; Wan, H. Ammonia-assisted synthesis towards a phyllosilicate-derived highly-dispersed and long-lived Ni/SiO2 catalyst. Catal. Sci. Technol. 2015, 5, 5095–5099. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, L.; Qin, Y.N. Study on the carbon deposition in steam reforming of ethanol over Co/CeO2 catalyst. Chem. Eng. J. 2008, 145, 25–31. [Google Scholar] [CrossRef]
- Natewong, P.; Prasongthum, N.; Mhadmhan, S.; Reubroycharoen, P. Fibrous platelet carbon nanofibers-silica fiber composite supports for a Co-based catalyst in the steam reforming of acetic acid. Appl. Catal. A Gen. 2018, 560, 215–224. [Google Scholar] [CrossRef]
- Mondal, T.; Pant, K.K.; Dalai, A.K. Catalytic oxidative steam reforming of bio-ethanol for hydrogen production over Rh promoted Ni/CeO2–ZrO2 catalyst. Int. J. Hydrogen Energy 2015, 40, 2529–2544. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, R.; Huang, S.; Chen, W.; Shi, Q. Ni/Y2O3-Al2O3 catalysts for hydrogen production from steam reforming of ethanol at low temperature. J. Rare Earths 2012, 30, 683–690. [Google Scholar] [CrossRef]
- Prasongthum, N.; Xiao, R.; Zhang, H.; Tsubaki, N.; Natewong, P.; Reubroycharoen, P. Highly active and stable Ni supported on CNTs-SiO2 fiber catalysts for steam reforming of ethanol. Fuel Process. Technol. 2017, 160, 185–195. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X.; Li, J. Hydrogen production from ethanol steam reforming over Ni/SiO2 catalysts: A comparative study of traditional preparation and microwave modification methods. Int. J. Energy Res. 2014, 38, 860–874. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalyst for ethanol steam reforming with enhanced activity and resistance to deactivation. J. Appl. Catal. B 2015, 176, 532–541. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, H.; Huang, Z.; Li, S.; Wu, G.; Ma, X.; Gong, J. Hydrogen Production via Steam Reforming of Ethanol on Phyllosilicate-Derived Ni/SiO2: Enhanced Metal-Support Interaction and Catalytic Stability. ACS Sustain. Chem. Eng. 2013, 1, 161–173. [Google Scholar] [CrossRef]
- Palacio, R.; Gallego, J.; Gabelica, Z.; Batiot-Dupeyrat, C.; Barrault, J.; Valange, S. Decomposition of ethanol into H2-rich gas and carbon nanotubes over Ni, Co and Fe supported on SBA-15 and Aerosil. Appl. Catal. A Gen. 2015, 504, 642–653. [Google Scholar] [CrossRef]
- Prasongthum, N.; Chaiya, C.; Samart, C.; Guan, G.; Natewong, P.; Reubroycharoen, P. Co-production of hydrogen and carbon nanotube-silica fiber composites from ethanol steam reforming over an Ni-silica fiber catalyst. Monatsh. Chem. 2017, 148, 1311–1321. [Google Scholar] [CrossRef]
- Peng, H.; Ma, Y.; Liu, W.; Xu, X.; Fang, X.; Lian, J.; Wang, X.; Li, C.; Zhou, W.; Yuan, P. Methane dry reforming on Ni/La2Zr2O7 treated by plasma in different atmospheres. J. Energy Chem. 2015, 24, 416–424. [Google Scholar] [CrossRef]
- Ratanathavorn, W.; Samart, C.; Reubroycharoen, P. Tinospora crispalike ZSM-5/silica fibers synthesized by electrospinning and hydrothermal method. Mater. Lett. 2015, 159, 135–137. [Google Scholar] [CrossRef]
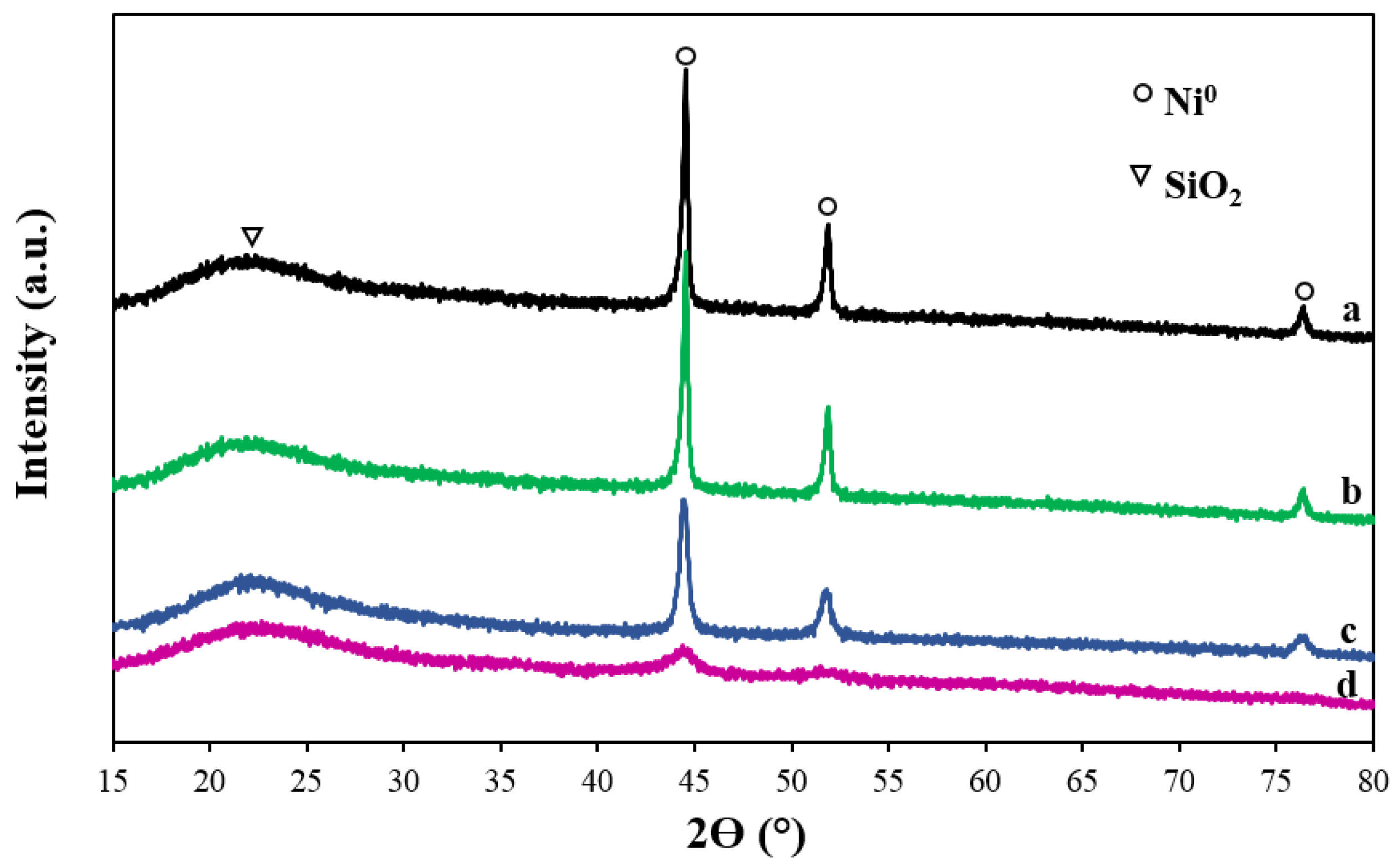
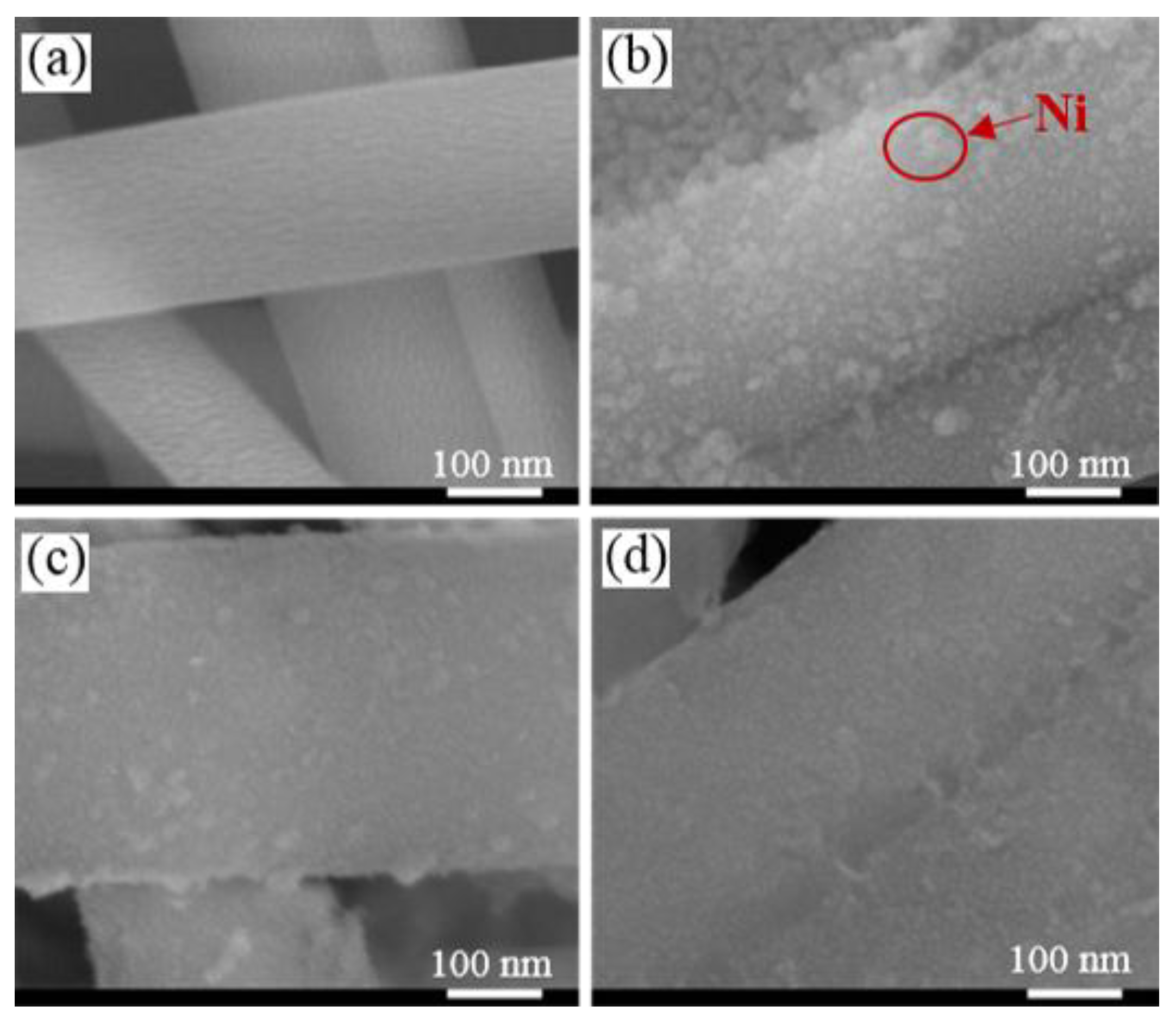
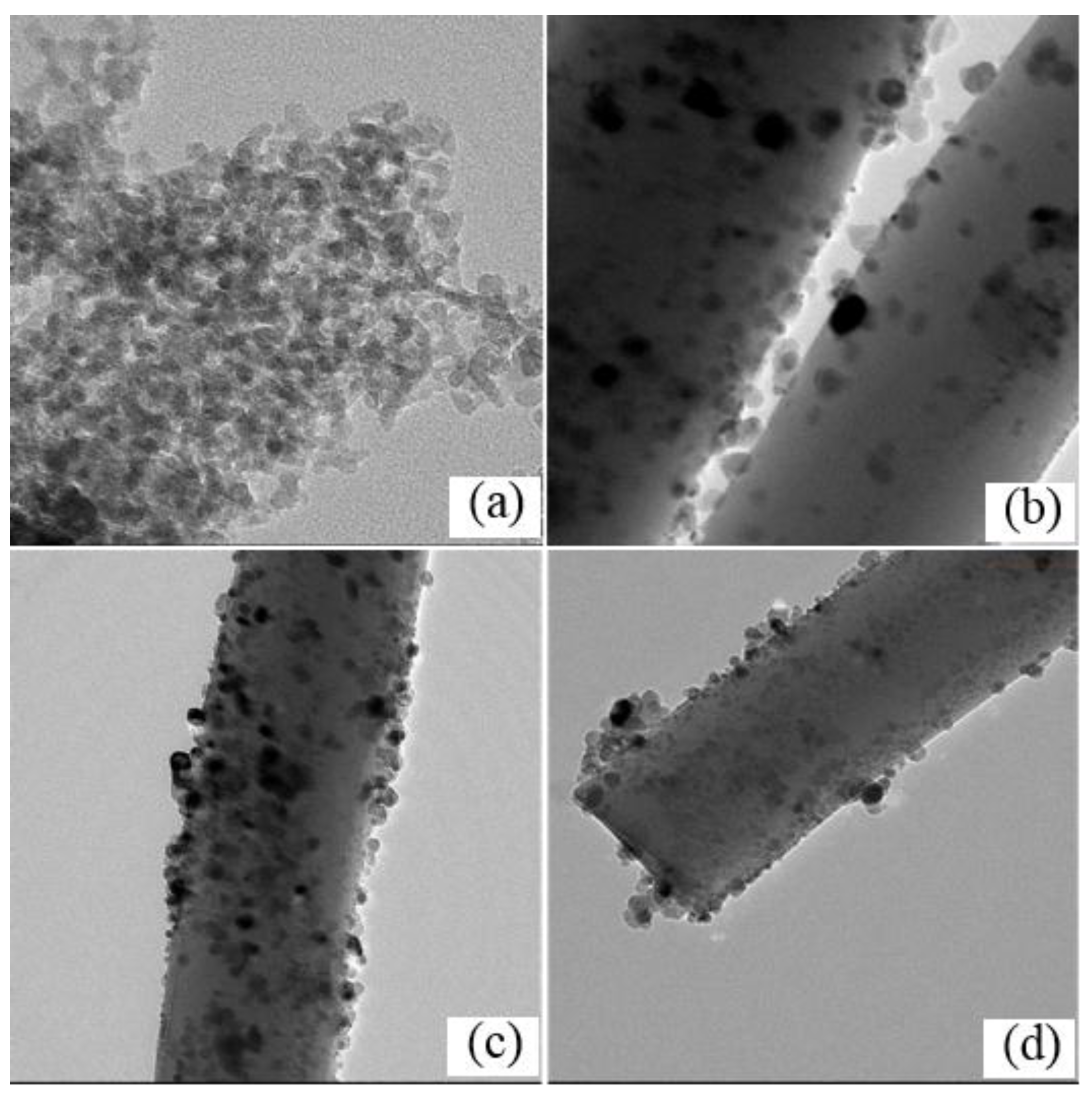

| Samples | SBET (m2/g) | Vp (cm3/g) | Dp (nm) | Ni Content a (%) | NiO Size (nm) | Ni0 Size (nm) | Ni Dispersion (%) e | |
|---|---|---|---|---|---|---|---|---|
| XRD b | TEM c | XRD d | ||||||
| SP | 236.3 | 1.161 | 12.4 | - | - | - | - | - |
| SF | 5.9 | 0.001 | 10.2 | - | - | - | - | - |
| Ni/SP-IM | 240.2 | 1.167 | 12.7 | 11 | 15 | 13 | 32 | 1.040 |
| Ni/SF-IM | 9.9 | 0.011 | 10.3 | 11 | 22 | 20 | 32 | 0.121 |
| Ni/SF-DP | 26.7 | 0.028 | 5.8 | 11 | 14 | 13 | 13 | 0.660 |
| Ni/SF-SEA | 35.9 | 0.029 | 5.9 | 8 | 7 | 7 | 8 | 0.829 |
| Samples | C Balance a (%) | Particle Size of Spent Ni0 (nm) | |||
|---|---|---|---|---|---|
| Cgas | Cliquid | Csolid | XRD b | TEM c | |
| Ni/SP-IM | 73.28 | - | 26.10 | 37 | 35 |
| Ni/SF-IM | 76.80 | - | 22.22 | 36 | 34 |
| Ni/SF-DP | 83.66 | - | 16.25 | 16 | 17 |
| Ni/SF-SEA | 83.43 | - | 16.17 | 8 | 8 |
| Catalysts | %Ni Loading | Preparation Method | Temp. (°C) | S/C (Mole Ratio) | YH2 (%) | Ref. |
|---|---|---|---|---|---|---|
| Ni/SF | 11 | DP | 500 | 9 | 35 | This study |
| Ni/SF | 8 | SEA | 500 | 9 | 45 | This study |
| Ni/SF | 11 | DP | 600 | 9 | 60 | This study |
| Ni/SF | 8 | SEA | 600 | 9 | 65 | This study |
| Ni/Al2O3 | 20 | IM | 500 | 13 | 25 | [31] |
| Ni/SiO2 fiber | 11 | IM | 500 | 9 | 40 | [32] |
| Ni/SiO2 | 10 | IM | 600 | 12 | 60 | [33] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhadmhan, S.; Natewong, P.; Prasongthum, N.; Samart, C.; Reubroycharoen, P. Investigation of Ni/SiO2 Fiber Catalysts Prepared by Different Methods on Hydrogen production from Ethanol Steam Reforming. Catalysts 2018, 8, 319. https://doi.org/10.3390/catal8080319
Mhadmhan S, Natewong P, Prasongthum N, Samart C, Reubroycharoen P. Investigation of Ni/SiO2 Fiber Catalysts Prepared by Different Methods on Hydrogen production from Ethanol Steam Reforming. Catalysts. 2018; 8(8):319. https://doi.org/10.3390/catal8080319
Chicago/Turabian StyleMhadmhan, Sareena, Paweesuda Natewong, Natthawan Prasongthum, Chanatip Samart, and Prasert Reubroycharoen. 2018. "Investigation of Ni/SiO2 Fiber Catalysts Prepared by Different Methods on Hydrogen production from Ethanol Steam Reforming" Catalysts 8, no. 8: 319. https://doi.org/10.3390/catal8080319