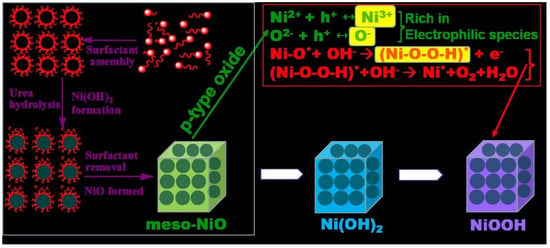
Engineering Mesoporous NiO with Enriched Electrophilic Ni3+ and O− toward Efficient Oxygen Evolution
Abstract
:1. Introduction
2. Results and Discussion
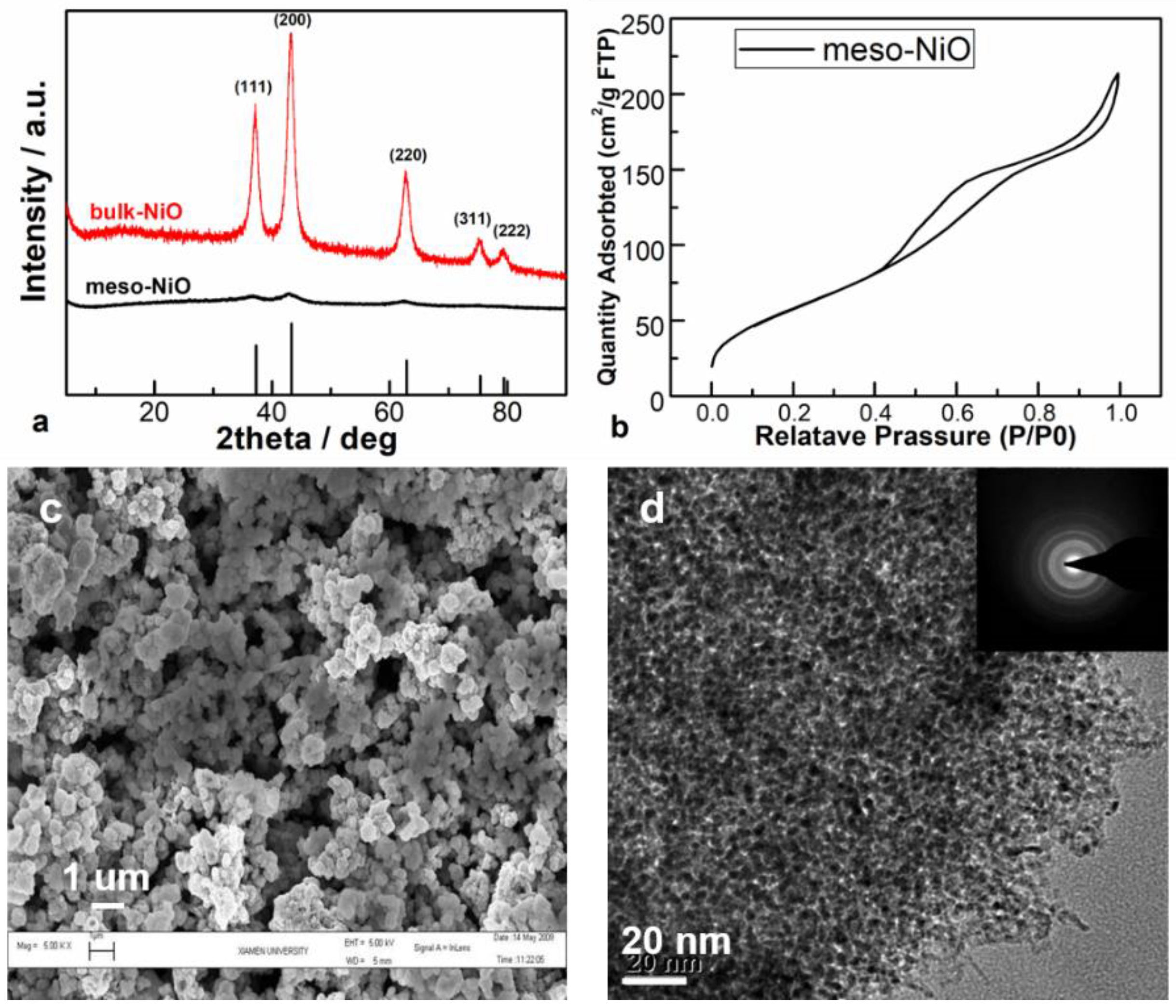
2.1. Texture Characterizations
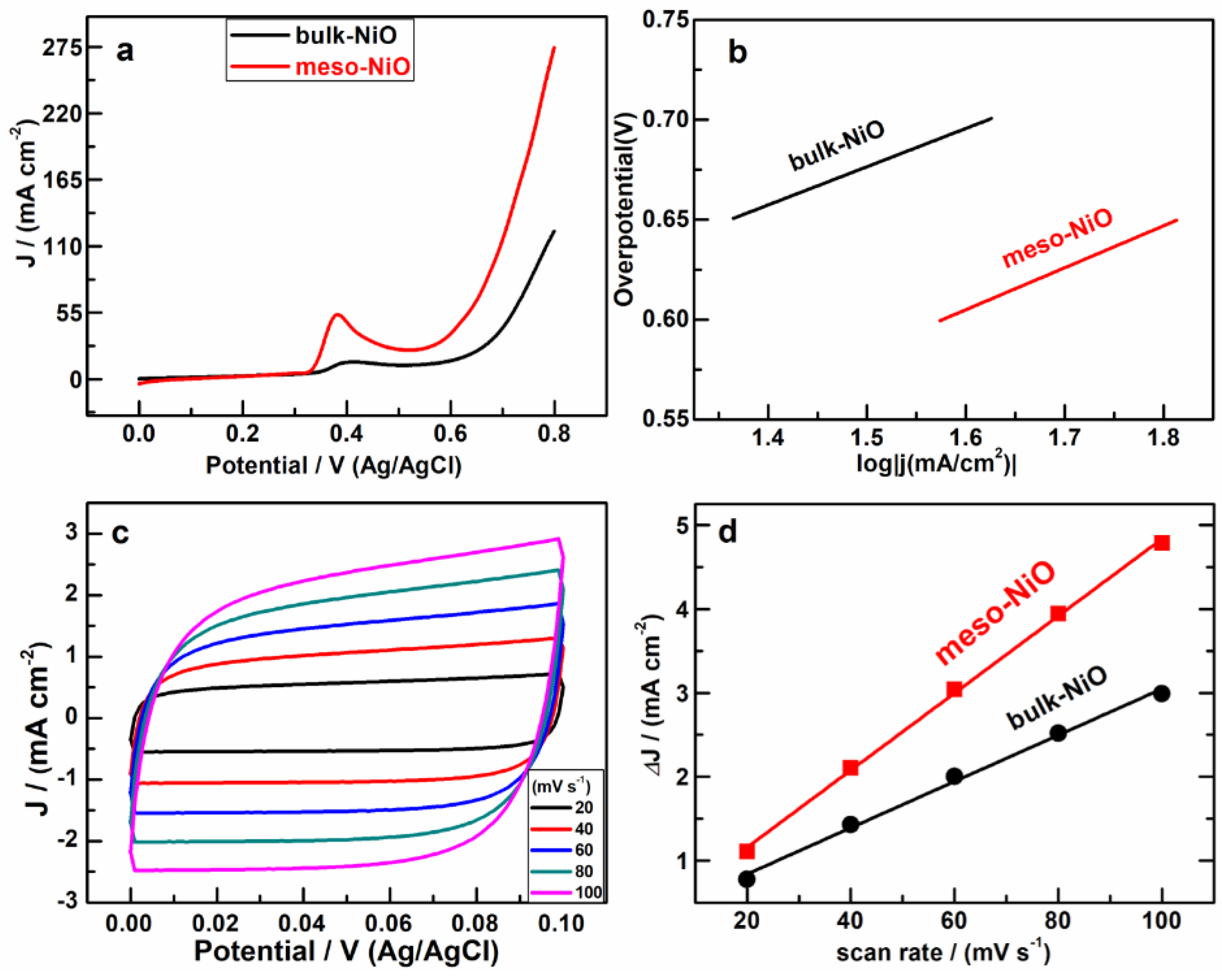
2.2. OER Activity
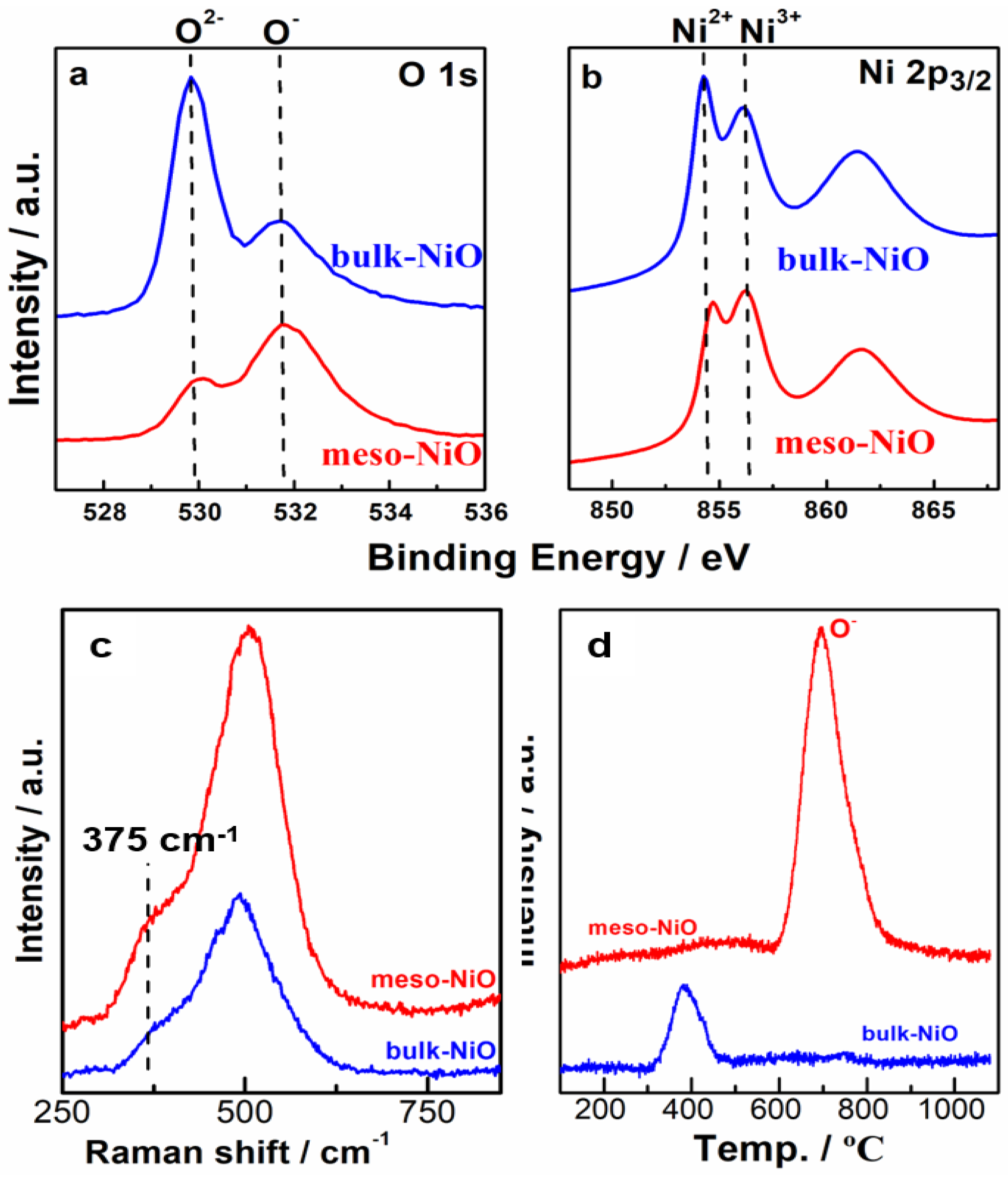
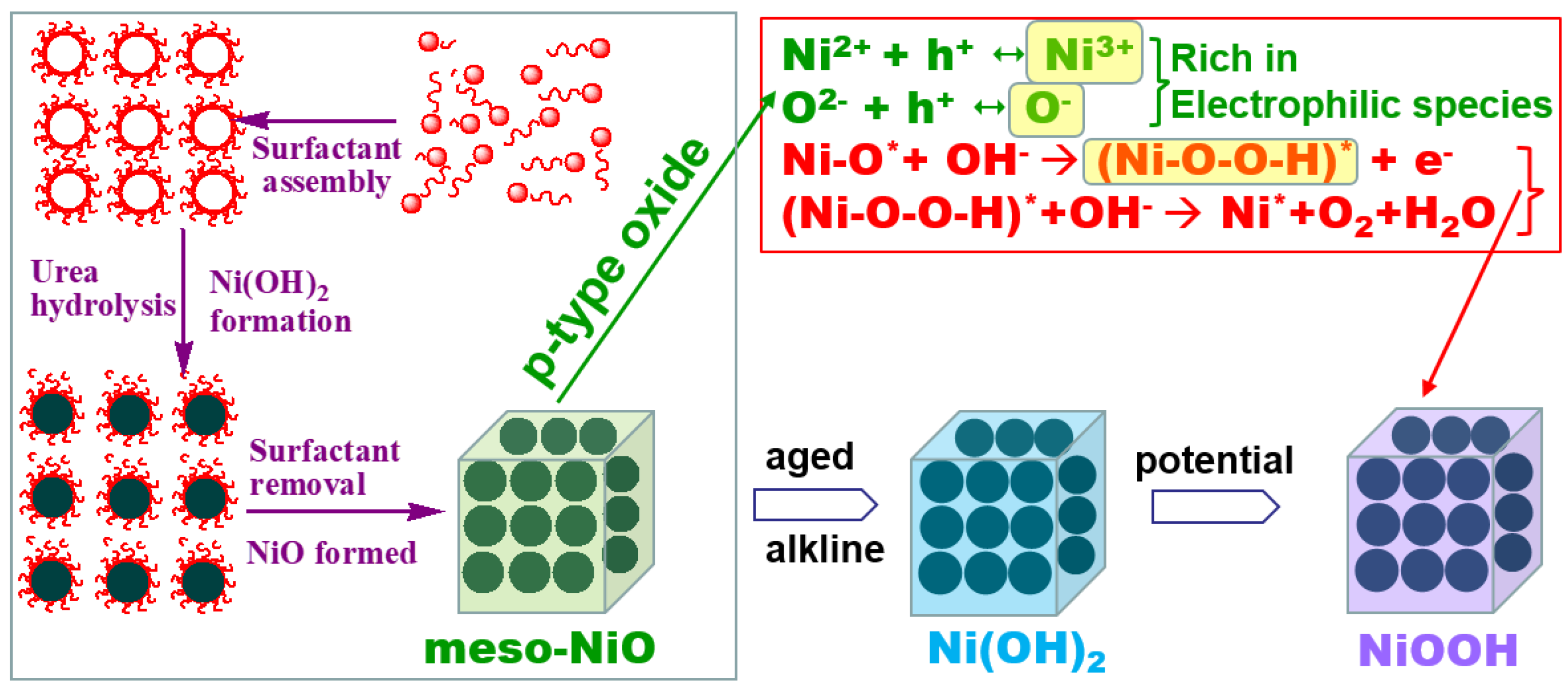
2.3. Characterizations
2.4. Discussion
3. Materials and Methods
3.1. Materials
3.2. Analytical Equipment
3.3. Synthesis of Nano-Nickel Oxide
3.4. Synthesis of Meso-Nickel Oxide
3.5. Characterizations
3.6. Electrode Preparation
3.7. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Jin, H.; Oh, A.; Baik, H.; Joo, S.H.; Lee, K. Synthesis of compositionally tunable, hollow mixed metal sulphide coxniysz octahedral nanocages and their composition-dependent electrocatalytic activities for oxygen evolution reaction. Nanoscale 2017, 13, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Domanska, M.; Norek, M.; Jóźwik, P.; Jankiewicz, B.; Stępniowski, W. Catalytic stability and surface analysis of microcrystalline Ni3Al thin foils in methanol decomposition. Appl. Surf. Sci. 2014, 293, 169–176. [Google Scholar] [CrossRef]
- Tang, C.; Asiri, A.M.; Sun, X. Highly-active oxygen evolution electrocatalyzed by a Fe-doped NiSe nanoflake array electrode. Chem. Commun. 2016, 52, 4529–4532. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Trotochaud, L.; Ranney, J.K.; Williams, K.N.; Boettcher, S.W. Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 2012, 134, 17253–17261. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Hsu, Y.Y.; Chen, R.; Chan, T.S.; Chen, H.M.; Liu, B. Ni3+-induced formation of active NiOOH on the spinel Ni-Co oxide surface for efficient oxygen evolution reaction. Adv. Energy Mater. 2015, 5, 395–417. [Google Scholar] [CrossRef]
- Matsumura, Y.; Tanaka, K.; Tode, N.; Yazawa, T.; Haruta, M. Catalytic methanol decomposition to carbon monoxide and hydrogen over nickel supported on silica. J. Mol. Catal. A Chem. 2000, 152, 157–165. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Micropor. Mesopor. Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Chang, J.; Park, M.; Ham, D.; Ogale, S.B.; Mane, R.S.; Han, S.H. Liquid-phase synthesized mesoporous electrochemical supercapacitors of nickel hydroxide. Electrochim. Acta 2008, 53, 5016–5021. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, B.; Su, L.; Zhang, X. Interface synthesis of mesoporous MnO2 and its electrochemical capacitive behaviors. J. Colloid Interface Sci. 2008, 322, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Xia, Y.Y. Electrochemical capacitance characterization of NiO with ordered mesoporous structure synthesized by template SBA-15. Electrochim. Acta 2006, 51, 3223–3227. [Google Scholar] [CrossRef]
- Xing, W.; Li, F.; Yan, Z.F.; Lu, G.Q. Synthesis and electrochemical properties of mesoporous nickel oxide. J. Power Sources 2004, 134, 324–330. [Google Scholar] [CrossRef]
- Shaju, K.M.; Jiao, F.; Débart, A.; Bruce, P.G. Mesoporous and nanowire Co3O4 as negative electrodes for rechargeable lithium batteries. Phys. Chem. Chem. Phys. 2007, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Wang, C.-C.; Huang, C.-J.; Sun, Y.-F.; Weng, W.-Z.; Wan, H.-L. Mesoporous nickel oxides as effective catalysts for oxidative dehydrogenation of propane to propene. Appl. Catal. A Gen. 2010, 382, 99–105. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Chen, T.; Weng, W.; Wan, H. Low-temperature catalytic performance for oxidative dehydrogenation of propane on nanosized Ti(Zr)-Ni-O prepared by modified sol-gel method. Catal. Commun. 2006, 7, 268–271. [Google Scholar] [CrossRef]
- Alberto, N.; Vigario, C.; Duarte, D.; Almeida, D.; Gonçalves, G. Characterization of graphene oxide coatings onto optical fibers for sensing applications. Mater. Today Proc. 2015, 2, 171–177. [Google Scholar] [CrossRef]
- Schenk, A.S.; Eiben, S.; Goll, M.; Reith, L.; Kulak, A.N.; Meldrum, F.C.; Jeske, H.; Wege, C.; Ludwigs, S. Virus-directed formation of electrocatalytically active nanoparticle-based Co3O4 tubes. Nanoscale 2017, 9, 6334–6345. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Dong, C.; Zhang, C.; Gao, Y.; Zhang, J.; Gao, H.; Wang, Y.; Zhang, Z. Dealloying-directed synthesis of efficient mesoporous CoFe-based catalysts towards the oxygen evolution reaction and overall water splitting. Nanoscale 2017, 9, 16467–16475. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.E.; Brandon, M.P. The oxygen evolution reaction on passive oxide covered transition metal electrodes in aqueous alkaline solution. Part 1-nickel. Int. J. Electrochem. Sci. 2008, 3, 1386–1424. [Google Scholar]
- Li, W.; Gao, X.; Wang, X.; Xiong, D.; Huang, P.P.; Song, W.G.; Bao, X.; Liu, L. From water reduction to oxidation: Janus Co-Ni-P nanowires as high-efficiency and ultrastable electrocatalysts for over 3000h water splitting. J. Power Sources 2016, 330, 156–166. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, X.; Cao, X.; Huang, X.; Tan, C.; Tian, J.; Liu, H.; Wang, J.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energ. Environ. Sci. 2013, 6, 2921–2924. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Mølhave, K.; Zhang, M.; Zhang, J. Simultaneous modulation of surface composition, oxygen vacancies and assembly in hierarchical Co3O4 mesoporous nanostructures for lithium storage and electrocatalytic oxygen evolution. Nanoscale 2017, 9, 14431–14441. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yu, H.L.; Ni, H.; Hu, S. Bimetallic (FexNi1−x)2P nanoarrays as exceptionally efficient electrocatalysts for oxygen evolution in alkaline and neutral media. Nano Energy 2017, 38, 553–560. [Google Scholar] [CrossRef]
- Stoyanova, M.; Konova, P.; Nikolov, P.; Naydenov, A.; Christoskova, S.; Mehandjiev, D. Alumina-supported nickel oxide for ozone decomposition and catalytic ozonation of CO and VOCs. Chem. Eng. J. 2006, 122, 41–46. [Google Scholar] [CrossRef]
- Cordoba-Torresi, S.I. Electrochromic behavior of nickel oxide electrodes. J. Electrochem. Soc. 1991, 138, 1554–1559. [Google Scholar] [CrossRef]
- Heracleous, E.; Lemonidou, A.A. Ni-nb-o mixed oxides as highly active and selective catalysts for ethene production via ethane oxidative dehydrogenation. Part i: Characterization and catalytic performance. J. Catal. 2006, 237, 162–174. [Google Scholar] [CrossRef]
- Fabbri, E.; Habereder, A.; Waltar, K.; Kötz, R.; Schmidt, T.J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2014, 4, 3800–3821. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, V.; Jones, T.E.; Wrabetz, S.; Massue, C.; Velasco Velez, J.J.; Arrigo, R.; Scherzer, M.; Piccinin, S.; Havecker, M.; Knop-Gericke, A. Reactive oxygen species in iridium-based oer catalysts. Chem. Sci. 2016, 7, 6791–6795. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, D.A.; Bendert, R.M. Effect of coprecipitated metal ions on the electrochemistry of nickel hydroxide thin films: Cyclic voltammetry in 1 M KOH. J. Electrochem. Soc. 1989, 20, 723–728. [Google Scholar] [CrossRef]
- Wehrens-Dijksma, M.; Notten, P.H.L. Electrochemical quartz microbalance characterization of Ni(OH)2-based thin film electrodes. Electrochim. Acta 2006, 51, 3609–3621. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Jóźwik, P.; Jankiewicz, B.J.; Bartosewicz, B.; Siemiaszko, D.; Stępniowski, W.J.; Bojar, Z. Study of Cyclic Ni3Al Catalyst Pretreatment Process for Uniform Carbon Nanotubes Formation and Improved Hydrogen Yield in Methanol Decomposition. Mater. Today Proc. 2016, 3S, S171–S177. [Google Scholar] [CrossRef]
- Chen, G.F.; Ma, T.Y.; Liu, Z.Q.; Li, N.; Su, Y.Z.; Davey, K.; Qiao, S.Z. Efficient and stable bifunctional electrocatalysts Ni/NixMy (M = P, S) for overall water splitting. Adv. Funct. Mater. 2016, 26, 3314–3323. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhai, Z.-Y.; Chen, Z.; Zhang, L.-Z.; Zhao, X.-F.; Si, F.-Z.; Li, J.-H. Engineering Mesoporous NiO with Enriched Electrophilic Ni3+ and O− toward Efficient Oxygen Evolution. Catalysts 2018, 8, 310. https://doi.org/10.3390/catal8080310
Liu X, Zhai Z-Y, Chen Z, Zhang L-Z, Zhao X-F, Si F-Z, Li J-H. Engineering Mesoporous NiO with Enriched Electrophilic Ni3+ and O− toward Efficient Oxygen Evolution. Catalysts. 2018; 8(8):310. https://doi.org/10.3390/catal8080310
Chicago/Turabian StyleLiu, Xiu, Zhi-Yuan Zhai, Zhou Chen, Li-Zhong Zhang, Xiu-Feng Zhao, Feng-Zhan Si, and Jian-Hui Li. 2018. "Engineering Mesoporous NiO with Enriched Electrophilic Ni3+ and O− toward Efficient Oxygen Evolution" Catalysts 8, no. 8: 310. https://doi.org/10.3390/catal8080310