The Roles of Graphene and Ag in the Hybrid Ag@Ag2O-Graphene for Sulfamethoxazole Degradation
Abstract
:1. Introduction
2. Results and Discussions
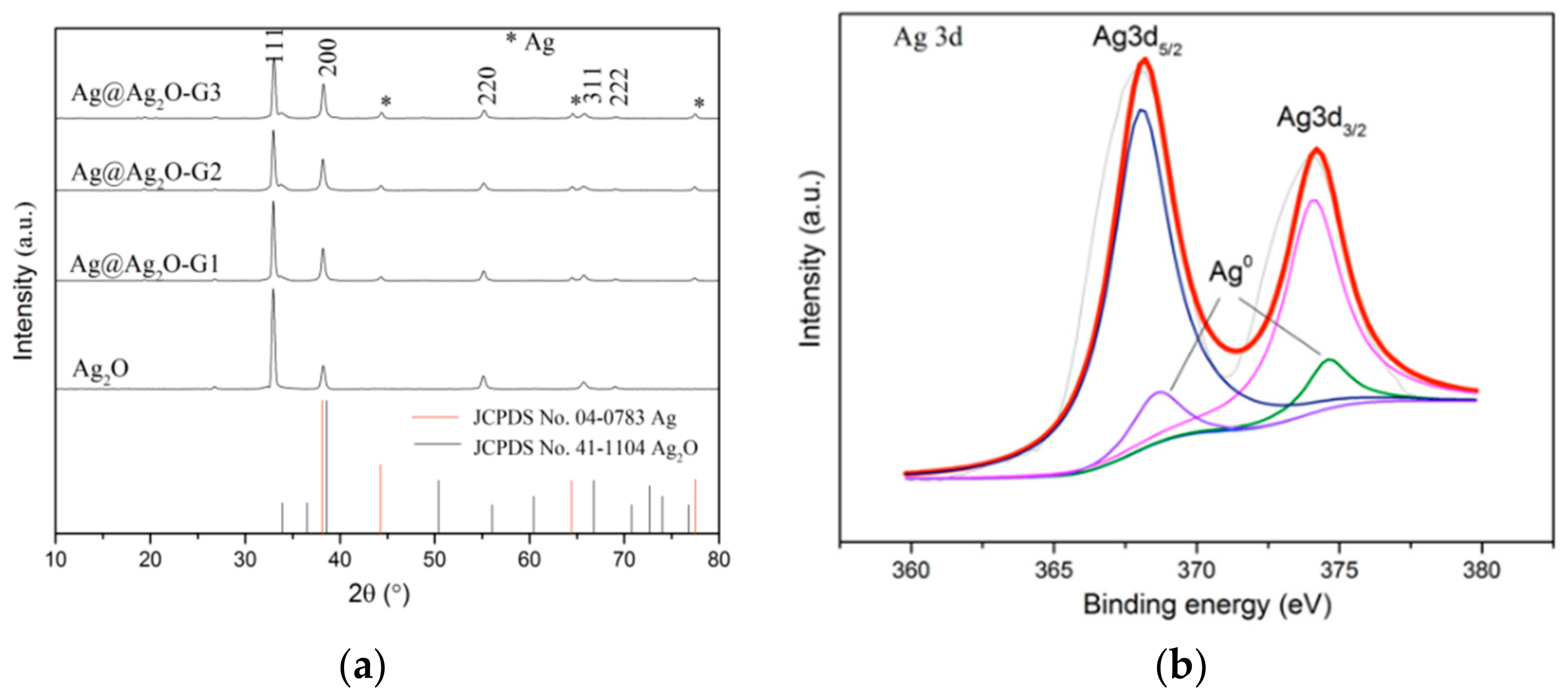
2.1. Catalyst Characterization
2.2. Photocatalytic Performance
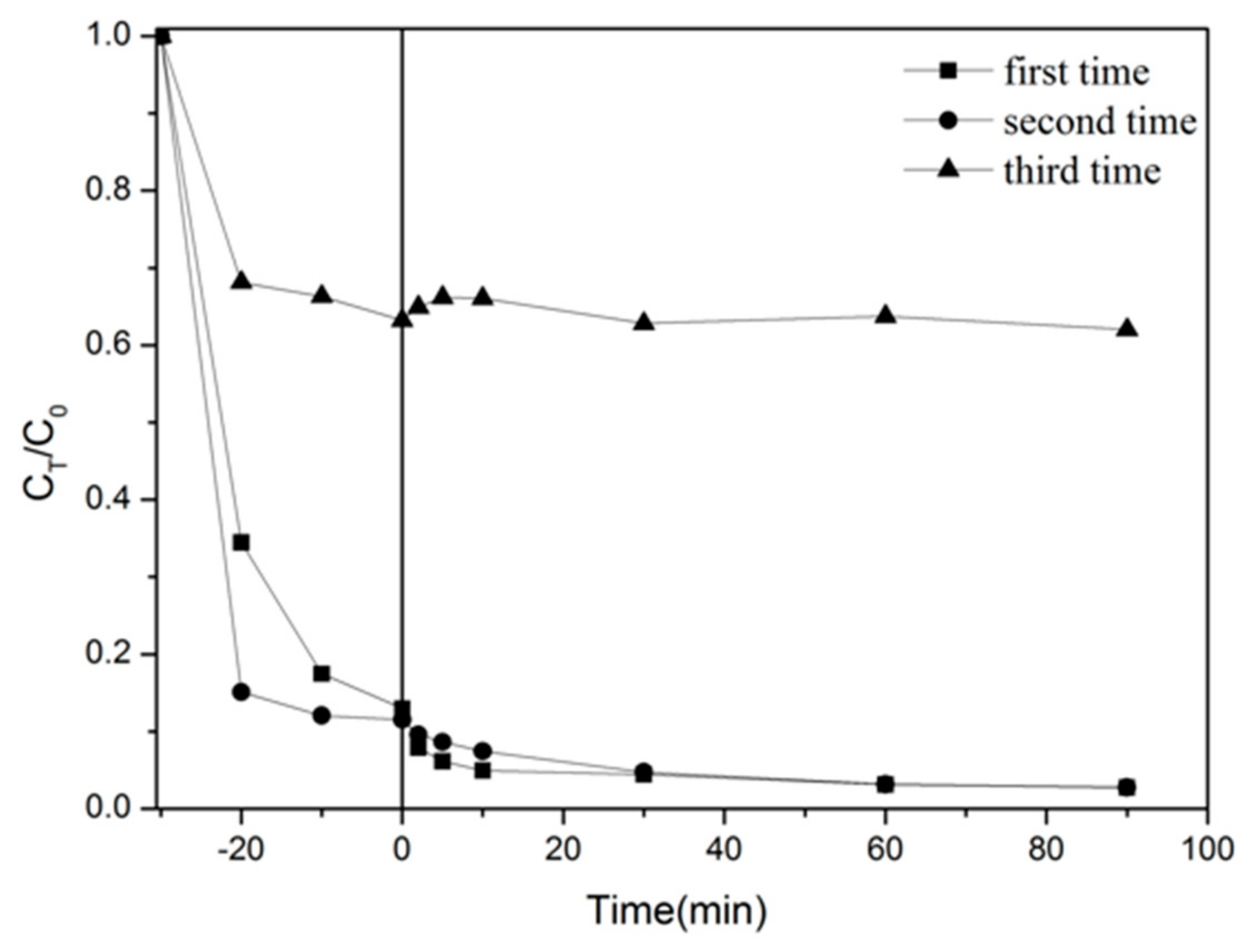
2.3. Photocatalyst Stability
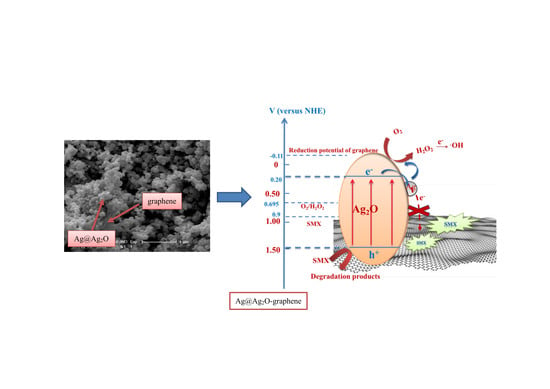
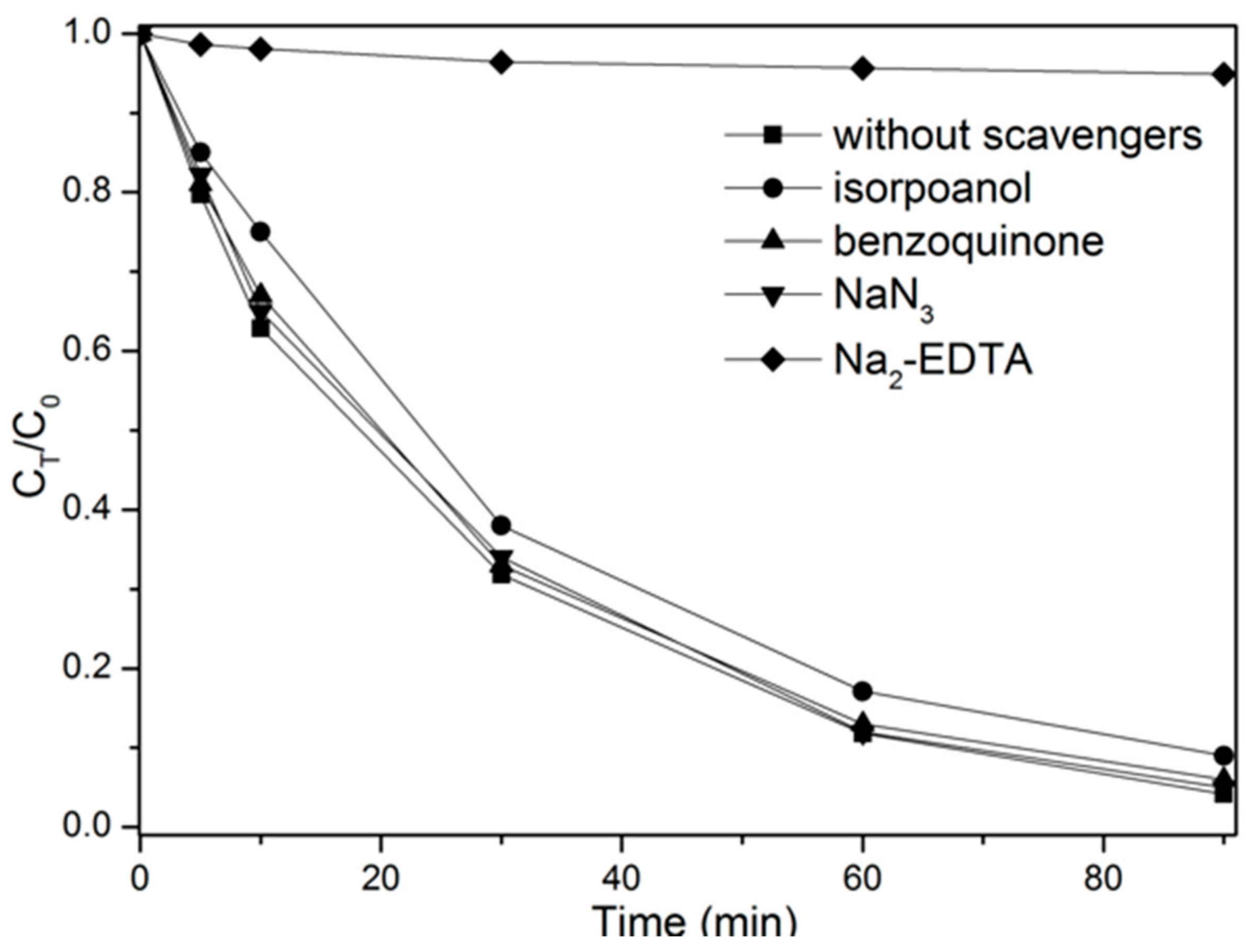
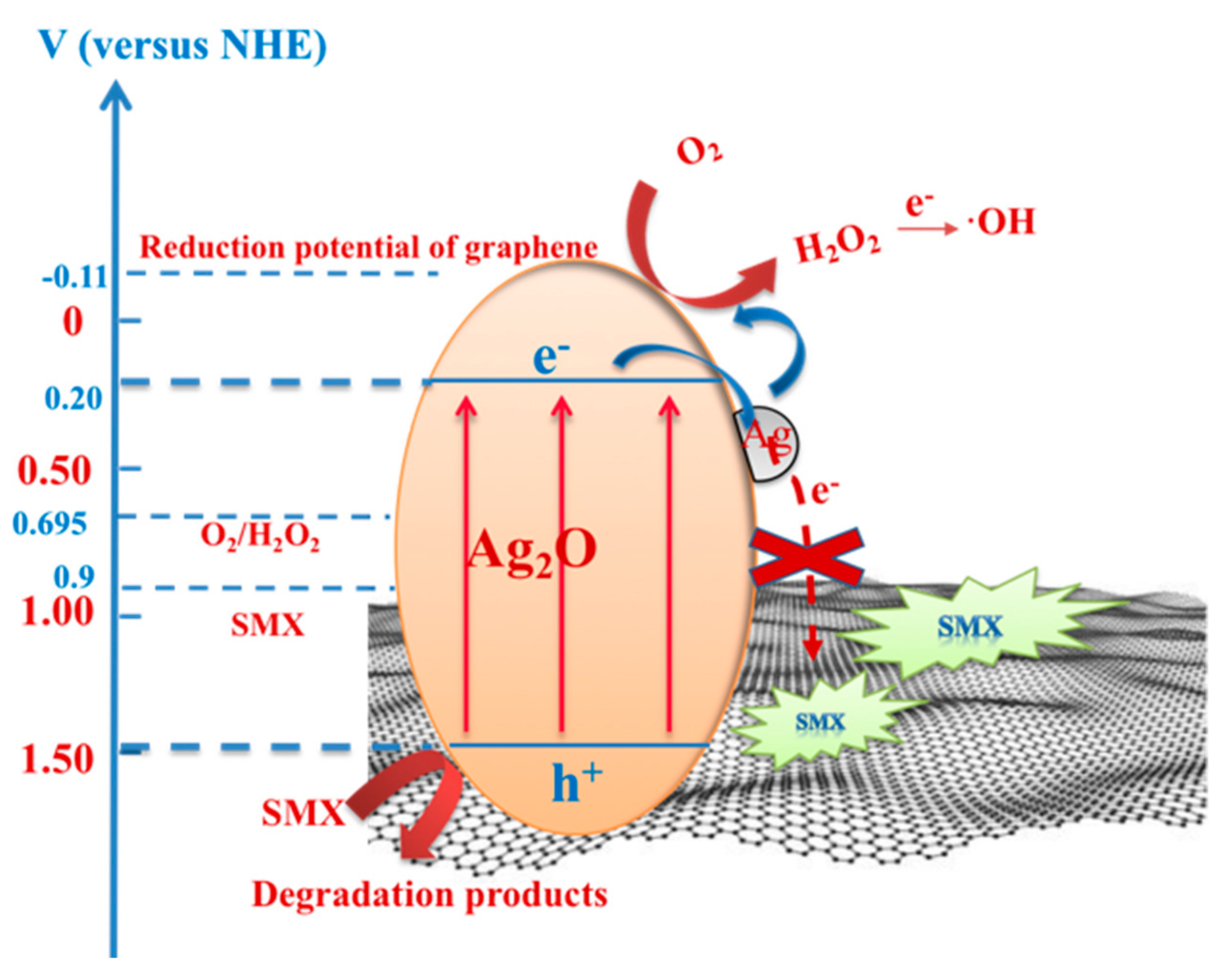
2.4. Mechanism Analysis
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
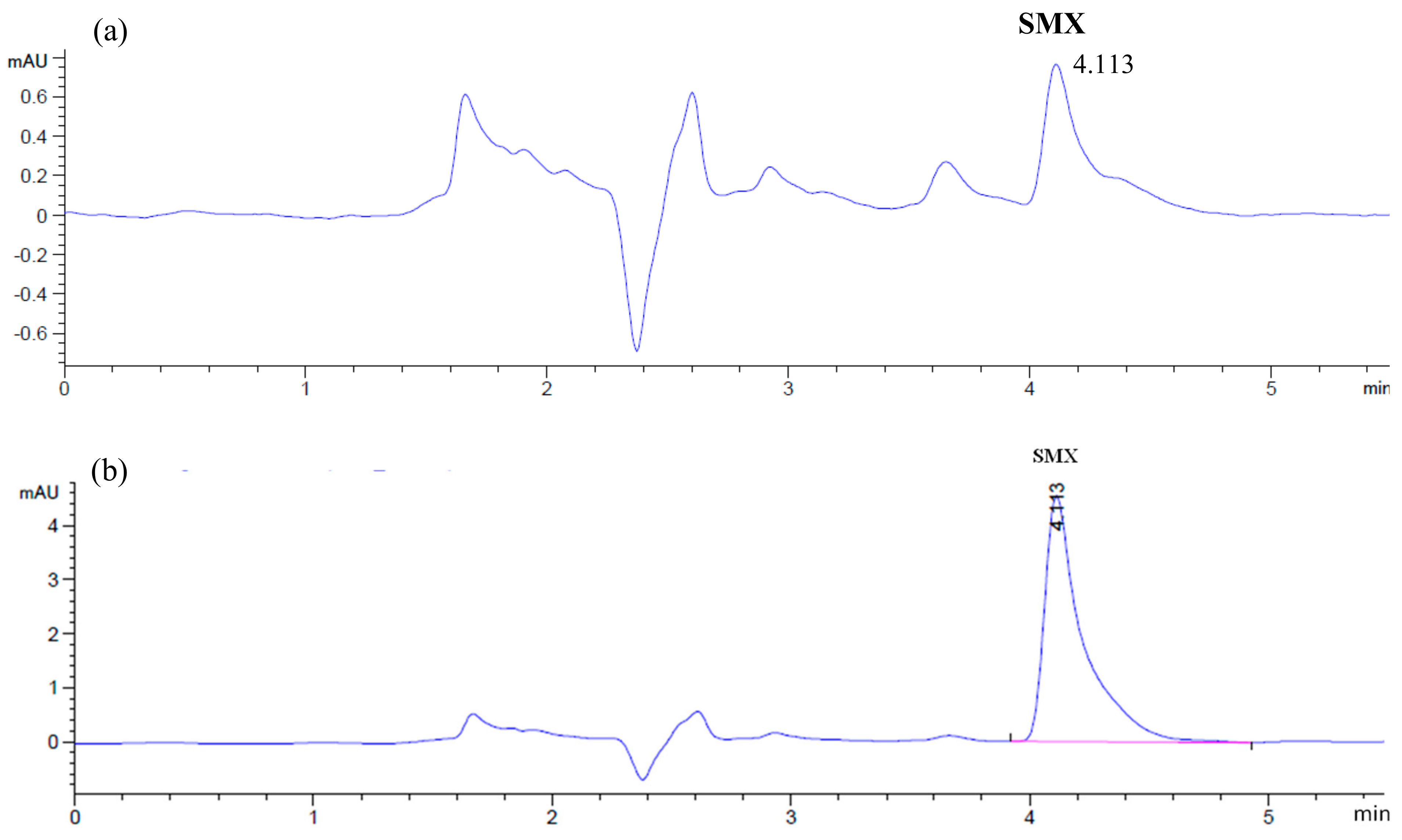
3.3. Analysis Method
3.4. Photocatalytic Degradation of SMX
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Alvarez, O.G.; Mazon, C.S.; Chen, L.; Deng, H.; Sui, M. The roles of conjugations of graphene and Ag in Ag3PO4-based photocatalysts for degradation of sulfamethoxazole. Catal. Sci. Technol. 2016, 6, 5972–5981. [Google Scholar] [CrossRef]
- Choi, M.; Shin, K.-H.; Jang, J. Plasmonic photocatalytic system using silver chloride/silver nanostructures under visible light. J. Colloid Interface Sci. 2010, 341, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xie, J.; Jia, W.; Wu, G.; Cao, Y. The formation of visible light-driven Ag/Ag2O photocatalyst with excellent property of photocatalytic activity and photocorrosion inhibition. J. Colloid Interface Sci. 2018, 516, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Wu, Z.; Yue, X.; Yuan, S.; Lu, H.; Liang, B. Silver Oxide as Superb and Stable Photocatalyst under Visible and Near-Infrared Light Irradiation and Its Photocatalytic Mechanism. Ind. Eng. Chem. Res. 2015, 54, 832–841. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Xing, Y.; Zong, L.; Yang, J. In situ anion-exchange synthesis and photocatalytic activity of AgBr/Ag2O heterostructure. Appl. Surf. Sci. 2015, 341, 190–195. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Cui, J. Ag2O/TiO2 Nanobelts Heterostructure with Enhanced Ultraviolet and Visible Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a New Visible-Light Photocatalyst: Self-Stability and High Photocatalytic Activity. Chem. Eur. J. 2011, 17, 7777–7780. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wu, J.; Gao, M.; Xin, Y.; Ma, T.; Sun, Y. Fabrication of Z-scheme g-C3N4/RGO/Bi2WO6 photocatalyst with enhanced visible-light photocatalytic activity. Chem. Eng. J. 2016, 290, 136–146. [Google Scholar] [CrossRef]
- Shen, H.; Wang, J.; Jiang, J.; Luo, B.; Mao, B.; Shi, W. All-solid-state Z-scheme system of RGO-Cu2O/Bi2O3 for tetracycline degradation under visible-light irradiation. Chem. Eng. J. 2017, 313, 508–517. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Tian, J.; Li, Y.; Cui, H. Towards full-spectrum (UV, visible, and near-infrared) photocatalysis: Achieving an all-solid-state Z-scheme between Ag2O and TiO2 using reduced graphene oxide as the electron mediator. Catal. Sci. Technol. 2017, 7, 4193–4205. [Google Scholar] [CrossRef]
- Ran, R.; Meng, X.; Zhang, Z. Facile preparation of novel graphene oxide-modified Ag2O/Ag3VO4/AgVO3 composites with high photocatalytic activities under visible light irradiation. Appl. Catal. B-Envrion. 2016, 196, 1–15. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A.; Ferraro, S.; Cespi, M.; Bonacucina, G.; Minicucci, M.; Di Cicco, A. Exfoliation of graphite into graphene in aqueous solution: An application as graphene/TiO2 nanocomposite to improve visible light photocatalytic activity. RSC Adv. 2016, 6, 93048–93055. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Visible-Light-Active TiO2-Based Hybrid Nanocatalysts for Environmental Applications. Catalysts 2017, 7, 100. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Yang, J.; Xu, Y.; Zhu, G.; Chen, K. Graphene Oxide Modified Ag2O Nanocomposites with Enhanced Photocatalytic Activity under Visible-Light Irradiation. Eur. J. Inorg. Chem. 2013, 2013, 6119–6125. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Cao, B.; Xin, S.; Guo, L.; Zhang, J.; Li, F. Ag3PO4/reduced graphite oxide sheets nanocomposites with highly enhanced visible light photocatalytic activity and stability. Appl. Catal. B-Envrion. 2013, 132–133, 45–53. [Google Scholar] [CrossRef]
- Moore, D.E.; Zhou, W. Photodegradation of sulfamethoxazole-a chemical-system capable of monitoring seasonal-changes in UVB intensity. Photochem. Photobiol. 1994, 59, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tian, J.; Li, T.; Cui, H. Synthesis of novel Ag/Ag2O heterostructures with solar full spectrum (UV, visible and near-infrared) light-driven photocatalytic activity and enhanced photoelectrochemical performance. Catal. Commun. 2016, 87, 82–85. [Google Scholar] [CrossRef]
- Dong, S.; Ding, X.; Guo, T.; Yue, X.; Han, X.; Sun, J. Self-assembled hollow sphere shaped Bi2WO6/RGO composites for efficient sunlight-driven photocatalytic degradation of organic pollutants. Chem. Eng. J. 2017, 316, 778–789. [Google Scholar] [CrossRef]
- Parra, S.; Olivero, J.; Pulgarin, C. Relationships between physicochemical properties and photoreactivity of four biorecalcitrant phenylurea herbicides in aqueous TiO2 suspension. Appl. Catal. B-Envrion. 2002, 36, 75–85. [Google Scholar] [CrossRef]
- Bandara, J.; Mielczarski, J.A.; Kiwi, J. 2. Photosensitized degradation of azo dyes on Fe, Ti, and Al oxides. Mechanism of charge transfer during the degradation. Langmuir 1999, 15, 7680–7687. [Google Scholar] [CrossRef]
- Shi, L.; Liang, L.; Ma, J.; Wang, F.; Sun, J. Enhanced photocatalytic activity over the Ag2O-g-C3N4 composite under visible light. Catal. Sci. Technol. 2014, 4, 758–765. [Google Scholar] [CrossRef]
- Gagné, F.; Blaise, C.; André, C. Occurrence of pharmaceutical products in a municipal effluent and toxicity to rainbow trout (Oncorhynchus mykiss) hepatocytes. Ecotoxicol. Environ. Saf. 2006, 64, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.F.; Xian, T.; Di, L.J.; Yang, H. Preparation of BiFeO3-Graphene Nanocomposites and Their Enhanced Photocatalytic Activities. J. Nanomater. 2013, 2013, 642897. [Google Scholar] [CrossRef]
- Yin, W.; Wang, W.; Sun, S. Photocatalytic degradation of phenol over cage-like Bi2MoO6 hollow spheres under visible-light irradiation. Catal. Commun. 2010, 11, 647–650. [Google Scholar] [CrossRef]
- Miyauchi, M. Photocatalysis and photoinduced hydrophilicity of WO3 thin films with underlying Pt nanoparticles. PCCP 2008, 10, 6258–6265. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, H.; Taniguchi, M.; Kaneco, S.; Suzuki, T. Photocatalytic degradation of bisphenol A by Ag3PO4 under visible light. Catal. Commun. 2013, 34, 30–34. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Abitorabi, M. Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible-light irradiation. J. Colloid Interface Sci. 2016, 480, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, L.; Lu, H.; Liu, L.; Wang, H.; Hu, C. Highly efficient and stable Ag/Ag3PO4 plasmonic photocatalyst in visible light. Catal. Commun. 2012, 17, 200–204. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, K.K.; Benayad, A.; Yoon, S.M.; Park, H.K.; Jung, I.S.; Jin, M.H.; Jeong, H.K.; Kim, J.M.; Choi, J.Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zou, G.; Deng, H. The Roles of Graphene and Ag in the Hybrid Ag@Ag2O-Graphene for Sulfamethoxazole Degradation. Catalysts 2018, 8, 272. https://doi.org/10.3390/catal8070272
Zhou L, Zou G, Deng H. The Roles of Graphene and Ag in the Hybrid Ag@Ag2O-Graphene for Sulfamethoxazole Degradation. Catalysts. 2018; 8(7):272. https://doi.org/10.3390/catal8070272
Chicago/Turabian StyleZhou, Li, Guoyan Zou, and Huiping Deng. 2018. "The Roles of Graphene and Ag in the Hybrid Ag@Ag2O-Graphene for Sulfamethoxazole Degradation" Catalysts 8, no. 7: 272. https://doi.org/10.3390/catal8070272