Electrocatalytic Performance of Carbon Supported WO3-Containing Pd–W Nanoalloys for Oxygen Reduction Reaction in Alkaline Media
Abstract
:1. Introduction
2. Results and Discussion
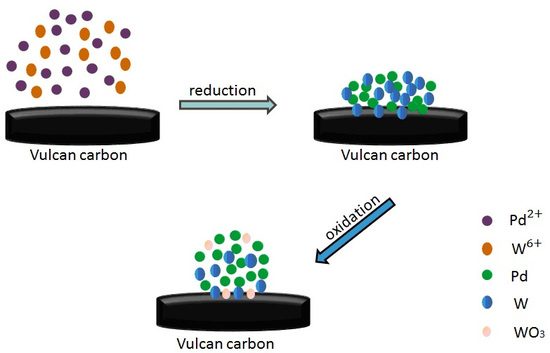
2.1. Characterization of Oxide-Rich PdW/C Catalysts
2.2. Electrochemical Performance
3. Materials and Methods
3.1. Preparation and Characterization of the Catalysts
3.2. Electrochemical Measurements
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Jeong, B.; Ocon, J.D. Oxygen electrocatalysis in chemical energy conversion and storage technologies. Curr. Appl. Phy. 2013, 13, 309–321. [Google Scholar] [CrossRef]
- Shin, D.; Jeong, B.; Choun, M.; Ocon, J.D.; Lee, J. Diagnosis of the measurement inconsistencies of carbon-based electrocatalysts for the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 1571–1580. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Fang, N.Z.B.; Li, H.; Bi, X.T.; Wang, H. Carbon-Supported Pt-Based Alloy Electrocatalysts for the Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells: Particle Size, Shape, and Composition Manipulation and Their Impact to Activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.L.; Xu, Y.Y.; Zhang, W.; Wu, T.; Xia, B.Y.; Wang, X. Unsupported Platinum-Based Electrocatalysts for Oxygen Reduction Reaction. ACS Energy Lett. 2017, 2, 2035–2043. [Google Scholar] [CrossRef]
- Wu, W.; Tang, Z.; Wang, K.; Liu, Z.; Li, L.; Chen, S. Peptide templated AuPt alloyed nanoparticles as highly efficient bi-functional electrocatalysts for both oxygen reduction reaction and hydrogen evolution reaction. Electrochim. Acta 2018, 260, 168–176. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Kühl, S.; Strasser, P. Oxygen Electrocatalysis on Dealloyed Pt Nanocatalysts. Top. Catal. 2016, 59, 1628–1637. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Chen, W. Recent developments in copper-based, non-noble metal electrocatalysts for the oxygen reduction reaction. Chin. J. Catal. 2016, 37, 1049–1061. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.; Yoon, T.; Kim, K.S. Nickel-Based Electrocatalysts for Energy-Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. ACS Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Chen, S.; Wu, Q.; Wang, X.; Hu, Z. A mini review on carbon-based metal-free electrocatalysts for oxygen reduction reaction. Chin. J. Catal. 2013, 34, 1986–1991. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Hamelin, J. Improved carbon nanostructures as a novel catalyst support in the cathode side of PEMFC: A critical review. Carbon 2015, 94, 705–728. [Google Scholar] [CrossRef]
- Bezerra, C.W.B.; Zhang, L.; Lee, K.; Liu, H.; Marques, A.L.B.; Marques, E.P.; Wang, H.; Zhang, J. A review of Fe–N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochim. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Risch, M.; Han, B.; Shao-Horn, Y. Recent Insights into Manganese Oxides in Catalyzing Oxygen Reduction Kinetics. ACS Catal. 2015, 5, 6021–6031. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Song, J.; Du, D.; Lin, Y. Metal-Organic Framework-Derived Non-Precious Metal Nanocatalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2017, 7, 1700363. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, N.; Lushington, A.; Sun, X. Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells. Catalysts 2016, 6, 116. [Google Scholar] [CrossRef]
- Choi, J.Y.; Higgins, D.; Jiang, G.; Hsu, R.; Qiao, J.; Chen, Z. Iron-tetracyanobenzene complex derived non-precious catalyst for oxygen reduction reaction. Electrochim. Acta 2015, 162, 224–229. [Google Scholar] [CrossRef]
- Thorseth, M.A.; Tornow, C.E.; Tse, E.C.M.; Gewirth, A.A. Cu complexes that catalyze the oxygen reduction reaction. Coord. Chem. Rev. 2013, 257, 130–139. [Google Scholar] [CrossRef]
- Fernández, J.L.; Mano, N.; Heller, A.; Bard, A.J. Optimization of “Wired” Enzyme O2–Electroreduction Catalyst compositions by Scanning Electrochemical Microscopy. Angew. Chem. Int. Ed. 2004, 43, 6355–6357. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Fernández, J.L.; Kim, Y.; Shin, W.; Bard, A.J.; Heller, A. Oxygen is Electroreduced to Water on a “Wired” Enzyme Electrode at a Lesser Overpotential than on Platinum. J. Am. Chem. Soc. 2003, 125, 15290–15291. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Babanova, S.; Rocha, R.C.; Desireddy, A.; Artyushkova, K.; Boncella, A.E.; Atanassov, P.; Martinez, J.S. A Hybrid DNA-Templated Gold Nanocluster for Enhanced Enzymatic Reduction of Oxygen. J. Am. Chem. Soc. 2015, 137, 11678–11687. [Google Scholar] [CrossRef] [PubMed]
- Husband, J.; Aaron, M.S.; Bains, R.K.; Lewis, A.R.; Warren, J.J. Catalytic reduction of dioxygen with modified Thermus thermophilus cytochrome c552. J. Inorg. Biochem. 2016, 157, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.; Suraniti, E.; Durand, F.; Mano, N. Oxygen reduction reactions of the thermostable bilirubin oxidase from Bacillus pumilus on mesoporous carbon-cryogel electrodes. Electrochim. Acta 2014, 117, 263–267. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, S.; Li, J.; Wang, F.; Kang, Y.; Lei, Z. Cerium carbide embedded in nitrogen-doped carbon as a highly active electrocatalyst for oxygen reduction reaction. J. Power Sources 2017, 359, 487–493. [Google Scholar] [CrossRef]
- Guoa, J.; Maob, Z.; Yana, X.; Suc, R.; Guanc, P.; Xua, B.; Zhangb, X.; Qinb, G.; Pennycookd, S.J. Ultrasmall tungsten carbide catalysts stabilized in graphitic layers for high-performance oxygen reduction reaction. Nano Energy 2016, 28, 261–268. [Google Scholar] [CrossRef]
- Bukola, S.; Merzougui, B.; Akinpelu, A.; Zeama, M. Cobalt and Nitrogen Co-Doped Tungsten Carbide Catalyst for Oxygen Reduction and Hydrogen Evolution Reactions. Electrochim. Acta 2016, 190, 1113–1123. [Google Scholar] [CrossRef]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Solla-Gullón, J.; Tammeveski, K.J. Recent progress in oxygen reduction electrocatalysis on Pd-based catalysts. Electroanal. Chem. 2016, 780, 327–336. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Zamani, P.; Yu, A.; Chen, Z. The application of graphene and its composites in oxygen reduction electrocatalysis: A perspective and review of recent progress. Energy Environ. Sci. 2016, 9, 357–390. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643−4667. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2009; Section 8; p. 23. [Google Scholar]
- Li, B.; Prakash, J. Oxygen reduction reaction on carbon supported Palladium–Nickel alloys in alkaline media. Electrochem. Commun. 2009, 11, 1162–1165. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Sarapuu, A.; Tammeveski, K.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Feliu, J.M. Electroreduction of oxygen on Vulcan carbon supported Pd nanoparticles and Pd–M nanoalloys in acid and alkaline solutions. Electrochim. Acta 2011, 56, 6702–6708. [Google Scholar] [CrossRef]
- Arenz, M.; Schmidt, T.J.; Wandelt, K.; Ross, P.N.; Markovic, N.M. The Oxygen Reduction Reaction on Thin Palladium Films Supported on a Pt(111) Electrode. J. Phys. Chem. B 2003, 107, 9813–9819. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Ren, X.; Mi, H.; Zhang, P.; Sun, L.; Deng, L.; Gao, Y. One-step rapid in-situ synthesis of nitrogen and sulfur co-doped three-dimensional honeycomb-ordered carbon supported PdNi nanoparticles as efficient electrocatalyst for oxygen reduction reaction in alkaline solution. Electrochim. Acta 2017, 253, 445–454. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Zhuo, H.; Wei, Z.; Zhuang, G.; Zhong, X.; Deng, S.; Li, X.; Wang, J. Oxygen vacancies on TiO2 promoted the activity and stability of supported Pd nanoparticles for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2264–2272. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Ke, T.; Zhang, P.; Ren, X.; Deng, L. Microwave-assisted synthesis of sulfur-doped graphene supported PdW nanoparticles as a high performance electrocatalyst for the oxygen reduction reaction. Electrochem. Commun. 2016, 69, 68–71. [Google Scholar] [CrossRef]
- Sun, L.; Liao, B.; Ren, X.; Li, Y.; Zhang, P.; Deng, L.; Gao, Y. Ternary PdNi-based nanocrystals supported on nitrogen-doped reduced graphene oxide as highly active electrocatalysts for the oxygen reduction reaction. Electrochim. Acta 2017, 235, 543–552. [Google Scholar] [CrossRef]
- Ren, X.; Liao, B.; Li, Y.; Zhang, P.; Deng, L.; Gao, Y. Facile synthesis of PdSnCo/nitrogen-doped reduced graphene as a highly active catalyst for lithium-air batteries. Electrochimica Acta 2017, 228, 36–44. [Google Scholar] [CrossRef]
- Yasmin, S.; Cho, S.; Jeon, S. Electrochemically reduced graphene-oxide supported bimetallic nanoparticles highly efficient for oxygen reduction reaction with excellent methanol tolerance. Appl. Surf. Sci. 2018, 434, 905–912. [Google Scholar] [CrossRef]
- Xue, Q.; Xu, G.; Mao, R.; Liu, H.; Zeng, J.; Jiang, J.; Chen, Y. Polyethyleneimine modified AuPd@PdAu alloy nanocrystals as advanced electrocatalysts towards the oxygen reduction reaction. J. Energy Chem. 2017, 26, 1153–1159. [Google Scholar] [CrossRef]
- Kabir, S.; Serov, A.; Artyushkova, K.; Atanassov, P. Nitrogen-Doped Three-Dimensional Graphene-Supported Palladium Nanocomposites: High-Performance Cathode Catalysts for Oxygen Reduction Reactions. ACS Catal. 2017, 7, 6609–6618. [Google Scholar] [CrossRef]
- Yu, T.H.; Sha, Y.; Merinov, B.V.; Goddard, W.A. Improved Non-Pt Alloys for the Oxygen Reduction Reaction at Fuel Cell Cathodes Predicted from Quantum Mechanics. J. Phys. Chem. C 2010, 114, 11527–11533. [Google Scholar] [CrossRef]
- Li, W.; Fan, F.-F.; Bard, A.J. The application of scanning electrochemical microscopy to the discovery of Pd–W electrocatalysts for the oxygen reduction reaction that demonstrate high activity, stability, and methanol tolerance. J. Solid State Electrochem. 2012, 16, 2563–2568. [Google Scholar] [CrossRef]
- Luo, Z.; Martí-Sànchez, S.; Nafria, R.; Joshua, G.S.; de la Mata, M.; Guardia, P.; Flox, C.; Martinez-Boubeta, C.; Simeonidis, K.; Llorca, J.; et al. Fe3O4@NiFexOy nanoparticles with enhanced electrocatalyticproperties for oxygen evolution in carbonate electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 29461–29469. [Google Scholar] [CrossRef] [PubMed]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong Interactions in Supported-Metal Catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Ocal, C.; Ferrer, S. The strong metal support interaction (SMSI) in Pt-TiO2 model catalysts, A new CO adsorption state on Pt-Ti atoms. J. Chem. Phys. 1986, 84, 6474–6478. [Google Scholar] [CrossRef]
- Zhang, S.; Plessow, P.N.; Willis, J.J.; Dai, S.; Xu, M.; Graham, G.W.; Cargnello, M.; Abild-Pedersen, F.; Pan, X. Dynamical Observation and Detailed Description of Catalysts under Strong Metal−Support Interaction. Nano Lett. 2016, 16, 4528–4534. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Zhu, Y.; Xu, S.; Wang, C.; Bian, C.; Meng, X.; Xiao, F.-S. Strong Metal−Support Interactions Achieved by Hydroxide-to-Oxide Support Transformation for Preparation of Sinter-Resistant Gold Nanoparticle Catalysts. ACS Catal. 2017, 7, 7461–7465. [Google Scholar] [CrossRef]
- Jia, Q.; Ghoshal, S.; Li, J.; Liang, W.; Meng, G.; Che, H.; Zhang, S.; Ma, Zi.; Mukerjee, S. Metal and Metal Oxide Interactions and Their Catalytic Consequences for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2017, 139, 7893–7903. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Guo, Z.; Li, W.; Pang, Z.; Tong, Q. Oxidation of Ethanol on Carbon-Supported Oxide-Rich Pd–W Bimetallic Nanoparticles in Alkaline Media. Russ. J. Phys. Chem. A 2017, 91, 1994–2001. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, T.; Li, W.; Zhang, C.; Zhang, D.; Pang, Z. Carbon Supported Oxide-Rich Pd-Cu Bimetallic Electrocatalysts for Ethanol Electrooxidation in Alkaline Media Enhanced by Cu/CuOx. Catalysts 2016, 6, 62. [Google Scholar] [CrossRef]
- Staikov, G. Monte Carlo method. In Electrocrystallization in Nanotechnology; Wiley-VCH: Weinheim, Germany, 2007; Chapter 2.3. [Google Scholar]
- Kolb, D.M.; Engelmann, G.E.; Ziegler, J.C. On the unusual electrochemical stability of nanofabricated copper clusters. Angew. Chem. Int. Ed. 2010, 39, 1123–1125. [Google Scholar] [CrossRef]
- Lv, M.; Li, W.; Liu, H.; Wen, W.; Dong, G.; Liu, J.; Peng, K. Enhancement of the formic acid electrooxidation activity of palladium using graphene/carbon black binary carbon supports. Chin. J. Catal. 2017, 38, 939–947. [Google Scholar] [CrossRef]
- Nutor, R.K.; Xu, X.; Fan, X.; Ren, S.; He, X.; Fang, Y. Structural anisotropy in FeCuNbSiB alloys: An in situ synchrotron XRD study. J. Magn. Magn. Mater. 2018, 454, 51–56. [Google Scholar] [CrossRef]
- Ohnuma, M.; Hono, K. Origin of the magnetic anisotropy induced by stress annealing in Fe-based nanocrystalline alloy. Appl. Phys. Lett. 2005, 86, 152513. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye–Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wen, W.; Li, W.; Yang, Y.; Hou, D.; Liu, C. Fabrication of high performance carbon-supported ternary Pd-Cu-Fe electrocatalysts for formic acid electrooxidation via partly galvanic sacrifice of tunable binary Cu-Fe alloy templates. Electrochim. Acta 2016, 196, 223–230. [Google Scholar] [CrossRef]
- Wu, Q.; Rao, Z.; Yuan, L.; Jiang, L.; Sun, G.; Ruan, J.; Zhou, Z.; Sang, S. Carbon supported PdO with improved activity and stability for oxygen reduction reaction in alkaline solution. Electrochim. Acta 2014, 150, 157–166. [Google Scholar] [CrossRef]
- Ji, X.; Ma, M.; Ge, R.; Ren, X.; Wang, H.; Liu, J.; Liu, Z.; Asiri, A.M.; Sun, X. WO3 Nanoarray: An Efficient Electrochemical Oxygen Evolution Catalyst Electrode Operating in Alkaline Solution. Inorg. Chem. 2017, 56, 14743–14746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Cui, Z.; Liu, C.; Lu, T.; Xing, W. Pd nanoparticles supported on WO3/C hybrid material as catalyst for oxygen reduction reaction. J. Power Sources 2008, 185, 941–945. [Google Scholar] [CrossRef]
- Foruzin, L.J.; Rezvani, Z.; Shishavan, Y.H. Biuck Habibi Ni2Zn0.5Fe-LDH modified carbon paste electrode as an efficient electrocatalyst for water oxidation in neutral media. Int. J. Hydrog. Energy 2018, 43, 150–160. [Google Scholar] [CrossRef]
- Habibi, B.; Ghaderi, S. Synthesis, characterization and electrocatalytic activity of Co@Pt nanoparticles supported on carbon-ceramic substrate for fuel cell applications. Int. J. Hydrog. Energy 2015, 40, 5115–5125. [Google Scholar] [CrossRef]
- Jung, W.; Han, J.; Ha, S. Analysis of palladium-based anode electrode using electrochemical impedance spectra in direct formic acid fuel cells. J. Power Sources 2007, 173, 53–59. [Google Scholar] [CrossRef]
- Ferreira, P.J.; la O’, G.J.; Shao-Horn, Y. Instability of Pt/C electrocatalysts in proton exchange membrane fuel cells. J. Electrochem. Soc. 2005, 152, A2256–A2271. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Zardari, P. Electrocatalysis of oxygen reduction on multi-walled carbon nanotube supported Ru-based catalysts in alkaline media. Int. J. Hydrog. Energy 2016, 41, 8803–8818. [Google Scholar] [CrossRef]
- Wen, W.J.; Li, C.Y.; Li, W.P.; Tian, Y. Carbon-supported Pd-Cr electrocatalysts for the electrooxidation of formic acid that demonstrate high activity and stability. Electrochim. Acta 2013, 109, 201–206. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709. [Google Scholar] [CrossRef]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, N.; Li, W.; Guo, Z.; Xu, X.; Zhao, H. Electrocatalytic Performance of Carbon Supported WO3-Containing Pd–W Nanoalloys for Oxygen Reduction Reaction in Alkaline Media. Catalysts 2018, 8, 225. https://doi.org/10.3390/catal8060225
Cui N, Li W, Guo Z, Xu X, Zhao H. Electrocatalytic Performance of Carbon Supported WO3-Containing Pd–W Nanoalloys for Oxygen Reduction Reaction in Alkaline Media. Catalysts. 2018; 8(6):225. https://doi.org/10.3390/catal8060225
Chicago/Turabian StyleCui, Nan, Wenpeng Li, Zengfeng Guo, Xun Xu, and Hongxia Zhao. 2018. "Electrocatalytic Performance of Carbon Supported WO3-Containing Pd–W Nanoalloys for Oxygen Reduction Reaction in Alkaline Media" Catalysts 8, no. 6: 225. https://doi.org/10.3390/catal8060225