Degradation and Loss of Antibacterial Activity of Commercial Amoxicillin with TiO2/WO3-Assisted Solar Photocatalysis
Abstract
:1. Introduction
2. Results
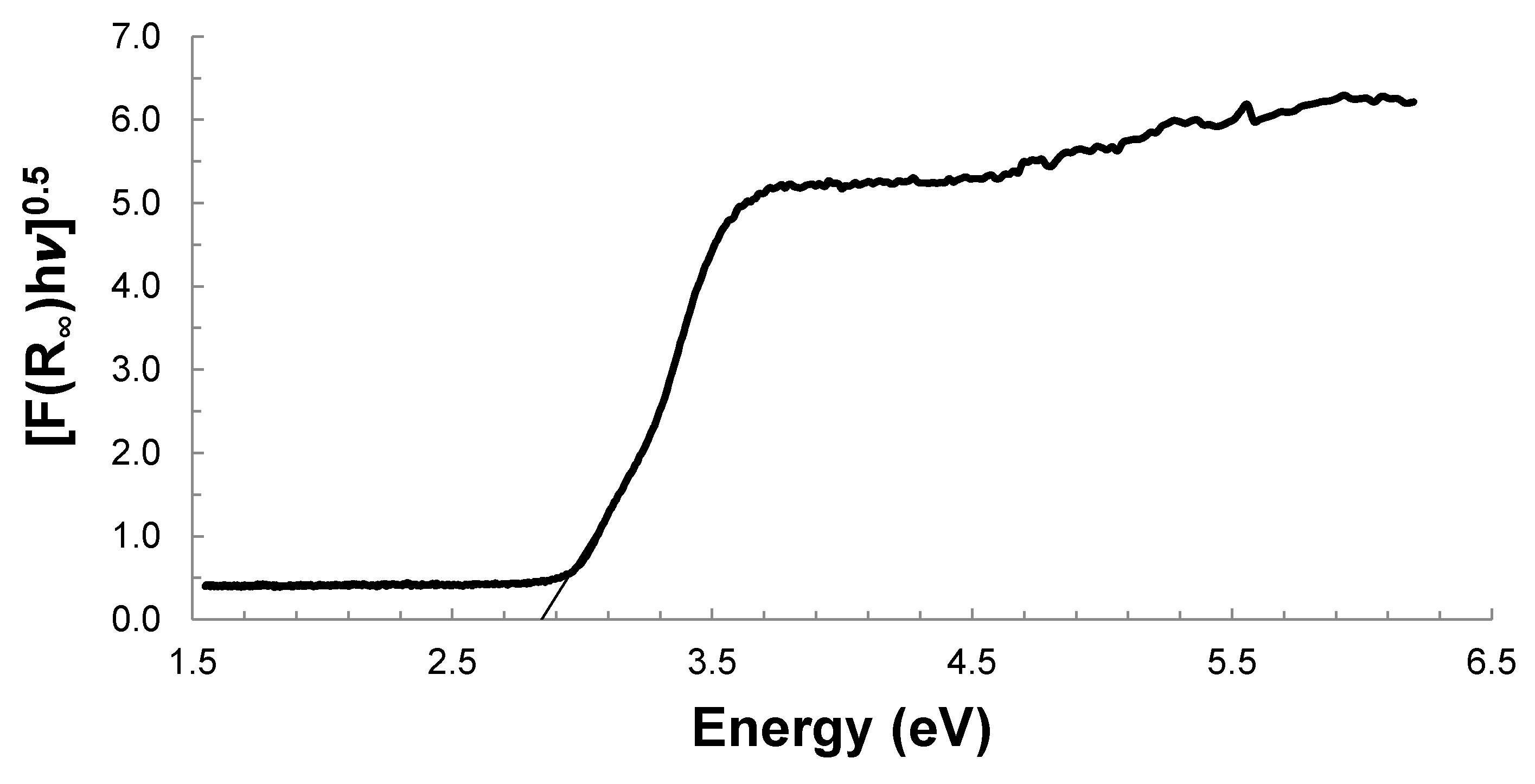
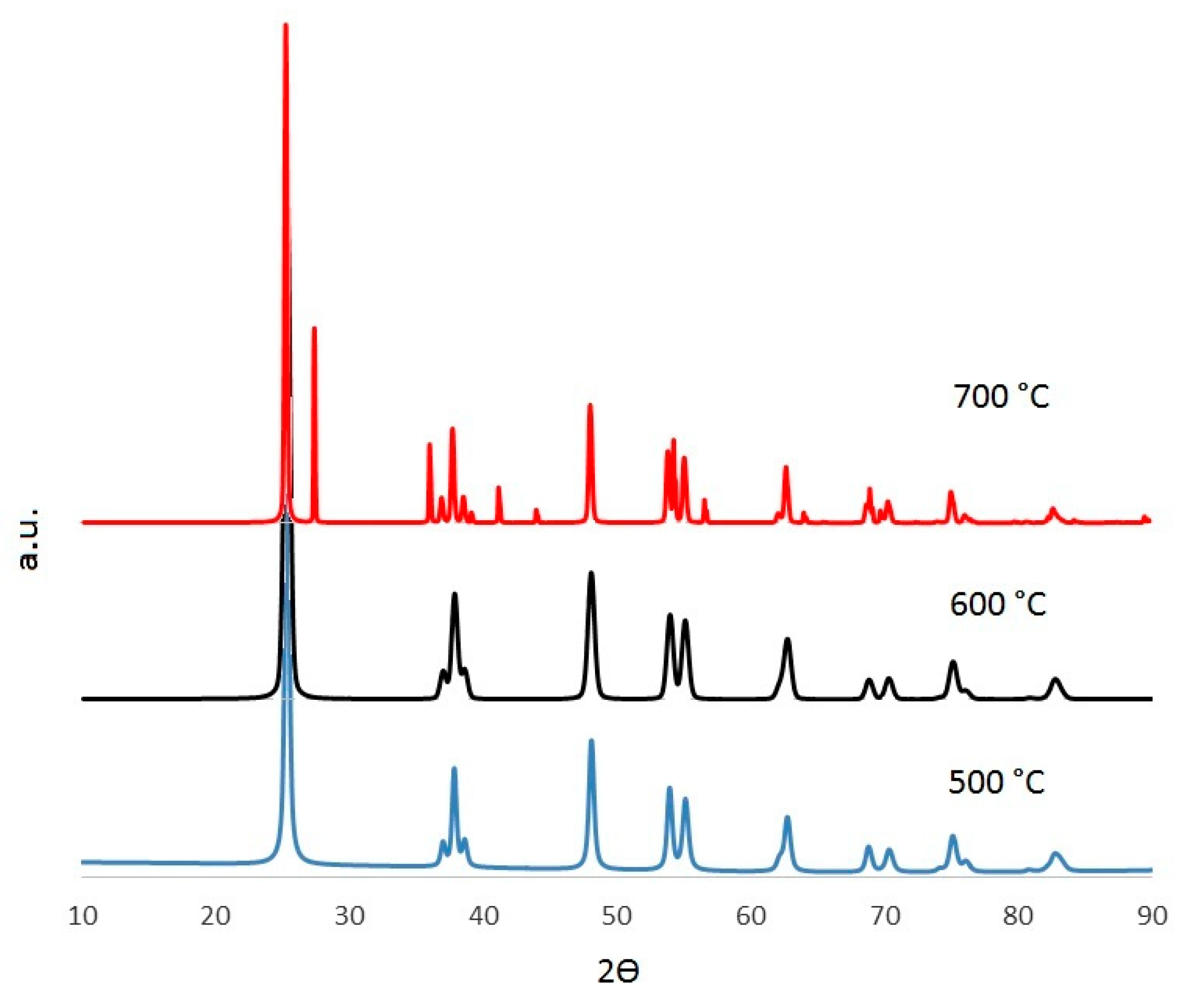
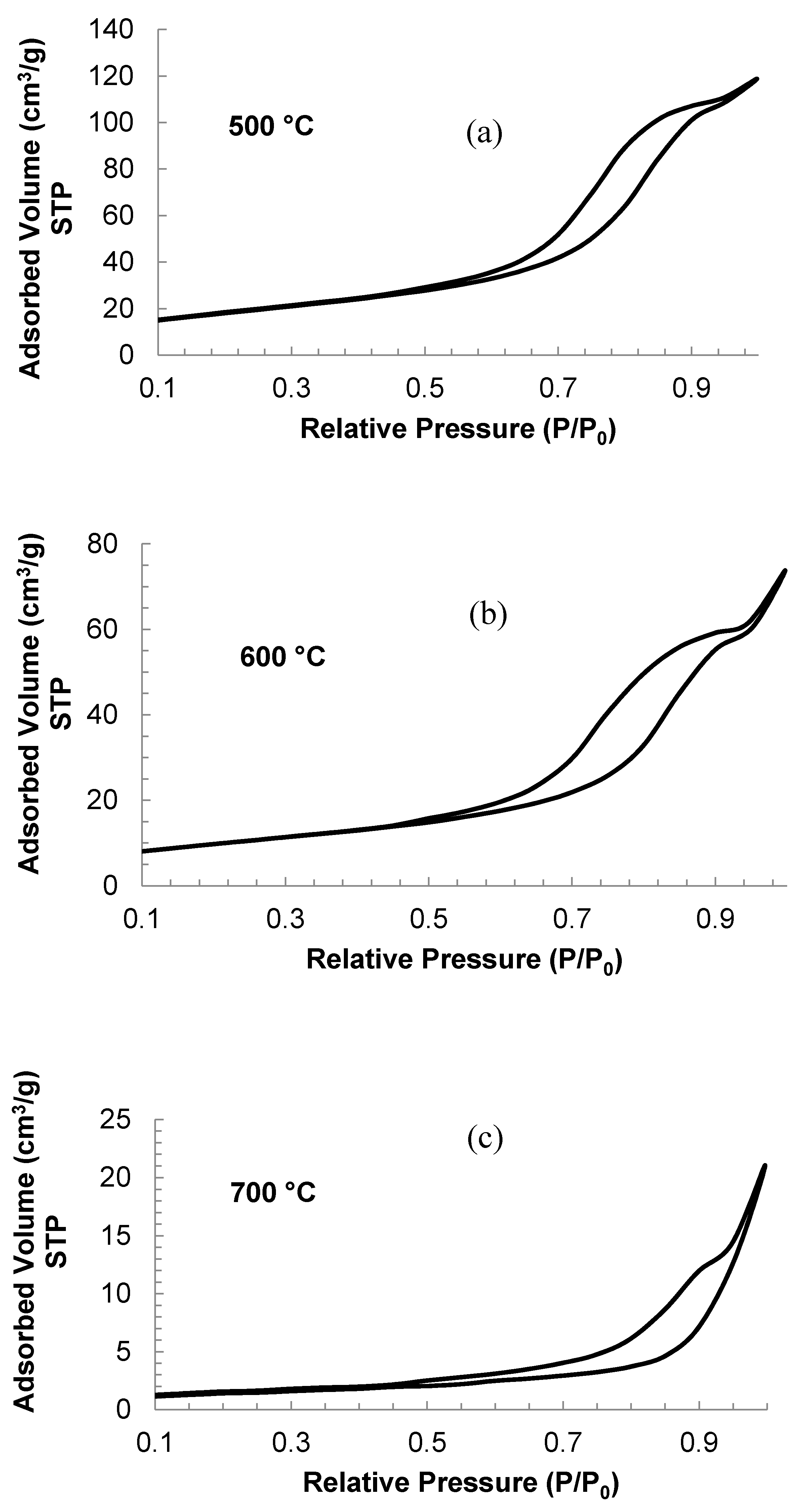
2.1. Effect of the Calcination Temperature on the TiO2/WO3 Characterization
2.2. Degradation and Loss of Antibacterial Activity of Commercial Amoxicillin by TiO2/WO3-Assisted Solar Photocatalysis
2.2.1. Effect of the Calcination Temperature
2.2.2. Effect of the Initial Amoxicillin Concentration
2.2.3. Effect of the Catalyst Load
2.2.4. Kinetic Analysis of the Amoxicillin Photocatalytic Degradation
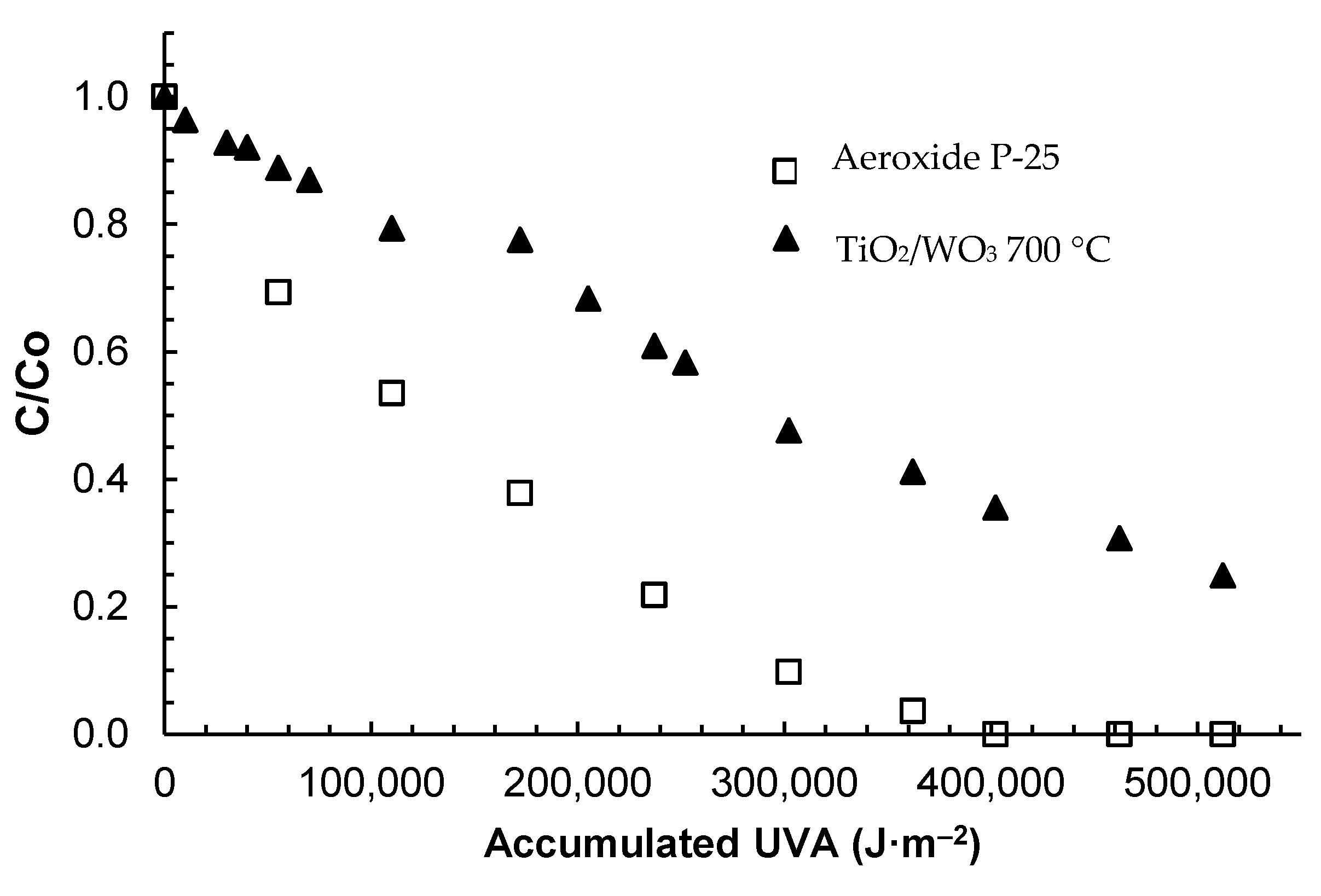
2.2.5. Loss of Antibiotic Power
3. Materials and Methods
3.1. Preparation of TiO2/WO3 Catalysts
3.2. Photocatalyst Characterization
3.3. Evaluation of the Photocatalytic Performance
3.4. Kinetic Analysis
3.5. Determination of Inhibition Halo
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Zhang, X.; Tryk, D. Heterogeneous photocatalysis: From water photolysis to applications in environmental cleanup. Int. J. Hydrogen Energy 2007, 32, 2664–2672. [Google Scholar] [CrossRef]
- Alarcon, D.C.; Maldonado, M.I.; Malato, S.; Gernjak, W. Photocatalytic decontamination and disinfection of water with solar collectors. Catal. Today 2007, 122, 137–149. [Google Scholar]
- Domènech, X.; Jardim, W.F.; Litter, M.I. Procesos avanzados de oxidación para la eliminación de contaminantes. In Eliminación de contaminantes por fotocatálisis heterogénea; Blesa, M.A., Ed.; CYTED: La Plata, Argentina, 2001; pp. 3–26. [Google Scholar]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review. Recent Patents Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Shen, S.H.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar]
- Ramos-Delgado, N.A.; Hinojosa-Reyes, L.; Guzman-Mar, J.L.; Gracia-Pinilla, M.A.; Hernández-Ramírez, A. Synthesis by sol–gel of WO3/TiO2 for solar photocatalytic degradation of malathion pesticide. Catal. Today 2013, 209, 35–40. [Google Scholar] [CrossRef]
- Ramos-Delgado, N.A.; Gracia-Pinilla, M.A.; Maya-Treviño, L.; Hinojosa-Reyes, L.; Guzman-Mar, J.L.; Hernández-Ramírez, A. Solar photocatalytic activity of TiO2 modified with WO3 on the degradation of an organophosphorus pesticide. J. Hazard. Mater. 2013, 263, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Liu, H.; Wang, C.; Liu, S.; Sun, P.; Wang, L.; Liu, Y. Heterostructured TiO2/WO3 porous microspheres: Preparation, characterization and photocatalytic properties. Catal. Today 2013, 201, 195–202. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Das, R.; Sarkar, S.; Chakraborty, S.; Choi, H.; Bhattacharjee, C. Remediation of Antiseptic Components in Wastewater by Photocatalysis Using TiO2 Nanoparticles. Ind. Eng. Chem. Res. 2014, 53, 3012–3020. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Vasquez, M.I.; Kümmerer, K. Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes—Degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 2011, 85, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Basile, T.; Petrella, A.; Petrella, M.; Boghetich, G.; Petruzzelli, V.; Colasuonno, S.; Petruzzelli, D. Review of Endocrine-Disrupting-Compound Removal Technologies in Water and Wastewater Treatment Plants: An EU Perspective. Ind. Eng. Chem. Res. 2011, 50, 8389–8401. [Google Scholar] [CrossRef]
- Klauson, D.; Babkina, J.; Stepanova, K.; Krichevskaya, M.; Prei, S. Aqueous photocatalytic oxidation of amoxicillin. Catal. Today 2010, 151, 39–45. [Google Scholar] [CrossRef]
- Chaudhuri, M.; Wahap, M.Z.B.A.; Affam, A.C. Treatment of aqueous solution of antibiotics amoxicillin and cloxacillin by modified photo-Fenton process. Desalin. Water Treat. 2013, 51, 7255–7268. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Mantzavinos, D. Boron-doped diamond oxidation of amoxicillin pharmaceutical formulation: Statistical evaluation of operating parameters, reaction pathways and antibacterial activity. J. Environ. Manag. 2017, 195, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2013, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Li, F.B.; Yang, C.L.; Ge, W.K. Photocatalytic activity of WOx-TiO2 under visible light irradiation. J. Photochem. Photobiol. A Chem. 2001, 141, 209–217. [Google Scholar] [CrossRef]
- Mccullagh, C.; Skillen, N.; Adams, M.; Robertson, P.K.J. Photocatalytic reactors for environmental remediation: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1002–1017. [Google Scholar] [CrossRef]
- Colina-Márquez, J.; Díaz, D.; Rendón, A.; López-Vásquez, A.; Machuca-Martínez, F. Photocatalytic treatment of a dye polluted industrial effluent with a solar pilot-scale CPC reactor. J. Adv. Oxid. Technol. 2009, 12, 93–99. [Google Scholar] [CrossRef]
- Xiaobo, C. Titanium Dioxide Nanomaterials and Their Energy Applications. Chin. J. Catal. 2009, 30, 839–851. [Google Scholar]
- Liu, K.; Hsueh, Y.; Su, C.; Perng, T. Photoelectrochemical application of mesoporous TiO2/WO3 nanohoneycomb prepared by sol e gel method. Int. J. Hydrogen Energy 2013, 38, 7750–7755. [Google Scholar] [CrossRef]
- Piszcz, M.; Tryba, B.; Grzmil, B.; Morawski, A.W. Photocatalytic Removal of Phenol Under UV Irradiation on WOx–TiO2 Prepared by Sol – Gel Method. Catal. Lett. 2009, 128, 190–196. [Google Scholar] [CrossRef]
- Weng, X.; Chen, Z.; Chen, Z.; Megharaj, M. Colloids and Surfaces A: Physicochemical and Engineering Aspects Clay supported bimetallic Fe/Ni nanoparticles used for reductive degradation of amoxicillin in aqueous solution: Characterization and kinetics. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 404–409. [Google Scholar] [CrossRef]
- Haider, A.J.; Anbari, R.H.A.; Kadhim, G.R.; Salame, C.T. Exploring potential Environmental applications of TiO2 Nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Reyes, A.; Colina-Marquez, J. Evaluation of the photocatalytic activity of iron oxide nanoparticles functionalized with titanium dioxide. J. Phys. Conf. Ser. 2016, 687, 012034. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The Effect of Calcination Temperature on the Surface Microstructure and Photocatalytic Activity of TiO2 Thin Films Prepared by Liquid Phase Deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Sánchez-Mora, E.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fís. 2007, 53, 18–22. [Google Scholar]
- Beranek, R.; Kisch, H. Tuning the optical and photoelectrochemical properties of surface-modified TiO2. Photochem. Photobiol. Sci. 2008, 7, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Enhancement of photoinduced hydrogen production from irradiated Pt/TiO2 suspensions with simultaneous degradation of azo-dyes. Appl. Catal. B Environ. 2006, 64, 171–179. [Google Scholar] [CrossRef]
- Bezrodna, T.; Gavrilko, T.; Puchkovska, G.; Shimanovska, V.; Baran, J. Spectroscopic study of TiO2 (rutile) –benzophenone heterogeneous systems. J. Mol. Struct. 2002, 614, 315–324. [Google Scholar] [CrossRef]
- Enríquez, J.M.H.; Serrano, L.A.G.; Soares, B.H.Z.; Alamilla, R.G.; Resendiz, B.B.Z.; Del Sánchez, T.; Hernández, , A.C. Síntesis y Caracterización de Nanopartículas de N-TiO2 – Anatasa. Superf. y Vacío 2008, 21, 1–5. [Google Scholar]
- Rungjaroentawon, N.; Onsuratoom, S.; Chavadej, S. Hydrogen production from water splitting under visible light irradiation using sensitized mesoporous-assembled TiO2-SiO2 mixed oxide photocatalysts. Int. J. Hydrogen Energy 2012, 37, 11061–11071. [Google Scholar] [CrossRef]
- Yang, H.; Shi, R.; Zhang, K.; Hu, Y.; Tang, A.; Li, X. Synthesis of WO3/TiO2 nanocomposites via sol–gel method. J. Alloys Compd. 2005, 398, 200–202. [Google Scholar] [CrossRef]
- Djaoued, Y.; Badilescu, S.; Ashrit, P.V.; Bersani, D.; Lottici, P.; Robichaud, J. Study of Anatase to Rutile Phase Transition in Nanocrystalline Titania Films. J. Sol-Gel Sci. Technol. 2002, 24, 255–264. [Google Scholar] [CrossRef]
- Colina-Márquez, J.; Machuca, F.; Puma, G.L. Radiation Absorption and Optimization of Solar Photocatalytic Reactors for Environmental Applications. Environ. Sci. Technol. 2010, 44, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Mueses, M.A.; Machuca-Martinez, F.; Hernández-Ramirez, A.; Puma, G.L. Effective radiation field model to scattering—Absorption applied in heterogeneous photocatalytic reactors. Chem. Eng. J. 2015, 279, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of photocatalytic activity of titanium dioxide using non-metal doping methods under visible light: A review. Int. J. Environ. Sci. Technol. 2017, 1–24. [Google Scholar] [CrossRef]
- Colina-Márquez, J.; Machuca-Martínez, F.; Puma, G.L. Photocatalytic mineralization of commercial herbicides in a pilot-scale solar CPC reactor: Photoreactor modeling and reaction kinetics constants independent of radiation field. Environ. Sci. Technol. 2009, 43, 8953–8960. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cooper, W.J.; Jung, J.; Song, W. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Gómez-Lus, M.L.; Rodríguez-Avial, C.; Martinez, L.M.; Vila, J. Procedimientos en Microbiología Clínica; SIEMC: Madrid, Spain, 2000; pp. 10–20. [Google Scholar]

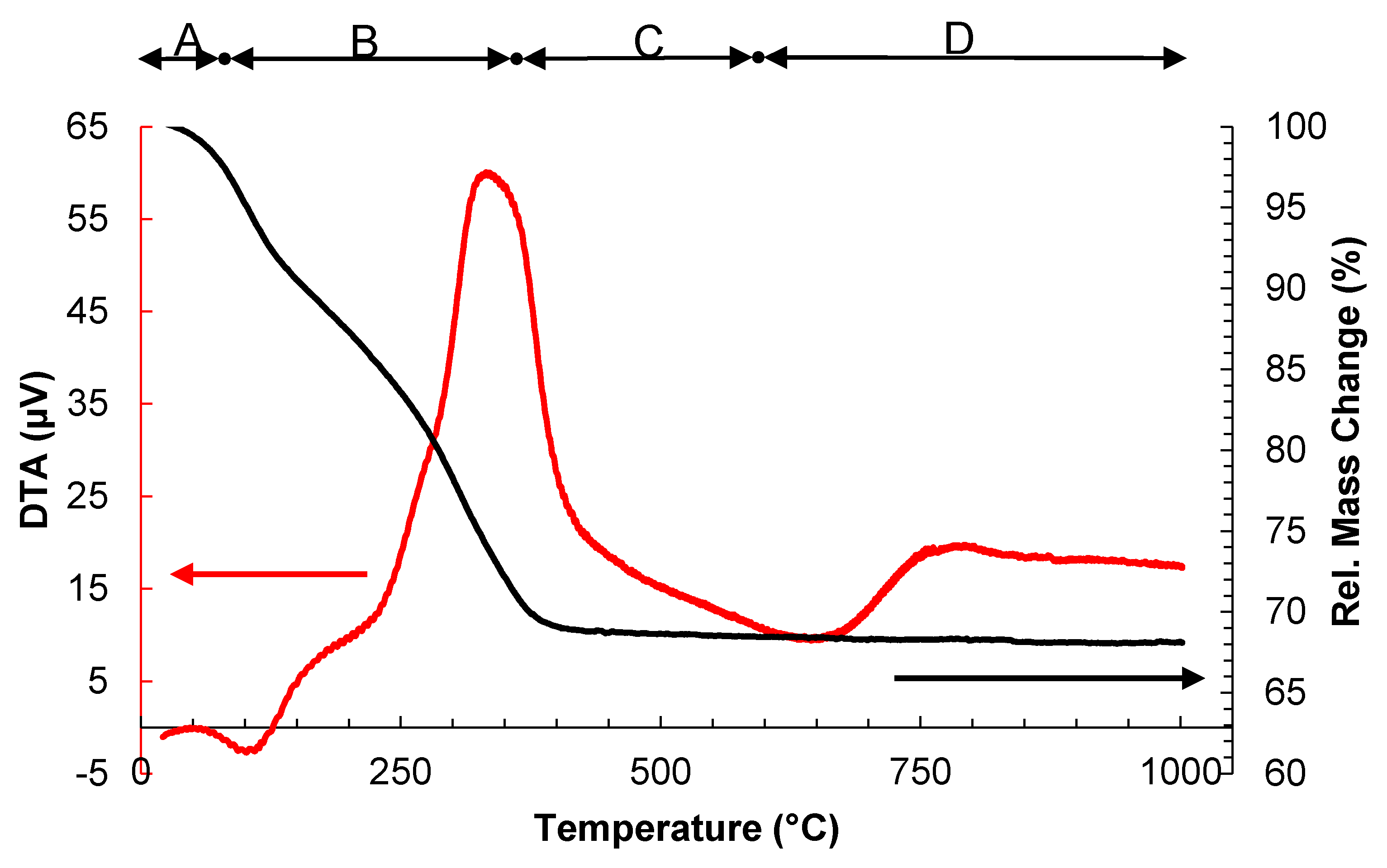


| Calcination Temperature | |||
|---|---|---|---|
| 500 °C | 600 °C | 700 °C | |
| Eg (eV) | 3.12 | 3.12 | 2.84 |
| λ (nm) | 397 | 397 | 436 |
| 500 °C | 600 °C | 700 °C | ||
|---|---|---|---|---|
| Anatase | Anatase | Anatase | Rutile | |
| Øperp (nm) | 59 | 58 | 116 | 820 |
| Øpara (nm) | 83 | 37 | 137 | 204 |
| Calcination Temperature (°C) | Surface Area (m2 g−1) | Average Pore Diameter (nm) |
|---|---|---|
| 500 | 66.45 | 77.53 |
| 600 | 35.93 | 77.31 |
| 700 | 4.970 | 122.40 |
| Calcination Temperature, °C | |||||||
|---|---|---|---|---|---|---|---|
| 500 | 600 | 700 | |||||
| Catalyst load [g L−1] | 0.05 | 0.10 | 0.05 | 0.10 | 0.05 | 0.10 | |
| Amoxicillin concentration [ppm] | 100 | 39.8 | 28.6 | 58.6 | 31.7 | 45.6 | 64.4 |
| 200 | 4.7 | 16.7 | 17.6 | 46.0 | 12.0 | 17.0 | |
| Catalyst | Kads (ppm−1) | kapp (ppm m2 J−1) |
|---|---|---|
| TiO2/WO3 | 0.0632 | 1.88 × 10−4 |
| Aeroxide P25 | 0.0087 | 1.14 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arce-Sarria, A.; Machuca-Martínez, F.; Bustillo-Lecompte, C.; Hernández-Ramírez, A.; Colina-Márquez, J. Degradation and Loss of Antibacterial Activity of Commercial Amoxicillin with TiO2/WO3-Assisted Solar Photocatalysis. Catalysts 2018, 8, 222. https://doi.org/10.3390/catal8060222
Arce-Sarria A, Machuca-Martínez F, Bustillo-Lecompte C, Hernández-Ramírez A, Colina-Márquez J. Degradation and Loss of Antibacterial Activity of Commercial Amoxicillin with TiO2/WO3-Assisted Solar Photocatalysis. Catalysts. 2018; 8(6):222. https://doi.org/10.3390/catal8060222
Chicago/Turabian StyleArce-Sarria, Augusto, Fiderman Machuca-Martínez, Ciro Bustillo-Lecompte, Aracely Hernández-Ramírez, and José Colina-Márquez. 2018. "Degradation and Loss of Antibacterial Activity of Commercial Amoxicillin with TiO2/WO3-Assisted Solar Photocatalysis" Catalysts 8, no. 6: 222. https://doi.org/10.3390/catal8060222