Activation of Persulfate Using an Industrial Iron-Rich Sludge as an Efficient Nanocatalyst for Landfill Leachate Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Activator Characterization
2.2. The Catalytic and Adsorptive Activity of CS and SL
2.3. Effective Parameters for the Leachate Treatment
2.3.1. Effect of pH
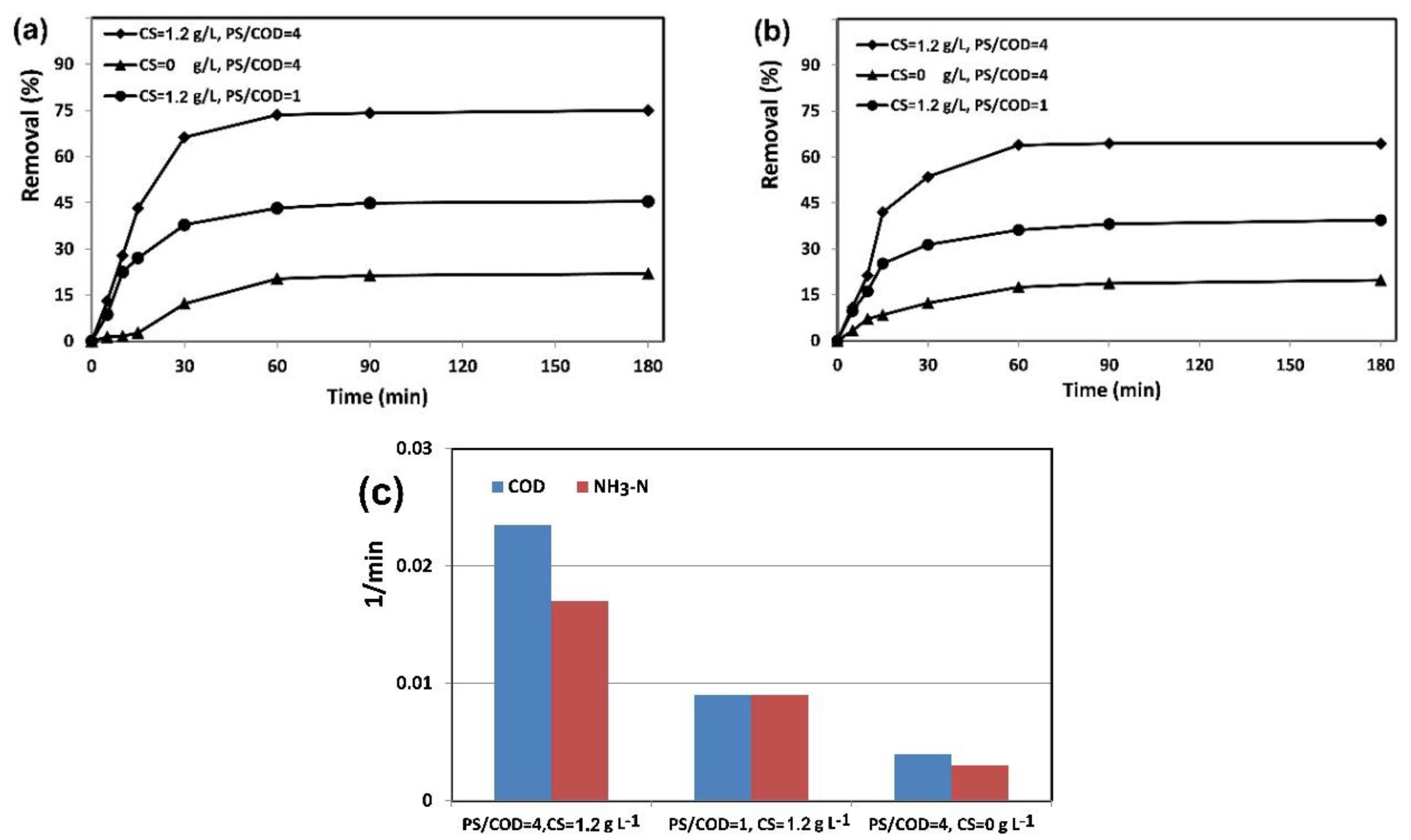
2.3.2. Effect of Converter Sludge Dosage and PS/COD Mass Ratio
2.3.3. Effect of Reaction Time and Kinetic Study
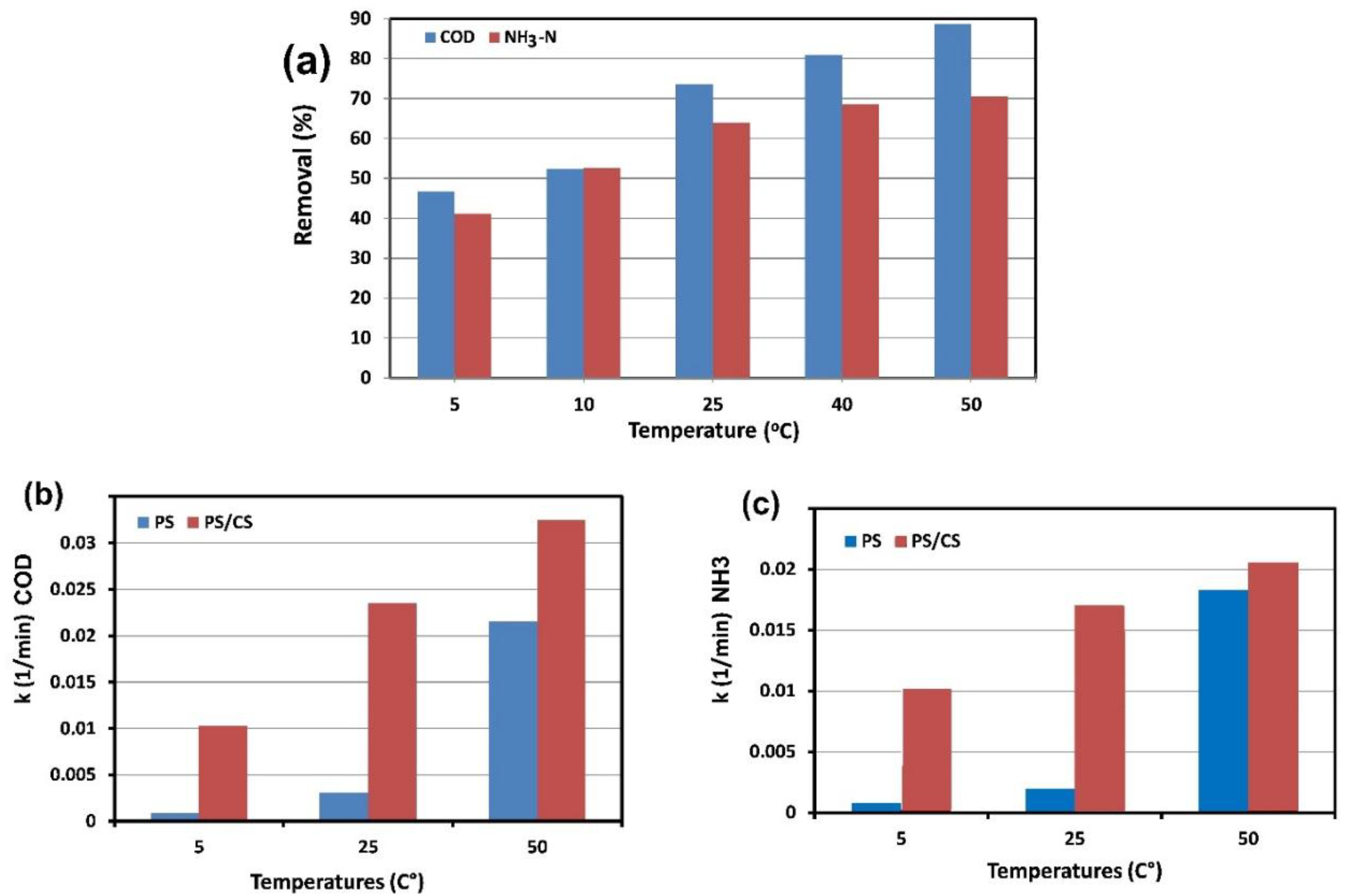
2.3.4. Effect of Temperature and Activation Energy
2.3.5. The Metal Release from CS during the CS/PS Process
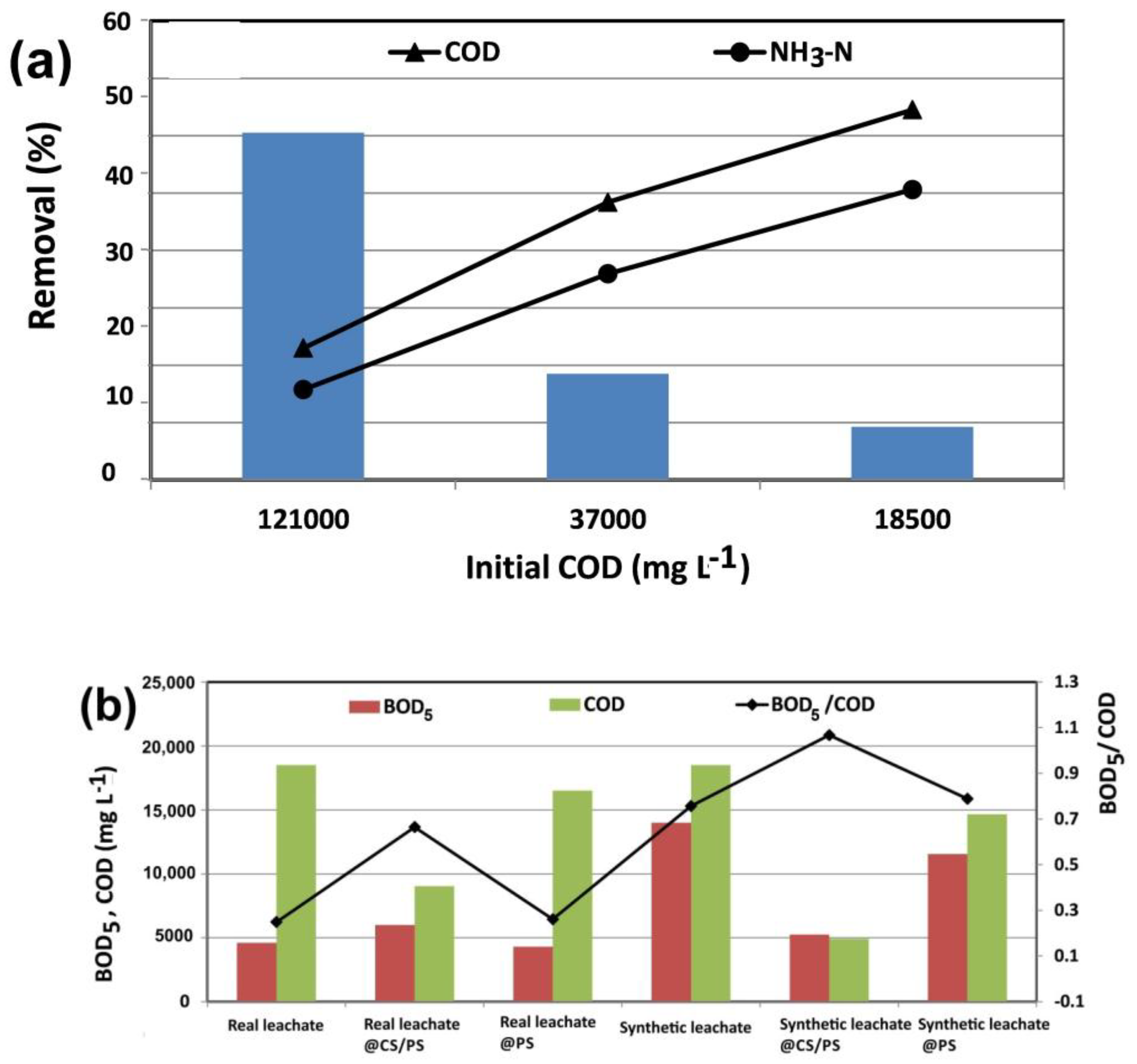
2.3.6. Treatment of Real Landfill Leachate and Biodegradability Investigation
3. Materials and Methods
3.1. Materials
3.2. Synthetic Leachate and Fresh Leachate Characteristics
3.3. Converter Sludge and Slag Characteristics
3.4. Analytical Methods
3.5. Oxidation Experiments
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abbas, A.A.; Jingsong, G.; Ping, L.Z.; Ya, P.Y.; Al-Rekabi, W.S. Review on landfill leachate treatments. Am. J. Appl. Sci. 2009, 6, 672–684. [Google Scholar] [CrossRef]
- Wu, L.; Chen, S.; Zhou, J.; Zhang, C.; Liu, J.; Luo, J.; Song, G.; Qian, G.; Song, L.; Xia, M. Simultaneous removal of organic matter and nitrate from bio-treated leachate via iron–carbon internal micro-electrolysis. RSC Adv. 2015, 5, 68356–68360. [Google Scholar] [CrossRef]
- Kulikowska, D.; Klimiuk, E. The effect of landfill age on municipal leachate composition. Bioresour. Technol. 2008, 99, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.-Y.; Kang, M.-S.; Yim, S.-K.; Choi, K.-H. Advanced landfill leachate treatment using an integrated membrane process. Desalination 2002, 149, 109–114. [Google Scholar] [CrossRef]
- Silva, A.C.; Dezotti, M.; Sant’Anna, G.L. Treatment and detoxification of a sanitary landfill leachate. Chemosphere 2004, 55, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ezyske, C.M. Sulfate radical-advanced oxidation process (SR-AOP) for simultaneous removal of refractory organic contaminants and ammonia in landfill leachate. Water Res. 2011, 45, 6189–6194. [Google Scholar] [CrossRef] [PubMed]
- Chemlal, R.; Abdi, N.; Drouiche, N.; Lounici, H.; Pauss, A.; Mameri, N. Rehabilitation of Oued Smar landfill into a recreation park: Treatment of the contaminated waters. Ecol. Eng. 2013, 51, 244–248. [Google Scholar] [CrossRef]
- Chemlal, R.; Azzouz, L.; Kernani, R.; Abdi, N.; Lounici, H.; Grib, H.; Mameri, N.; Drouiche, N. Combination of advanced oxidation and biological processes for the landfill leachate treatment. Ecol. Eng. 2014, 73, 281–289. [Google Scholar] [CrossRef]
- Soubh, A.; Mokhtarani, N. The post treatment of composting leachate with a combination of ozone and persulfate oxidation processes. RSC Adv. 2016, 6, 76113–76122. [Google Scholar] [CrossRef]
- Abu Amr, S.S.; Aziz, H.A.; Adlan, M.N.; Bashir, M.J.K. Pretreatment of stabilized leachate using ozone/persulfate oxidation process. Chem. Eng. J. 2013, 221, 492–499. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Zhao, Y.; Chai, X.; Niu, D. Enhanced dewaterability of sewage sludge in the presence of Fe(II)-activated persulfate oxidation. Bioresour. Technol. 2012, 116, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Kang, S.G.; Kim, D.W.; Chiu, P.C. Degradation of 2,4-dinitrotoluene by persulfate activated with iron sulfides. Chem. Eng. J. 2011, 172, 641–646. [Google Scholar] [CrossRef]
- House, D.A. Kinetics and Mechanism of Oxidations by Peroxydisulfate. Chem. Rev. 1962, 62, 185–203. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, W.-X.; Brown, D.G.; Sethi, D. Oxidation of Lindane with Fe(II)-Activated Sodium Persulfate. Environ. Eng. Sci. 2008, 25, 221–228. [Google Scholar] [CrossRef]
- Lin, Y.T.; Liang, C.; Chen, J.H. Feasibility study of ultraviolet activated persulfate oxidation of phenol. Chemosphere 2011, 82, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Santos, A.; Vicente, F.; González, C. Diuron abatement using activated persulphate: Effect of pH, Fe(II) and oxidant dosage. Chem. Eng. J. 2010, 162, 257–265. [Google Scholar] [CrossRef]
- Diao, Z.; Xu, X.; Chen, H.; Jiang, D.; Yang, Y.; Kong, L.; Sun, Y.; Hu, Y.; Hao, Q.; Liu, L. Simultaneous removal of Cr (VI) and phenol by persulfate activated with bentonite-supported nanoscale zero-valent iron: Reactivity and mechanism. J. Hazard. Mater. 2016, 316, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Nachiappan, S.; Gopinath, K.P. Treatment of pharmaceutical effluent using novel heterogeneous fly ash activated persulfate system. J. Environ. Chem. Eng. 2015, 3, 2229–2235. [Google Scholar] [CrossRef]
- Karchegani, S.M.; Hoodaji, M.; Kalbasi, M. The effect of steel converter slag application along with sewage sludge in iron nutrition and corn plant yield. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 96–104. [Google Scholar]
- Bozkurt, M.A.; Akdeniz, H.; Keskin, B.; Yilmaz, I.H. Possibilities of using sewage sludge as nitrogen fertilizer for maize. Acta Agric. Scand. Sect. B Soil Plant Sci. 2006, 56, 37–41. [Google Scholar] [CrossRef]
- Brofas, G.; Michopoulos, P.; Alifragis, D. Sewage Sludge as an Amendment for Calcareous Bauxite Mine Spoils Reclamation. J. Environ. Qual. 2000, 29, 811–816. [Google Scholar] [CrossRef]
- Mahmood, T.; Elliott, A. A review of secondary sludge reduction technologies for the pulp and paper industry. Water Res. 2006, 40, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Kalbasi, M.; Hajrasuliha, S. Effect of converter sludge, and its mixtures with organic matter, elemental sulfur and sulfuric acid on availability of iron, phosphorus and manganese of 3 calcareous soils from central Iran. Afr. J. Agric. Res. 2012, 7, 568–576. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Z.S.; Bruell, C.J. Influence of pH on persulfate oxidation of TCE at ambient temperatures. Chemosphere 2007, 66, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Shiying, Y.; Wang, P.; Yang, X.; Wei, G.; Zhang, W.; Shan, L. A novel advanced oxidation process to degrade organic pollutants in wastewater: Microwave-activated persulfate oxidation. J. Environ. Sci. 2009, 21, 1175–1180. [Google Scholar] [CrossRef]
- Yan, J.; Lei, M.; Zhu, L.; Anjum, M.N.; Zou, J.; Tang, H. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate. J. Hazard. Mater. 2011, 186, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Dionysiou, D.D.; Al-abed, S.R.; Zhou, D. Applied Catalysis B: Environmental Superoxide radical driving the activation of persulfate by magnetite nanoparticles: Implications for the degradation of PCBs. Appl. Catal. B Environ. 2013, 129, 325–332. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, H.; Xue, X. Ultrasound enhanced heterogeneous activation of peroxydisulfate by bimetallic Fe-Co/GAC catalyst for the degradation of Acid Orange 7 in water. J. Environ. Sci. 2012, 26, 1267–1273. [Google Scholar] [CrossRef]
- Virtanen, S.; Schmuki, P.; Davenport, A.J.; Vitus, C.M. Dissolution of Thin Iron Oxide Films Used as Models for Iron Passive Films Studied by In Situ X-ray Absorption Near-Edge Spectroscopy. J. Electrochem. Soc. 1997, 144, 198. [Google Scholar] [CrossRef]
- Liang, C.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere 2004, 55, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xiu, Z.; Liu, F.; Wu, G.; Adamson, D.; Newell, C.; Vikesland, P.; Tsai, A.L.; Alvarez, P.J. Perfluorooctanoic acid degradation in the presence of Fe(III) under natural sunlight. J. Hazard. Mater. 2013, 262, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Govindan, K.; Raja, M.; Maheshwari, S.U.; Noel, M. Analysis and understanding of amido black 10B dye degradation in aqueous solution by electrocoagulation with the conventional oxidants peroxomonosulfate, peroxodisulfate and hydrogen peroxide. Environ. Sci. Water Res. Technol. 2014, 1, 108–119. [Google Scholar] [CrossRef]
- Liang, C.; Guo, Y.Y. Remediation of diesel-contaminated soils using persulfate under alkaline condition. Water Air. Soil Pollut. 2012, 223, 4605–4614. [Google Scholar] [CrossRef]
- Furman, O.S.; Teel, A.M.Y.L.; Watts, R.J. Mechanism of Base Activation of Persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Quan, X.; Chen, S. Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zero-valent iron and peroxydisulfate at ambient temperature. Sep. Purif. Technol. 2010, 71, 302–307. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, H.W.; Park, J.M.; Park, H.S.; Yoon, C. Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J. Hazard. Mater. 2009, 168, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, J.M.; Durán, A.; González, R.; Expósito, A.J. In situ chemical oxidation of carbamazepine solutions using persulfate simultaneously activated by heat energy, UV light, Fe2+ ions, and H2O2. Appl. Catal. B Environ. 2015, 176–177, 120–129. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Shao, Y.; Gao, N.; Deng, Y.; Tan, C.; Zhou, S. Zero-valent iron/persulfate(Fe0/PS) oxidation acetaminophen in water. Int. J. Environ. Sci. Technol. 2013, 11, 881–890. [Google Scholar] [CrossRef]
- Xu, X.R.; Li, X.Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep. Purif. Technol. 2010, 72, 105–111. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, W.; Shi, P.; Guo, J.; Cheng, J. Characterization of dissolved organic matter in landfill leachate during the combined treatment process of air stripping, Fenton, SBR and coagulation. Waste Manag. 2015, 41, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Münnich, K.; Fricke, K.; Harborth, P. Removal of nitrogen from MBT residues by leachate recirculation in combination with intermittent aeration. Waste Manag. Res. 2014, 32, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hilles, A.H.; Abu Amr, S.S.; Hussein, R.A.; El-Sebaie, O.D.; Arafa, A.I. Performance of combined sodium persulfate/H2O2 based advanced oxidation process in stabilized landfill leachate treatment. J. Environ. Manag. 2016, 166, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, S.; Gandhimathi, R.; Muthukkumaran, K. Bioclogging in porous media: Influence in reduction of hydraulic conductivity and organic contaminants during synthetic leachate permeation. J. Environ. Health Sci. Eng. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hrapovic, L.; Rowe, R.K. Intrinsic degradation of volatile fatty acids in laboratory-compacted clayey soil. J. Contam. Hydrol. 2002, 58, 221–242. [Google Scholar] [CrossRef]
- Pazoki, M.; Abdoli, M.A.; Karbassi, A.; Mehrdadi, N.; Yaghmaeian, K. Attenuation of municipal landfill leachate through land treatment. Int. J. Environ. Heal. Sci. Eng. 2014, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamsi, M.A.; Thomson, N.R. Treatment of organic compounds by activated persulfate using nanoscale zerovalent iron. Ind. Eng. Chem. Res. 2013, 52, 13564–13571. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad, A.L.; Hameed, B.H. Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chem. Eng. J. 2008, 136, 164–172. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Fulgione, I.; Frontistis, Z.; Rizzo, L.; Mantzavinos, D. Solar light-induced photoelectrocatalytic degradation of bisphenol-A on TiO2/ITO film anode and BDD cathode. Catal. Today 2013, 209, 74–78. [Google Scholar] [CrossRef]





| Temperature | COD | NH3 | ||||
|---|---|---|---|---|---|---|
| kCS/PS, * 10−3/min | kPS, * 10−3/min | E% | kCS/PS, * 10−3/min | kPS, * 10−3/min | E% | |
| 5 | 10.3 | 0.9 | 91.3 | 10 | 0.8 | 92 |
| 25 | 23.5 | 3.1 | 86.8 | 17 | 2 | 88.2 |
| 50 | 32.5 | 21.5 | 33.8 | 20.5 | 18.3 | 10.7 |
| Metal Elements | mg L−1 | Metal Elements | mg L−1 |
|---|---|---|---|
| Al | 2.34 | Ag | <0.02 |
| Fe | 91.13 | As | <0.05 |
| Si | 55.35 | Cd | <0.02 |
| Co | <0.05 | ||
| Cr | <0.05 | ||
| Cu | <0.05 | ||
| Hg | <0.05 | ||
| Pb | <0.05 | ||
| Se | <0.05 | ||
| Ni | <0.05 | ||
| Ti | <0.05 | ||
| Sn | <0.05 |
| Quantity | Components | |
|---|---|---|
| 18,500 | COD (mg L−1) | General indicators |
| 775 | NH3–N(mg L−1) | |
| 14,000 | BOD5 (mg L−1) | |
| 24 ± 2 | Temperature (°C) | |
| 5.8–6.0 | pH (adjusted using NaOH) | |
| 2 | Butyric (butanoic) acid | Volatile fatty acids (VFA)s) (mL L−1) |
| 6 | Propionic (propanoic) acid | |
| 10 | Acetic (ethanoic) acid | |
| 325 | K2CO3 | Inorganic nutrients (mg L−1) |
| 3015 | NaHCO3 | |
| 312 | KHCO3 | |
| 30 | K2HPO4 | |
| 50 | NaNO3 | |
| 1440 | NaCl | |
| 695 | CO(NH2)2, urea | |
| 1653 | NH4Cl | |
| 2882 | CaCl2 | |
| 156 | MgSO4 | |
| 1647 | MgCl2 | |
| 896 | Mg(OH)2 |
| Parameter | Range | Average * | Unit |
|---|---|---|---|
| COD | 109,000–133,000 | 121,000 | mg L−1 |
| BOD5 | 27,000–34,200 | 30,600 | mg L−1 |
| NH3-N | 6440–7100 | 6770 | mg L−1 |
| TDS | 44,500–51,500 | 48,000 | mg L−1 |
| EC | 79.3–97.1 | 88.2 | mS cm−1 |
| pH | 6.7–7.9 | 7.3 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soubh, A.M.; Baghdadi, M.; Abdoli, M.A.; Aminzadeh, B. Activation of Persulfate Using an Industrial Iron-Rich Sludge as an Efficient Nanocatalyst for Landfill Leachate Treatment. Catalysts 2018, 8, 218. https://doi.org/10.3390/catal8050218
Soubh AM, Baghdadi M, Abdoli MA, Aminzadeh B. Activation of Persulfate Using an Industrial Iron-Rich Sludge as an Efficient Nanocatalyst for Landfill Leachate Treatment. Catalysts. 2018; 8(5):218. https://doi.org/10.3390/catal8050218
Chicago/Turabian StyleSoubh, Alaa Mohamad, Majid Baghdadi, Mohammad Ali Abdoli, and Behnoush Aminzadeh. 2018. "Activation of Persulfate Using an Industrial Iron-Rich Sludge as an Efficient Nanocatalyst for Landfill Leachate Treatment" Catalysts 8, no. 5: 218. https://doi.org/10.3390/catal8050218






