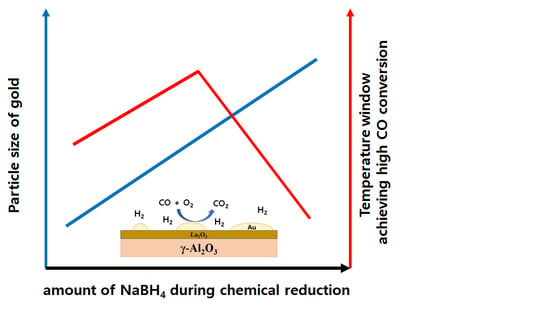
Preferential CO Oxidation in H2 over Au/La2O3/Al2O3 Catalysts: The Effect of the Catalyst Reduction Method
Abstract
:1. Introduction
2. Results
2.1. CO-PROX Performance
2.2. Characterization of the Catalysts
2.2.1. TEM Analysis
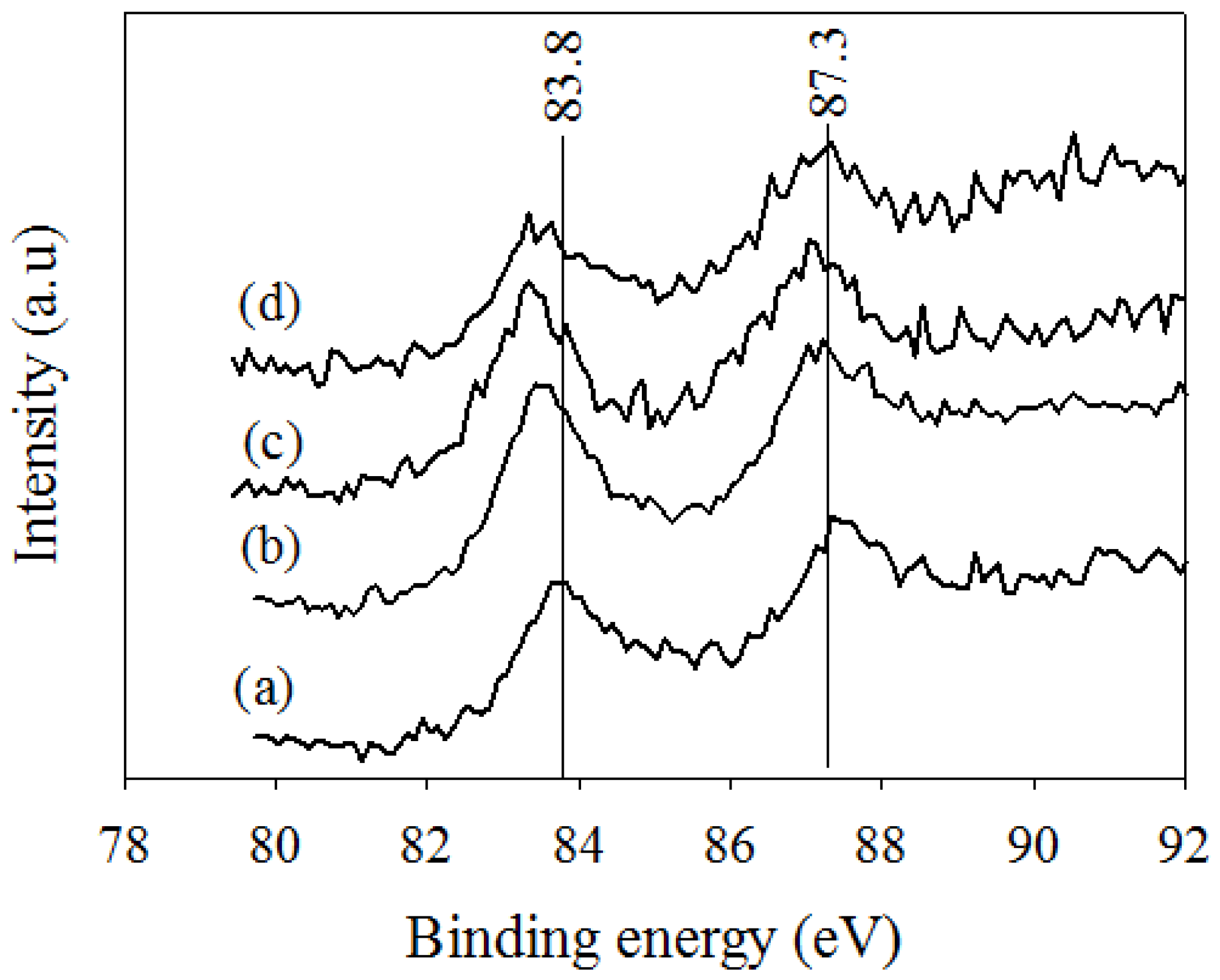
2.2.2. XPS Analysis
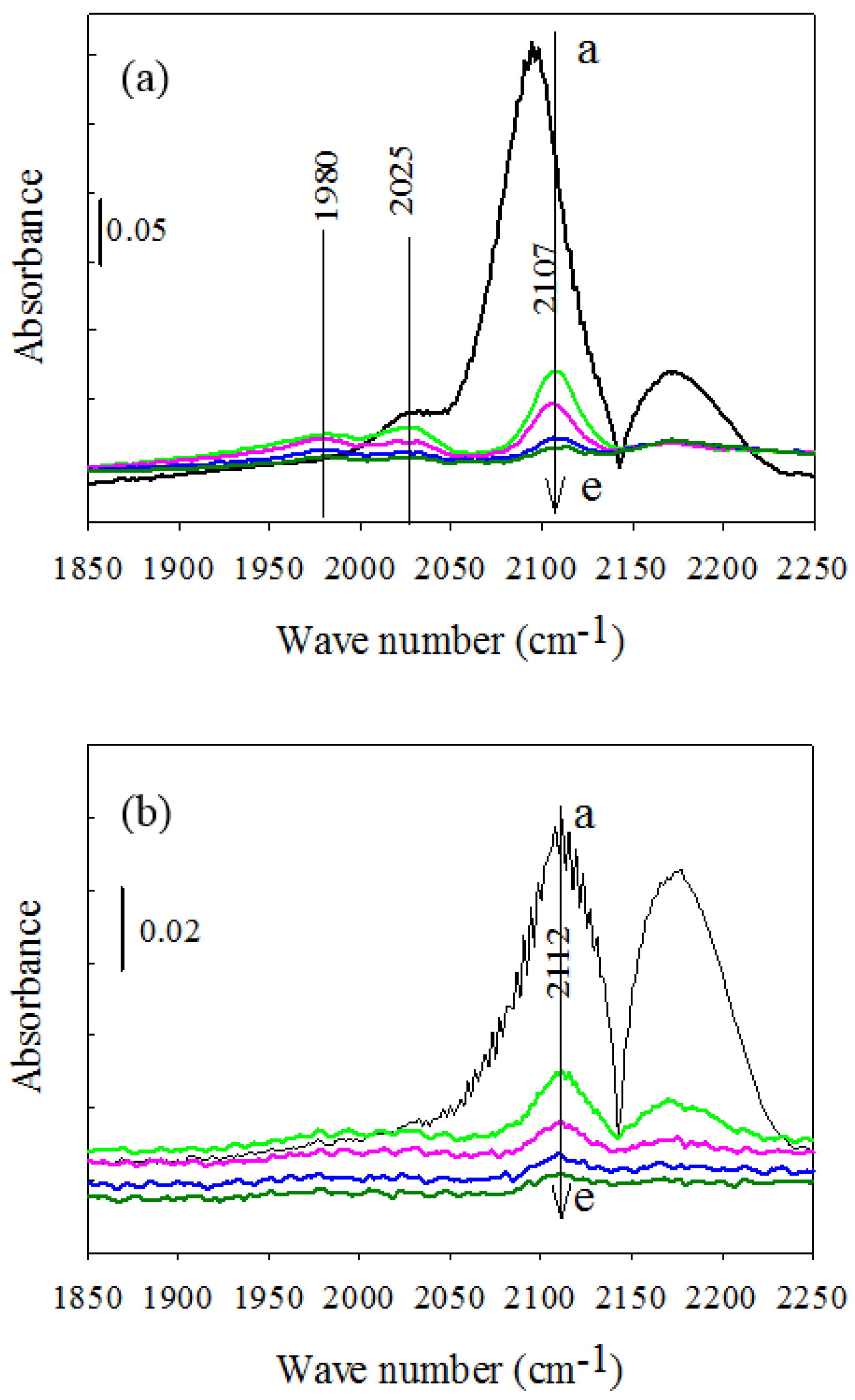
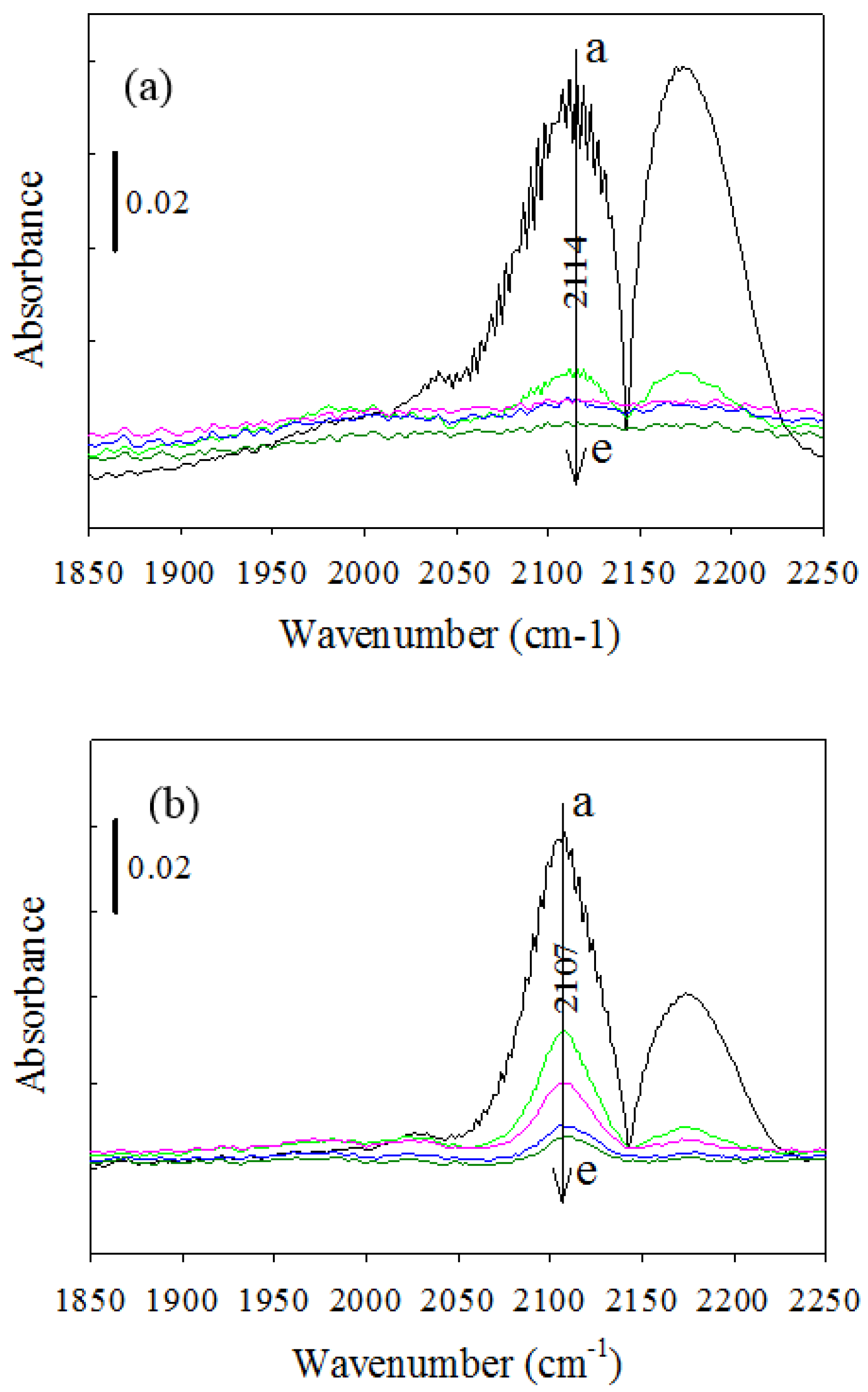
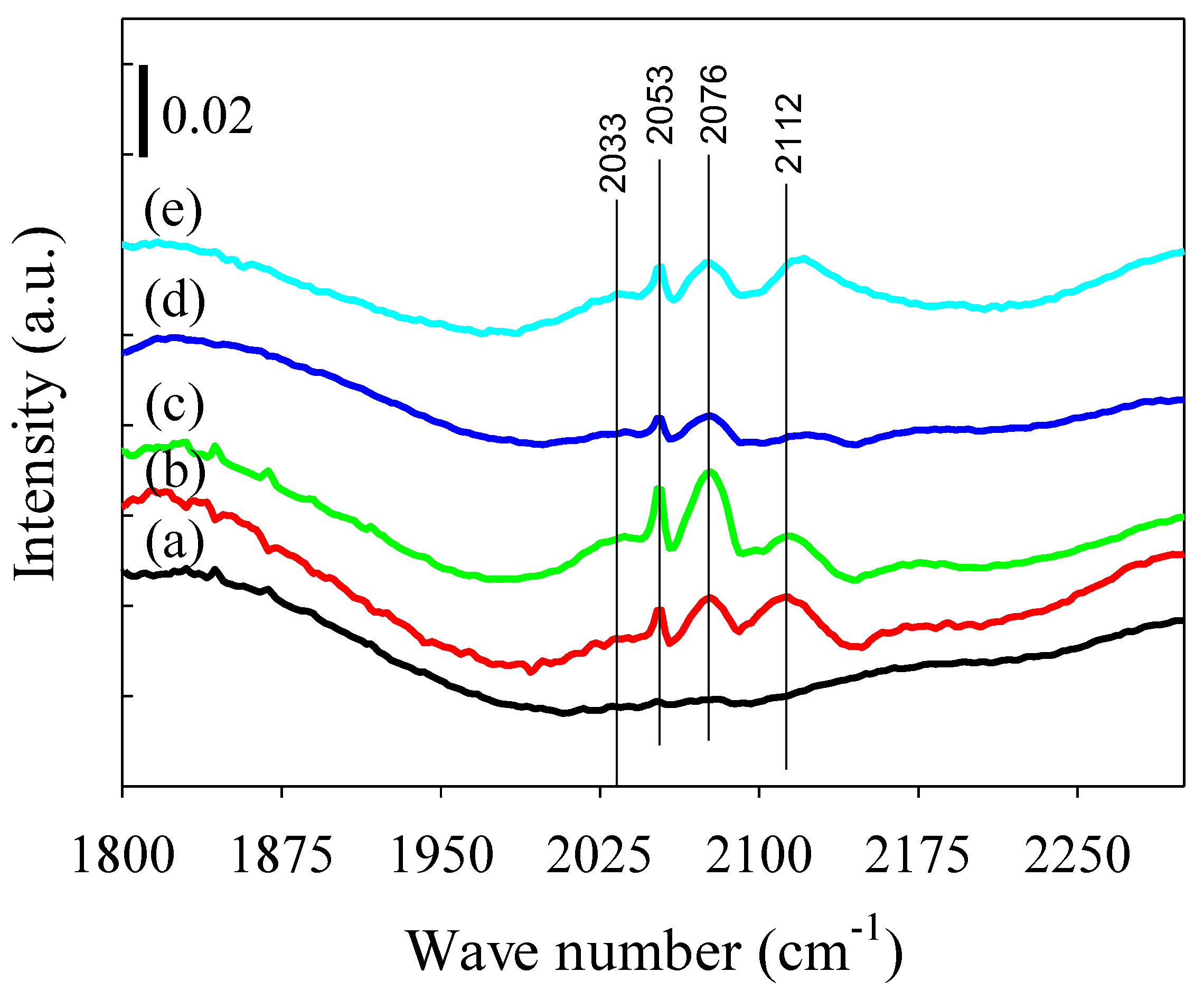
2.2.3. CO-DRIFTS Measurements
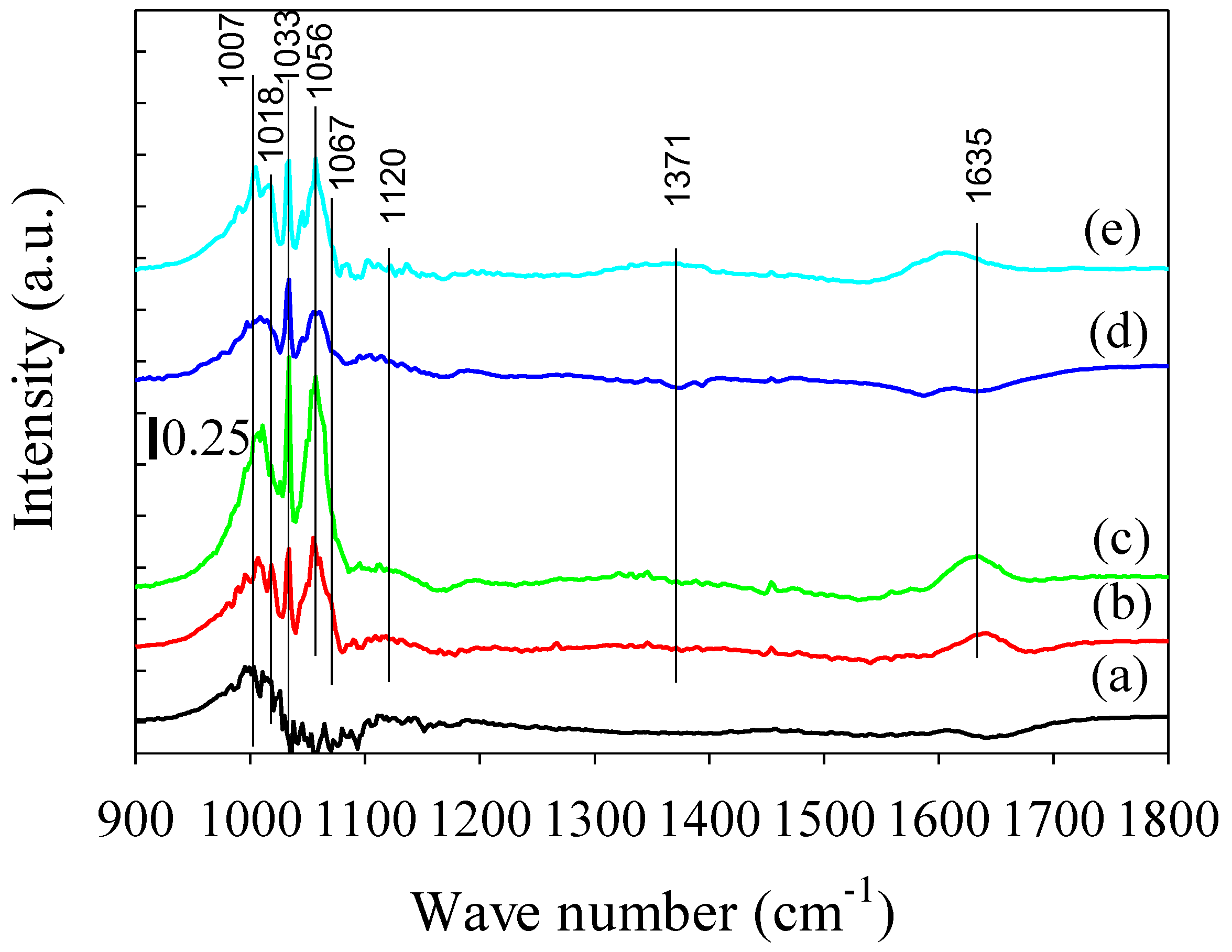
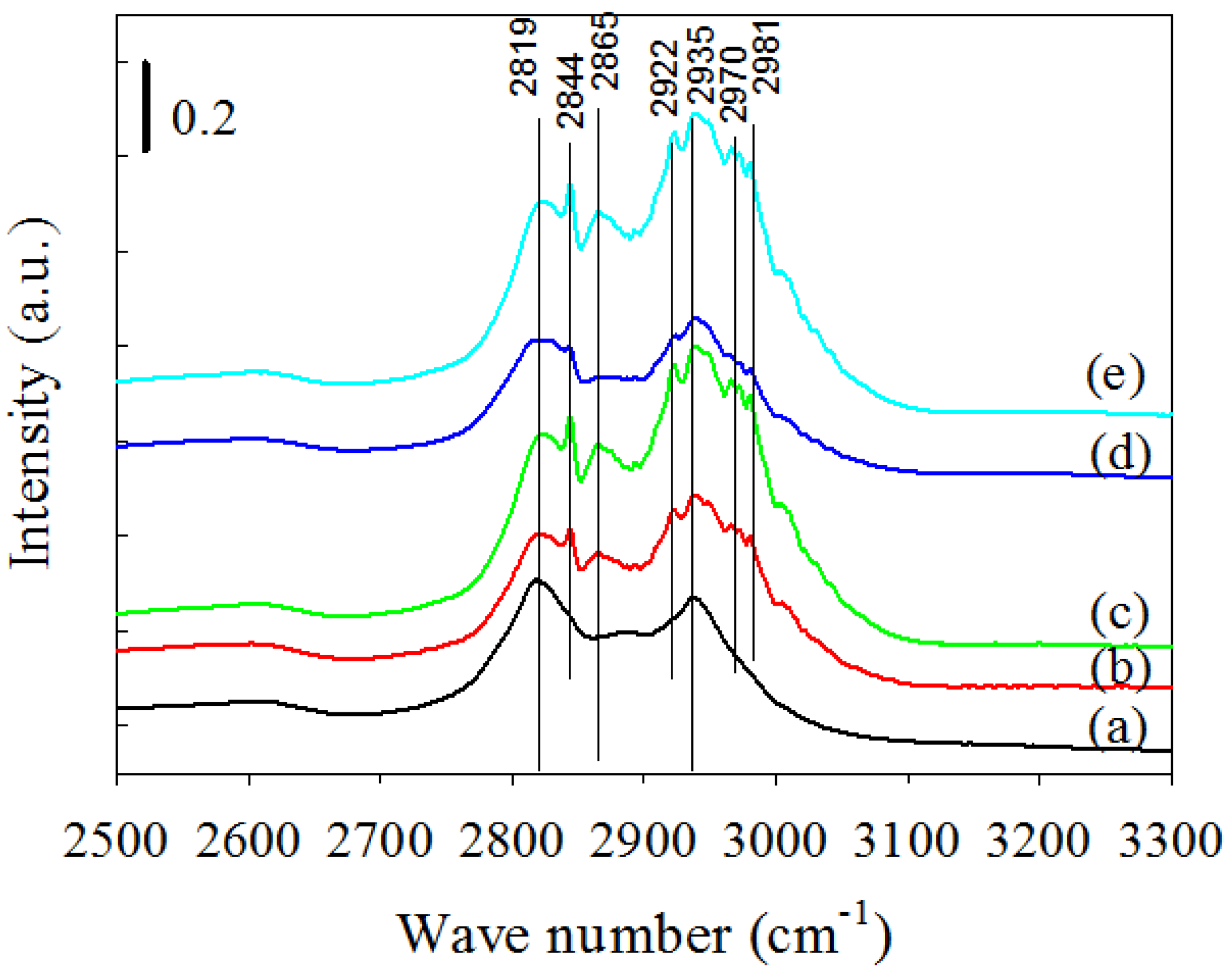
2.2.4. DRIFTS Analysis during Methanol Decomposition
3. Discussion
4. Materials and Methods
4.1. Preparation of Catalysts
4.2. Characterization of Catalysts
4.3. Catalytic Activity Tests
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Park, E.D.; Lee, D.; Lee, H.C. Recent progress in selective CO removal in a H2-rich stream. Catal. Today 2009, 139, 280–290. [Google Scholar] [CrossRef]
- Liu, K.; Wang, A.; Zhang, T. Recent advances in preferential oxidation of CO Reaction over platinum group metal catalysts. ACS Catal. 2012, 2, 1165–1178. [Google Scholar] [CrossRef]
- Torres Sanchez, R.M.; Ueda, A.; Tanaka, K.; Haruta, M. Selective oxidation of CO in hydrogen over gold supported on manganese oxides. J. Catal. 1997, 168, 125–127. [Google Scholar] [CrossRef]
- Okumura, M.; Nakamura, S.; Tsubota, S.; Nakamura, T.; Azuma, M.; Haruta, M. Chemical vapor deposition of gold on Al2O3, SiO2, and TiO2 for the oxidation of CO and of H2. Catal. Lett. 1998, 51, 53–58. [Google Scholar] [CrossRef]
- Bond, G.C.; Thompson, D.T. Catalysis by gold. Catal. Rev. Sci. Eng. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Ko, E.Y.; Park, E.D.; Seo, K.W.; Lee, H.C.; Lee, D.; Kim, S. A comparative study of catalysts for the preferential CO oxidation in excess hydrogen. Catal. Today 2006, 116, 377–383. [Google Scholar] [CrossRef]
- Kandoi, S.; Gokhale, A.A.; Grabow, L.C.; Dumesic, J.A.; Mavrikakis, M. Why Au and Cu are more selective than Pt for preferential oxidation of CO at low temperature. Catal. Lett. 2004, 93, 93–100. [Google Scholar] [CrossRef]
- Bond, G.C.; Louis, C.; Thompson, D.T. Catalysis by Gold; Imperial College Press: London, UK, 2006. [Google Scholar]
- Mohr, C.; Hofmeister, H.; Claus, P. The influence of real structure of gold catalysts in the partial hydrogenation of acrolein. J. Catal. 2003, 213, 86–94. [Google Scholar] [CrossRef]
- Zanella, R.; Louis, C.; Giorgio, S.; Touroude, R. Crotonaldehyde hydrogenation by gold supported on TiO2: Structure sensitivity and mechanism. J. Catal. 2004, 223, 328–339. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Park, J.E.; Park, E.D. Recent advances in preferential oxidation of CO in H2 over gold catalysts. Catal. Surv. Asia 2014, 18, 75–88. [Google Scholar] [CrossRef]
- Grisel, R.J.H.; Nieuwenhuys, B.E. Selective oxidation of CO, over supported Au catalysts J. Catal. 2001, 199, 48–59. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M. Supported gold catalysts for CO oxidation and preferential oxidation of CO in H2 stream: Support effect. Catal. Today 2010, 158, 56–62. [Google Scholar] [CrossRef]
- Quinet, E.; Morfin, F.; Diehl, F.; Avenier, P.; Caps, V.; Rousset, J.L. Hydrogen effect on the preferential oxidation of carbon monoxide over alumina-supported gold nanoparticles. Appl. Catal. B 2008, 80, 195–201. [Google Scholar] [CrossRef]
- Bethke, G.K.; Kung, H.H. Selective CO oxidation in a hydrogen-rich stream over Au/γ-Al2O3 catalysts. Appl. Catal. A 2000, 194, 43–53. [Google Scholar] [CrossRef]
- Quinet, E.; Piccolo, L.; Morfin, F.; Avenier, P.; Diehl, F.; Caps, V.; Rousset, J.L. On the mechanism of hydrogen-promoted gold-catalyzed CO oxidation. J. Catal. 2009, 268, 384–389. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Kustov, L.M. Effects of the support on the morphology and electronic properties of supported metal clusters: Modern concepts and progress in 1990s. Appl. Catal. A 1999, 188, 3–35. [Google Scholar] [CrossRef]
- Mojet, B.L.; Miller, J.T.; Ramaker, D.E.; Koningsberger, D.C. A new model describing the metal–support interaction in noble metal catalysts. J. Catal. 1999, 186, 373–386. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 17, 3393–3403. [Google Scholar] [CrossRef]
- Boronat, M.; Illas, F.; Corma, A. Active sites for H2 adsorption and activation in Au/TiO2 and the role of the support. J. Phys. Chem. A 2009, 113, 3750. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Q.; Qiao, B.; Huang, Y.; Li, L.; Lin, J.; Liu, X.Y.; Wang, A.; Li, W.C.; Zhang, T. La-doped Al2O3 supported Au nanoparticles: highly active and selective catalysts for PROX under PEMFC operation conditions. Chem. Commun. 2014, 50, 2721–2725. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, P.; Park, J.E.; Kim, B.; Park, E.D. Preferential oxidation of CO in a hydrogen-rich stream over Au/MOx/Al2O3 (M=La, Ce, and Mg) catalysts. Catal. Today 2016, 265, 19–26. [Google Scholar] [CrossRef]
- Hugon, A.; El Kolli, N.; Louis, C. Advances in the preparation of supported gold catalysts: Mechanism of deposition, simplification of the procedures and relevance of the elimination of chlorine. J. Catal. 2010, 274, 239–250. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Upare, P.P.; Le, N.T.; Hwang, Y.K.; Hwang, D.W.; Lee, U.H.; Kim, H.R.; Chang, J.S. Facile synthesis of CeO2-supported gold nanoparticle catalysts for selective oxidation of glycerol into lactic acid. Appl. Catal. A 2013, 468, 260–268. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; Maciejewski, M.; Becker, O.S.; Fabrizioli, P.; Baiker, A. Comparative study of Au/TiO2 and Au/ZrO2 catalysts for low temperature CO Oxidation. J. Catal. 1999, 186, 458–469. [Google Scholar] [CrossRef]
- Centeno, M.A.; Paulis, M.; Montes, M.; Odriozola, J.A. Catalytic combustion of volatile organic compounds on Au/CeO2/Al2O3 and Au/Al2O3 catalysts. Appl. Catal. A 2002, 234, 65–78. [Google Scholar] [CrossRef]
- Ilieva, L.; Pantaleo, G.; Sobczak, J.W.; Ivanov, I.; Venezia, A.M.; Andreeva, D. NO reduction by CO in the presence of water over gold supported catalysts on CeO2-Al2O3 mixed support, prepared by mechanochemical activation. Appl. Catal B 2007, 76, 107–114. [Google Scholar] [CrossRef]
- Delannoy, L.; Fajerwerg, K.; Lakshmanan, P.; Potvin, C.; Methivier, C.; Louis, C. Supported gold catalysts for the decomposition of VOC: Total oxidation of propene in low concentration as model reaction. Appl. Catal. B 2010, 94, 117–124. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M. FTIR study of the electronic effects of CO adsorbed on gold nanoparticles supported on titania. Surf. Sci. 2000, 454, 942–946. [Google Scholar] [CrossRef]
- Hadjiivanov, K.J.; Vayssilov, G.N. Advances in Catalysis; Academic Press: New York, NY, USA, 2002. [Google Scholar]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzoli, M.; Andreeva, D.; Tabakova, T. FTIR study of the low-temperature water–gas shift reaction on Au/Fe2O3 and Au/TiO2 Catalysts. J. Catal. 1999, 188, 176–185. [Google Scholar] [CrossRef]
- Frank, B.; Jentoft, F.C.; Soerijanto, H.; Kröhnert, J.; Schlögl, R.; Schomäcker, R. Steam reforming of methanol over copper-containing catalysts: Influence of support material on microkinetics. J. Catal. 2007, 246, 177–192. [Google Scholar] [CrossRef]
- Matter, P.H.; Ozkan, U.S. Effect of pretreatment conditions on Cu/Zn/Zr-based catalysts for the steam reforming of methanol to H2. J. Catal. 2005, 234, 463–475. [Google Scholar] [CrossRef]
- Jacobs, G.; Davis, B.H. In situ DRIFTS investigation of the steam reforming of methanol over Pt/ceria. Appl. Catal. A. Gen. 2005, 285, 43–49. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Tabakova, T.; Boccuzzi, F.; Manzoli, M.; Andreeva, D. FTIR study of low-temperature water-gas shift reaction on gold/ceria catalyst. Appl. Catal. A 2003, 252, 385. [Google Scholar] [CrossRef]
- Date, M.; Imai, H.; Tsubota, S.; Haruta, M. In situ measurements under flow condition of the CO oxidation over supported gold nanoparticles. Catal. Today 2007, 122, 222–225. [Google Scholar] [CrossRef]
- Wu, N.; Fu, L.; Su, M.; Aslam, M.; Chun Wong, K.; Dravid, V.P. Interaction of Fatty Acid Monolayers with Cobalt Nanoparticles. Nano Lett. 2004, 4, 383–386. [Google Scholar] [CrossRef]
- Nor Hidawati, E.; Mimi Sakinah, A.M. Treatment of glycerin pitch from biodiesel production. Int. J. Chem. Environ. Eng. 2011, 2, 309–313. [Google Scholar]
- Kubelkova, L.; Cejka, J.; Novakova, J. Surface reactivity of ZSM-5 zeolites in interaction with ketones at ambient temperature (a FT-i.r. study). Zeolites 1991, 11, 48–53. [Google Scholar] [CrossRef]
- Yoda, E.; Ootawa, A. Dehydration of glycerol on H-MFI zeolite investigated by FT-IR. Appl. Catal. A 2009, 360, 66–70. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Campisi, S.; Wang, D.; Prati, L.; Villa, A. Selective oxidation of raw glycerol using supported AuPd nanoparticles. Catalysts 2015, 5, 131–144. [Google Scholar] [CrossRef]
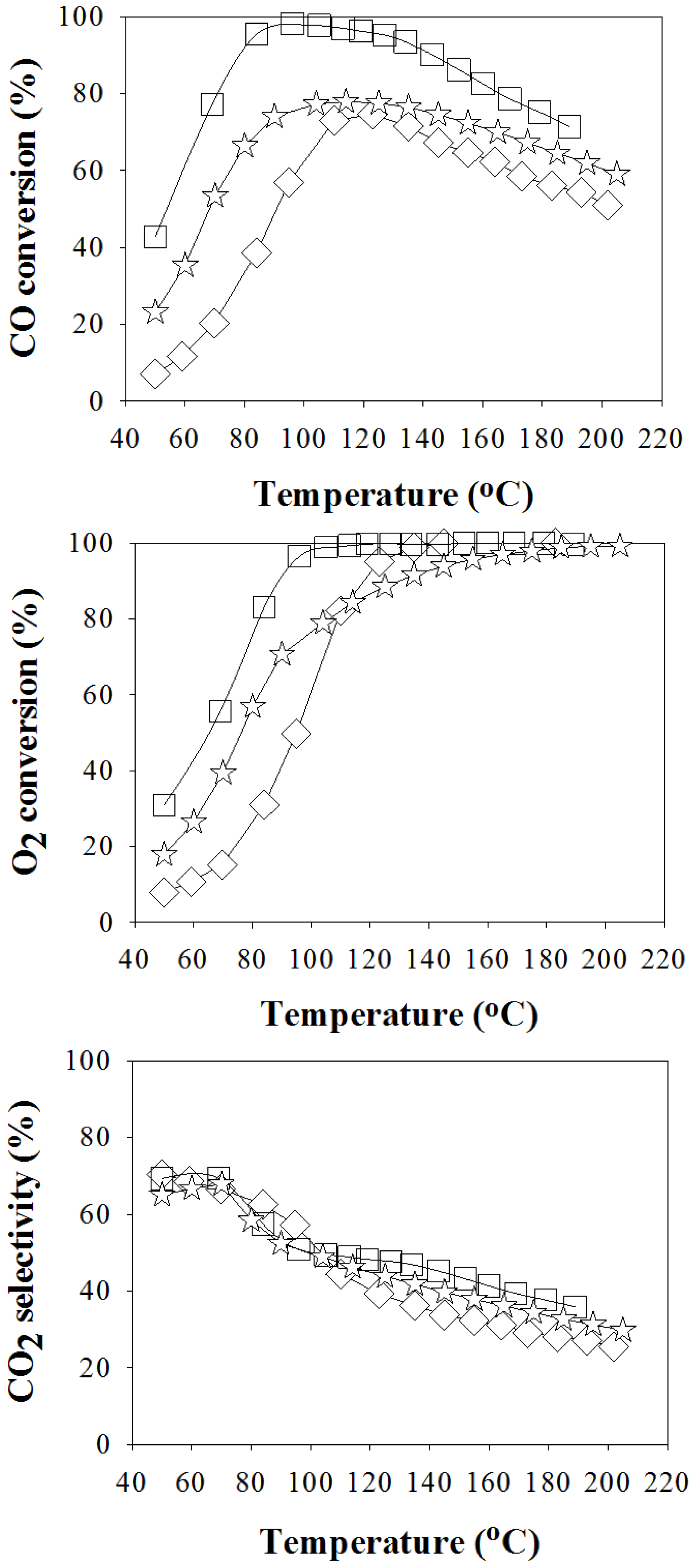
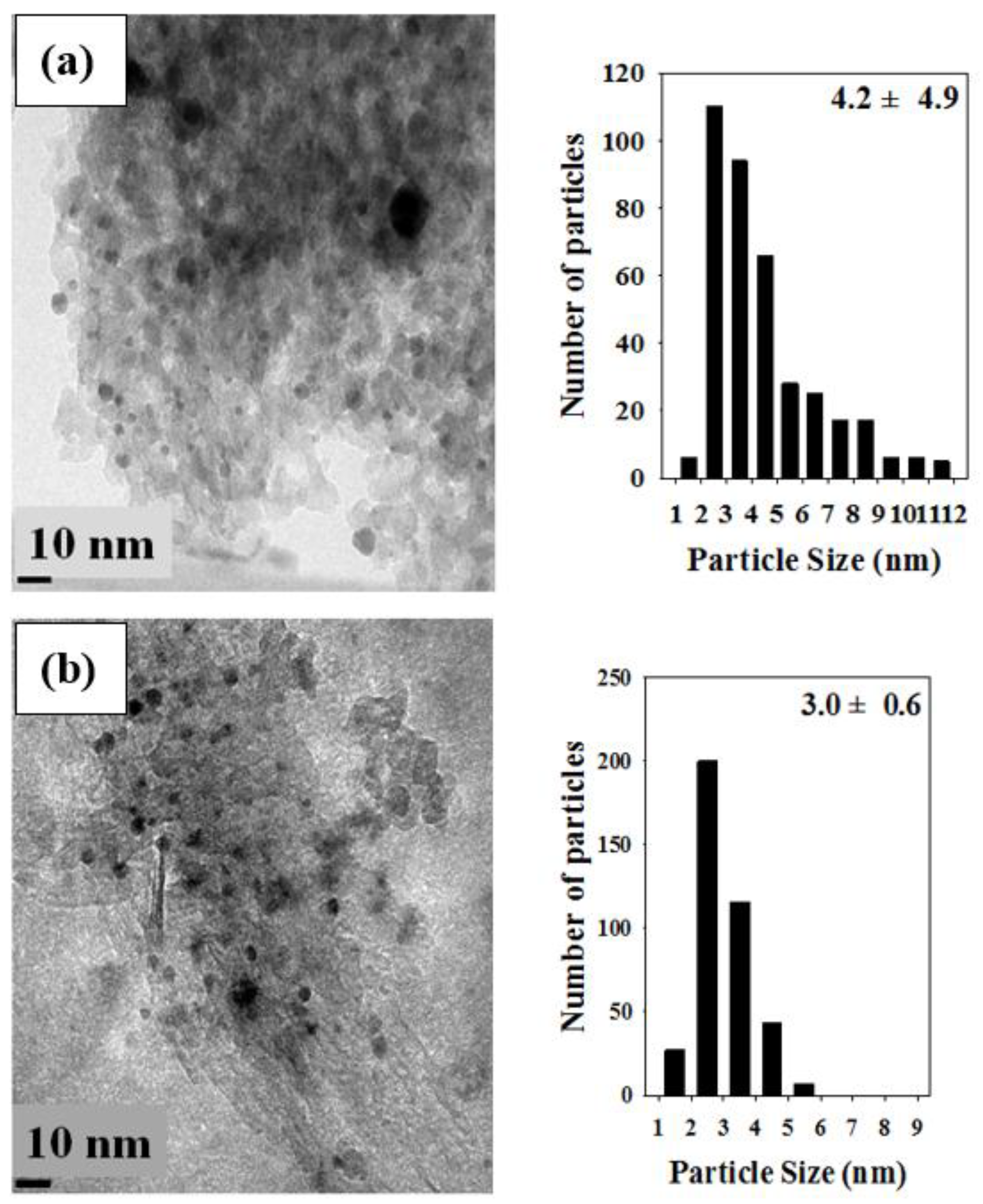
| Catalyst | Reducing Agents a | Average Particle Size of Gold (nm) b | Temperatures Achieving CO Conversions Higher than 97% (°C) c | Reference |
|---|---|---|---|---|
| Au/La2O3/Al2O3 (H) | H2 | 2.9 ± 0.7 | 61 | [22] |
| Au/La2O3/Al2O3 (S5) | NaBH4 (5) + H2 | 2.2 ± 0.2 | 61–74 | [22] |
| Au/La2O3/Al2O3 (S35) | NaBH4 (35) + H2 | 4.2 ± 4.9 | 95–115 | In this study |
| Au/La2O3/Al2O3 (S115) | NaBH4 (115) + H2 | n.d. | - | In this study |
| Au/La2O3/Al2O3 (G) | Glycerol + H2 | 3.0 ± 0.6 | - | In this study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakshmanan, P.; Park, E.D. Preferential CO Oxidation in H2 over Au/La2O3/Al2O3 Catalysts: The Effect of the Catalyst Reduction Method. Catalysts 2018, 8, 183. https://doi.org/10.3390/catal8050183
Lakshmanan P, Park ED. Preferential CO Oxidation in H2 over Au/La2O3/Al2O3 Catalysts: The Effect of the Catalyst Reduction Method. Catalysts. 2018; 8(5):183. https://doi.org/10.3390/catal8050183
Chicago/Turabian StyleLakshmanan, Pandian, and Eun Duck Park. 2018. "Preferential CO Oxidation in H2 over Au/La2O3/Al2O3 Catalysts: The Effect of the Catalyst Reduction Method" Catalysts 8, no. 5: 183. https://doi.org/10.3390/catal8050183