Investigation of Iron Vanadates for Simultaneous Carbon Soot Abatement and NH3-SCR
Abstract
:1. Introduction
2. Results and Discussion
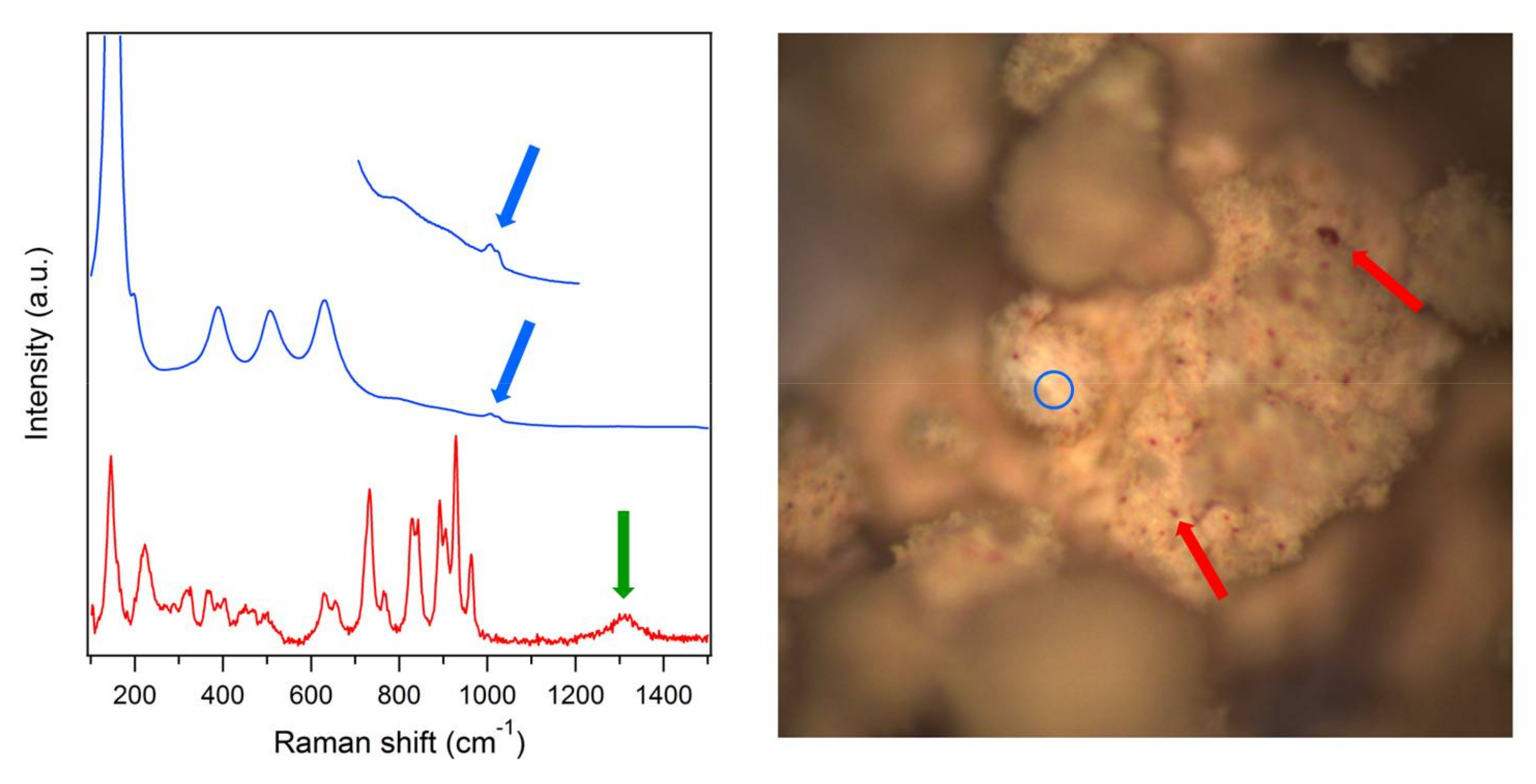
2.1. Catalysts Characterization
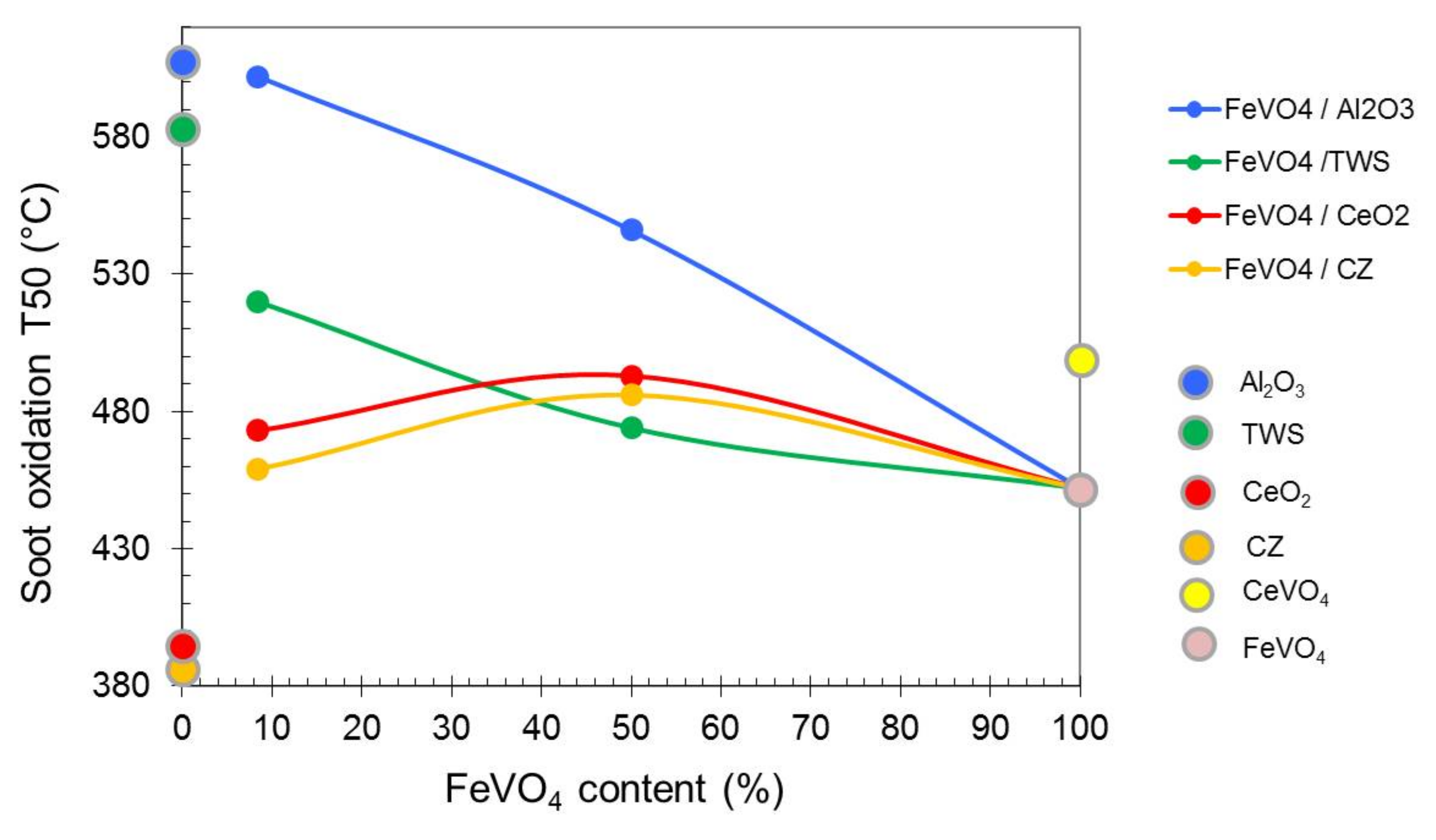
2.2. Soot Oxidation Activity
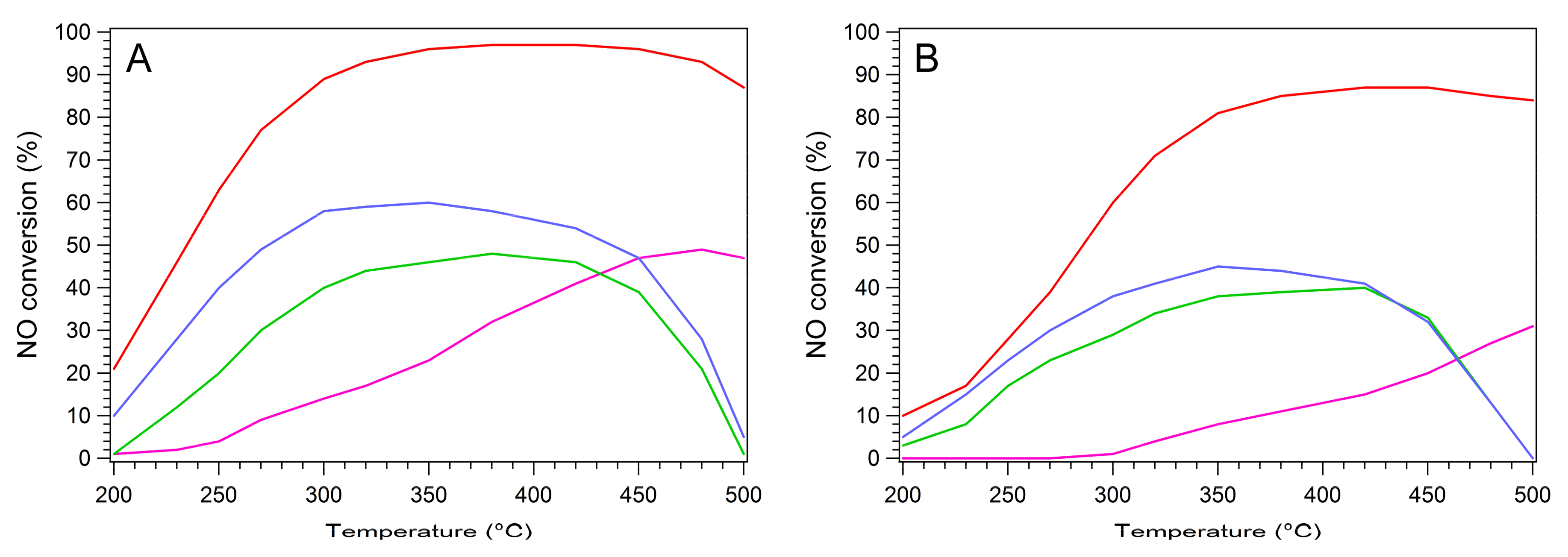
2.3. NH3-SCR Activity
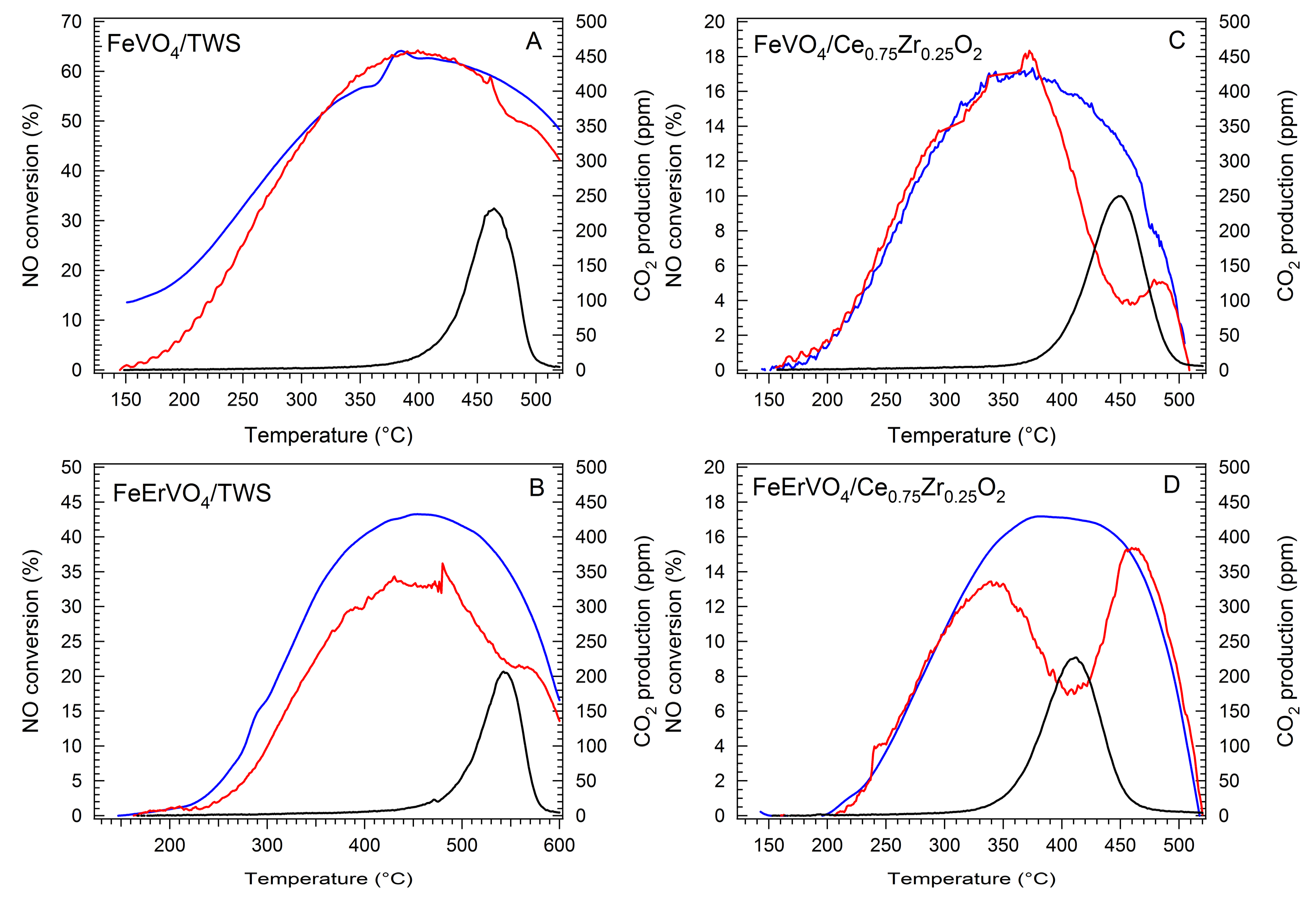
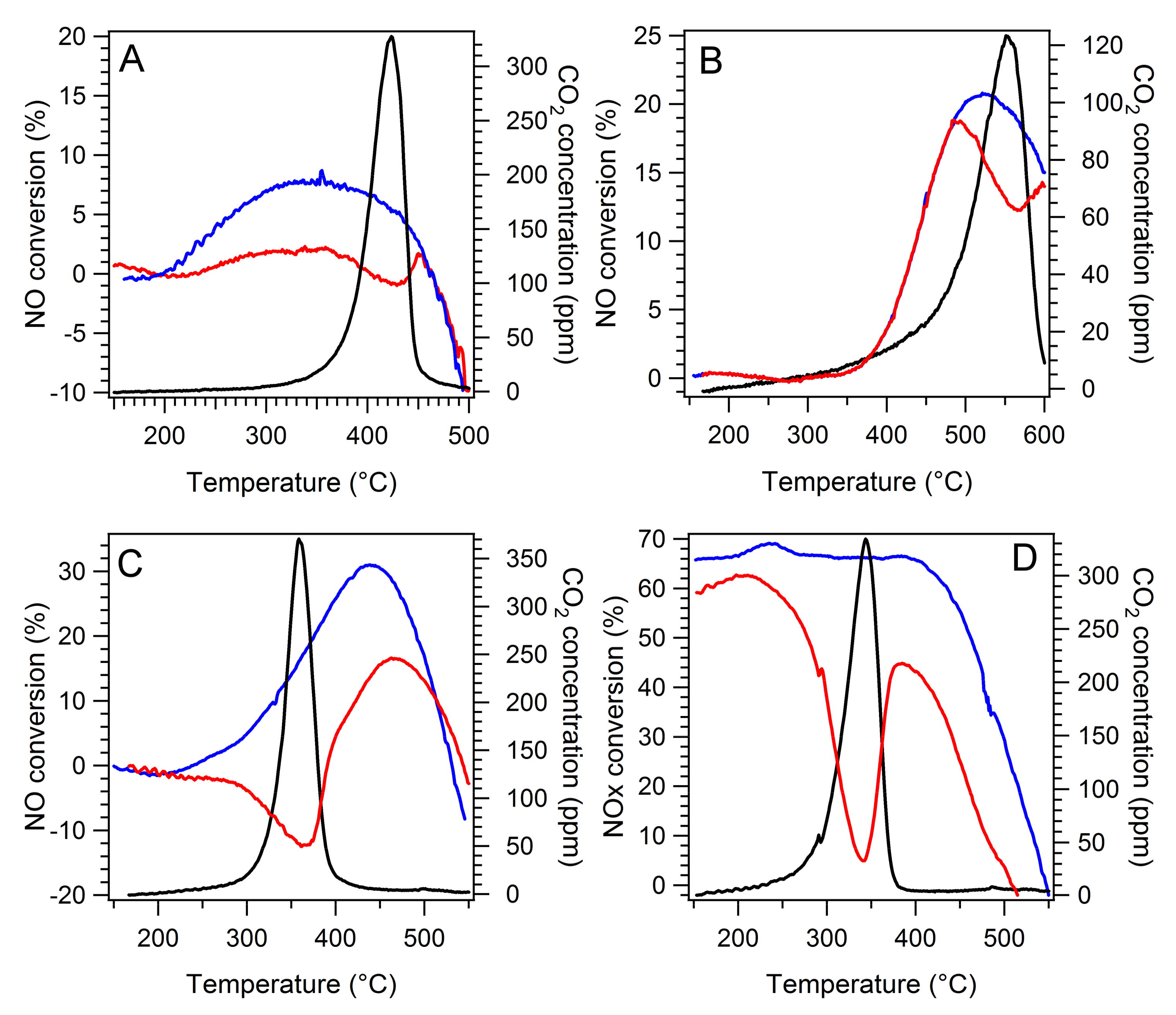
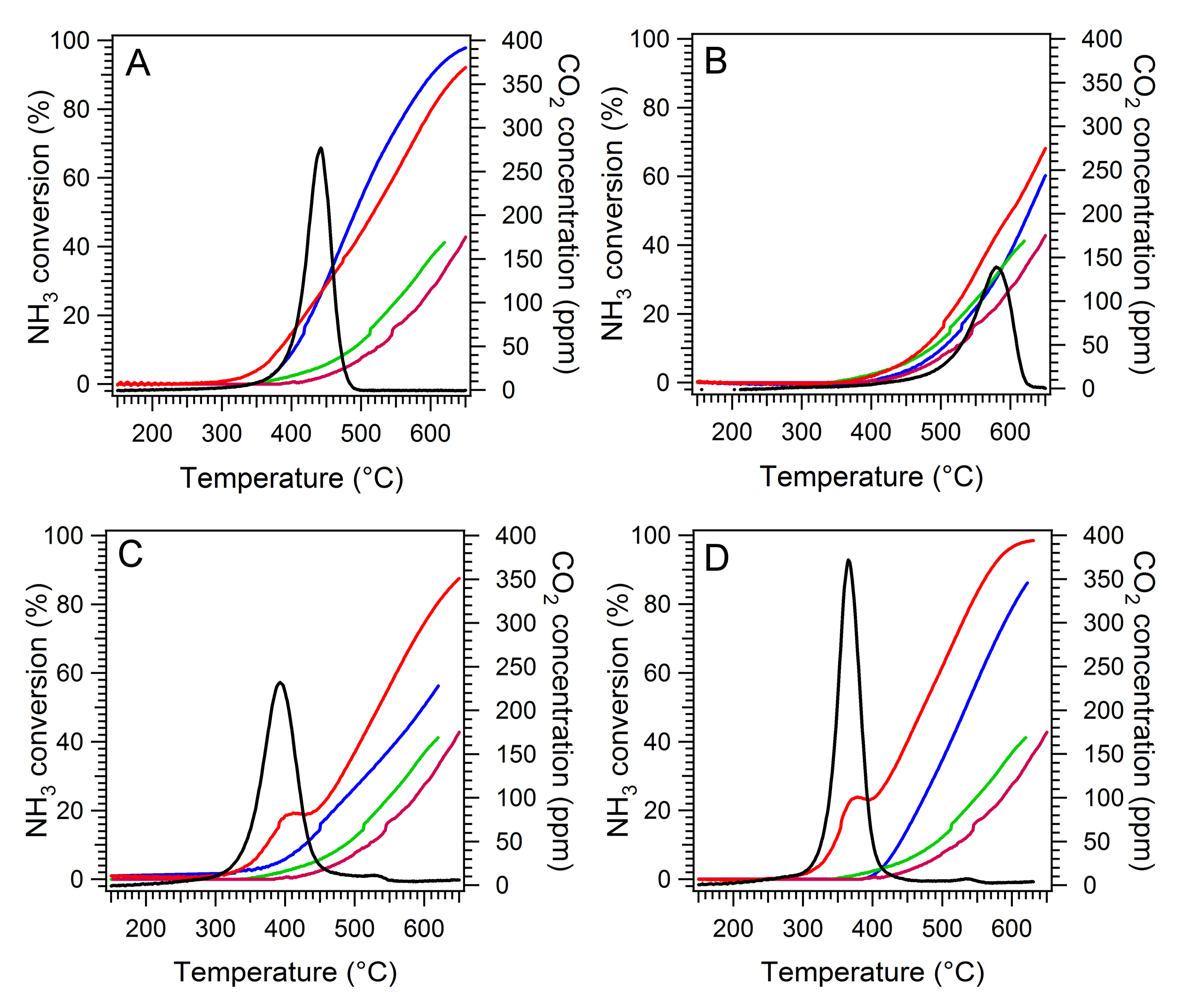
2.4. Simultaneous NH3-SCR and Soot Oxidation Activity
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalytic Tests
3.3. Characterization of the Catalysts
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bassou, B.; Guilhaume, N.; Iojoiu, E.E.; Farrusseng, D.; Lombaert, K.; Bianchi, D.; Mirodatos, C. High-throughput approach to the catalytic combustion of diesel soot II: Screening of oxide-based catalysts. Catal. Today 2011, 159, 138–143. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx abatement systems for mobile sources: From three-way to lean burn after-treatment technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Woo, S.I. Recent advances in catalytic deNO(x) science and technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Brandenberger, S.; Krocher, O.; Tissler, A.; Althoff, R. The state of the art in selective catalytic reduction of NOx by ammonia using metal-exchanged zeolite catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Boger, T. Integration of SCR functionality into diesel particulate filters. In Urea-Scr Technology for Denox after Treatment of Diesel Exhausts; Nova, I., Tronconi, E., Eds.; Springer: New York, NY, USA, 2014; pp. 623–655. [Google Scholar]
- Johnson, T.V. Review of selective catalytic reduction (SCR) and related technologies for mobile applications. In Urea-SCR Technology for deNOx after Treatment of Diesel Exhausts; Nova, I., Tronconi, E., Eds.; Springer: New York, NY, USA, 2014; pp. 3–31. [Google Scholar]
- Weibel, M.; Waldbusser, N.; Wunsch, R.; Chatterjee, D.; Bandl-Konrad, B.; Krutzsch, B. A novel approach to catalysis for NOx reduction in diesel exhaust gas. Top. Catal. 2009, 52, 1702–1708. [Google Scholar] [CrossRef]
- Watling, T.C.; Ravenscroft, M.R.; Avery, G. Development, validation and application of a model for an SCR catalyst coated diesel particulate filter. Catal. Today 2012, 188, 32–41. [Google Scholar] [CrossRef]
- Song, X.; Johnson, J.H.; Naber, J.D. A review of the literature of selective catalytic reduction catalysts integrated into diesel particulate filters. Int. J. Engine Res. 2015, 16, 738–749. [Google Scholar] [CrossRef]
- Cavataio, G.; Warner, J.R.; Girard, J.W.; Ura, J.; Dobson, D.; Lambert, C.K. Laboratory study of soot, propylene and diesel fuel impact on zeolite-based SCR filter catalysts. SAE Int. J. Fuels Lubr. 2009, 2, 342–368. [Google Scholar] [CrossRef]
- Casanova, M.; Schermanz, K.; Llorca, J.; Trovarelli, A. Improved high temperature stability of NH3-SCR catalysts based on rare earth vanadates supported on TiO2-WO3-SiO2. Catal. Today 2012, 184, 227–236. [Google Scholar] [CrossRef]
- Vargas, M.A.L.; Casanova, M.; Trovarelli, A.; Busca, G. An IR study of thermally stable V2O5-WO3-TiO2 SCR catalysts modified with silica and rare-earths (Ce, Tb, Er). Appl. Catal. B Environ. 2007, 75, 303–311. [Google Scholar] [CrossRef]
- Casanova, M.; Llorca, J.; Sagar, A.; Schermanz, K.; Trovarelli, A. Mixed iron-erbium vanadate NH3-SCR catalysts. Catal. Today 2015, 241, 159–168. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Sagar, A.; Schermanz, K.; Krocher, O. Generation of NH3 selective catalytic reduction active catalysts from decomposition of supported FeVO4. ACS Catal. 2015, 5, 4180–4188. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Lian, Z.; Shan, W.; Xie, L.; Asakura, K.; Yang, W.; Deng, H. Highly dispersed iron vanadate catalyst supported on TiO2 for the selective catalytic reduction of NOx with NH3. J. Catal. 2013, 307, 340–351. [Google Scholar] [CrossRef]
- Gillot, S.; Tricot, G.; Vezin, H.; Dacquin, J.-P.; Dujardin, C.; Granger, P. Development of stable and efficient CeVO4 systems for the selective reduction of NOx by ammonia: Structure-activity relationship. Appl. Catal. B Environ. 2017, 218, 338–348. [Google Scholar] [CrossRef]
- Bueno-Lopez, A. Diesel soot combustion ceria catalysts. Appl. Catal. B Environ. 2014, 146, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Casanova, M.; Nodari, L.; Sagar, A.; Schermanz, K.; Trovarelli, A. Preparation, characterization and NH3-SCR activity of FeVO4 supported on TiO2-WO3-SiO2. Appl. Catal. B Environ. 2015, 176, 699–708. [Google Scholar] [CrossRef]
- Wu, Z.L.; Rondinone, A.J.; Ivanov, I.N.; Overbury, S.H. Structure of vanadium oxide supported on ceria by multiwavelength raman spectroscopy. J. Phys. Chem. C 2011, 115, 25368–25378. [Google Scholar] [CrossRef]
- Went, G.T.; Leu, L.J.; Bell, A.T. Quantitative structural-analysis of dispersed vanadia species in TiO2(anatase)-supported V2O5. J. Catal. 1992, 134, 479–491. [Google Scholar] [CrossRef]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- Haber, J.; Machej, T.; Serwicka, E.M.; Wachs, I.E. Mechanism of surface spreading in vanadia-titania system. Catal. Lett. 1995, 32, 101–114. [Google Scholar] [CrossRef]
- Gaur, K.; Lal, H.B. Electrical transport in heavy rare-earth vanadates. J. Mater. Sci. 1986, 21, 2289–2296. [Google Scholar] [CrossRef]
- Casanova, M.; Sagar, A.; Schermanz, K.; Trovarelli, A. Enhanced stability of Fe2O3-doped FeVO4/TiO2-WO3-SiO2 SCR catalysts. Top. Catal. 2016, 59, 996–1001. [Google Scholar] [CrossRef]
- Vermaire, D.C.; Vanberge, P.C. The preparation of WO3/TiO2 and WO3/Al2O3 and characterization by temperature-programmed reduction. J. Catal. 1989, 116, 309–317. [Google Scholar] [CrossRef]
- Giordano, F.; Trovarelli, A.; de Leitenburg, C.; Giona, M. A model for the temperature-programmed reduction of low and high surface area ceria. J. Catal. 2000, 193, 273–282. [Google Scholar] [CrossRef]
- Daturi, M.; Finocchio, E.; Binet, C.; Lavalley, J.-C.; Fally, F.; Perrichon, V.; Vidal, H.; Hickey, N.; Kašpar, J. Reduction of high surface area CeO2−ZrO2 mixed oxides. J. Phys. Chem. B 2000, 104, 9186–9194. [Google Scholar] [CrossRef]
- Gallert, T.; Casanova, M.; Puzzo, F.; Strazzolini, P.; Trovarelli, A. SO2 resistant soot oxidation catalysts based on orthovanadates. Catal. Commun. 2017, 97, 120–124. [Google Scholar] [CrossRef]
- Machida, M.; Murata, Y.; Kishikawa, K.; Zhang, D.J.; Ikeue, K. On the reasons for high activity of CeO2 catalyst for soot oxidation. Chem. Mater. 2008, 20, 4489–4494. [Google Scholar] [CrossRef]
- Soler, L.; Casanovas, A.; Escudero, C.; Pérez-Dieste, V.; Aneggi, E.; Trovarelli, A.; Llorca, J. Ambient pressure photoemission spectroscopy reveals the mechanism of carbon soot oxidation in ceria-based catalysts. ChemCatChem 2016, 8, 2748–2751. [Google Scholar] [CrossRef]
- Zouaoui, N.; Issa, M.; Kehrli, D.; Jeguirim, M. CeO2 catalytic activity for soot oxidation under NO/O2 in loose and tight contact. Catal. Today 2012, 189, 65–69. [Google Scholar] [CrossRef]
- Tronconi, E.; Nova, I.; Marchitti, F.; Koltsakis, G.; Karamitros, D.; Maletic, B.; Markert, N.; Chatterjee, D.; Hehle, M. Interaction of NOx reduction and soot oxidation in a DPF with Cu-Zeolite SCR coating. Emiss. Control Sci. Technol. 2015, 1, 134–151. [Google Scholar] [CrossRef]
- Mehring, M.; Elsener, M.; Kröcher, O. Selective catalytic reduction of NOx with ammonia over soot. ACS Catal. 2012, 2, 1507–1518. [Google Scholar] [CrossRef]
 TiO2-anatase,
TiO2-anatase,  Fe2O3,
Fe2O3,  CeO2 or CeZrO2, ▼ CeVO4,
CeO2 or CeZrO2, ▼ CeVO4,  ErVO4,
ErVO4,  Al2O3.
Al2O3.
 TiO2-anatase,
TiO2-anatase,  Fe2O3,
Fe2O3,  CeO2 or CeZrO2, ▼ CeVO4,
CeO2 or CeZrO2, ▼ CeVO4,  ErVO4,
ErVO4,  Al2O3.
Al2O3.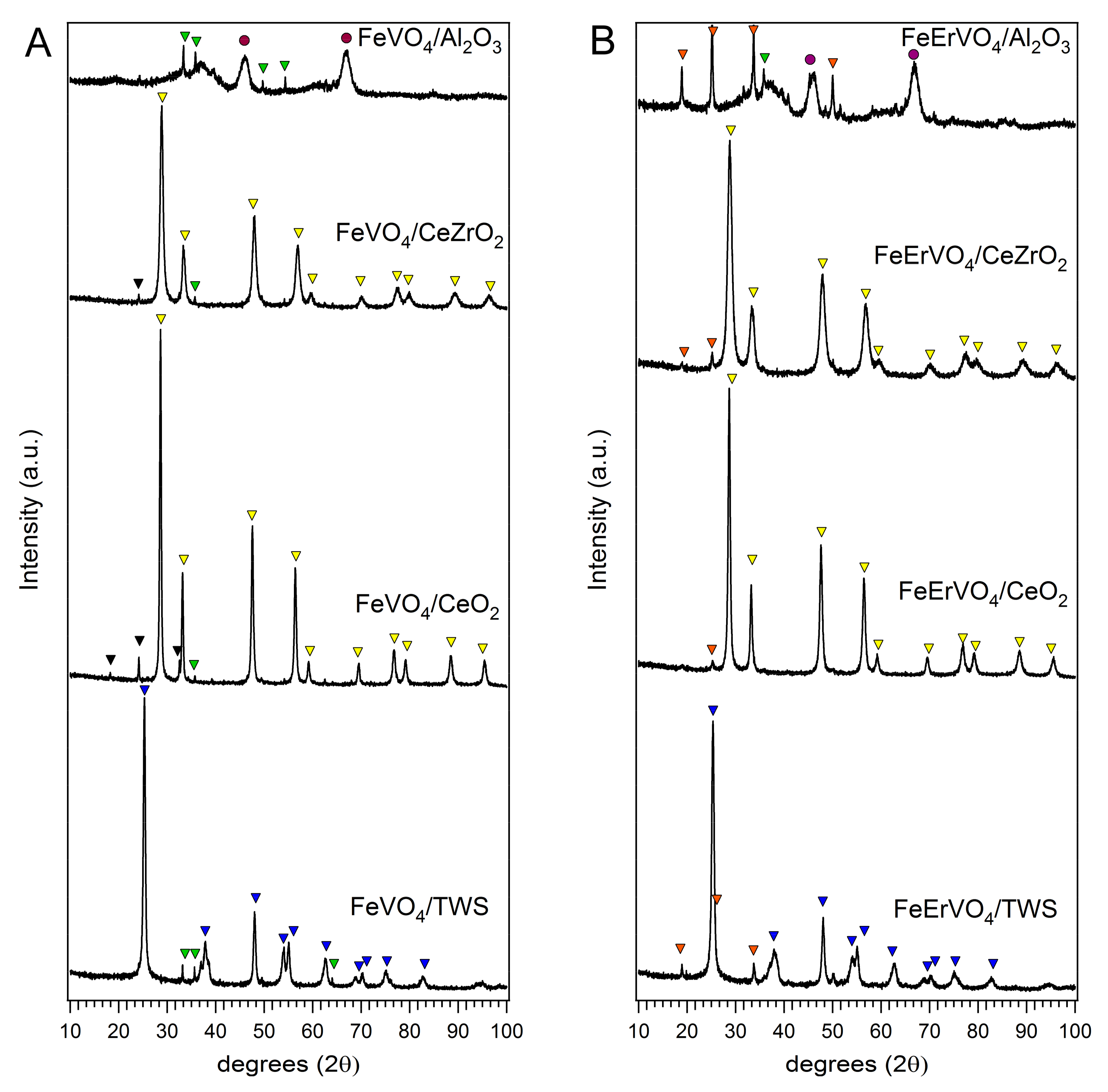
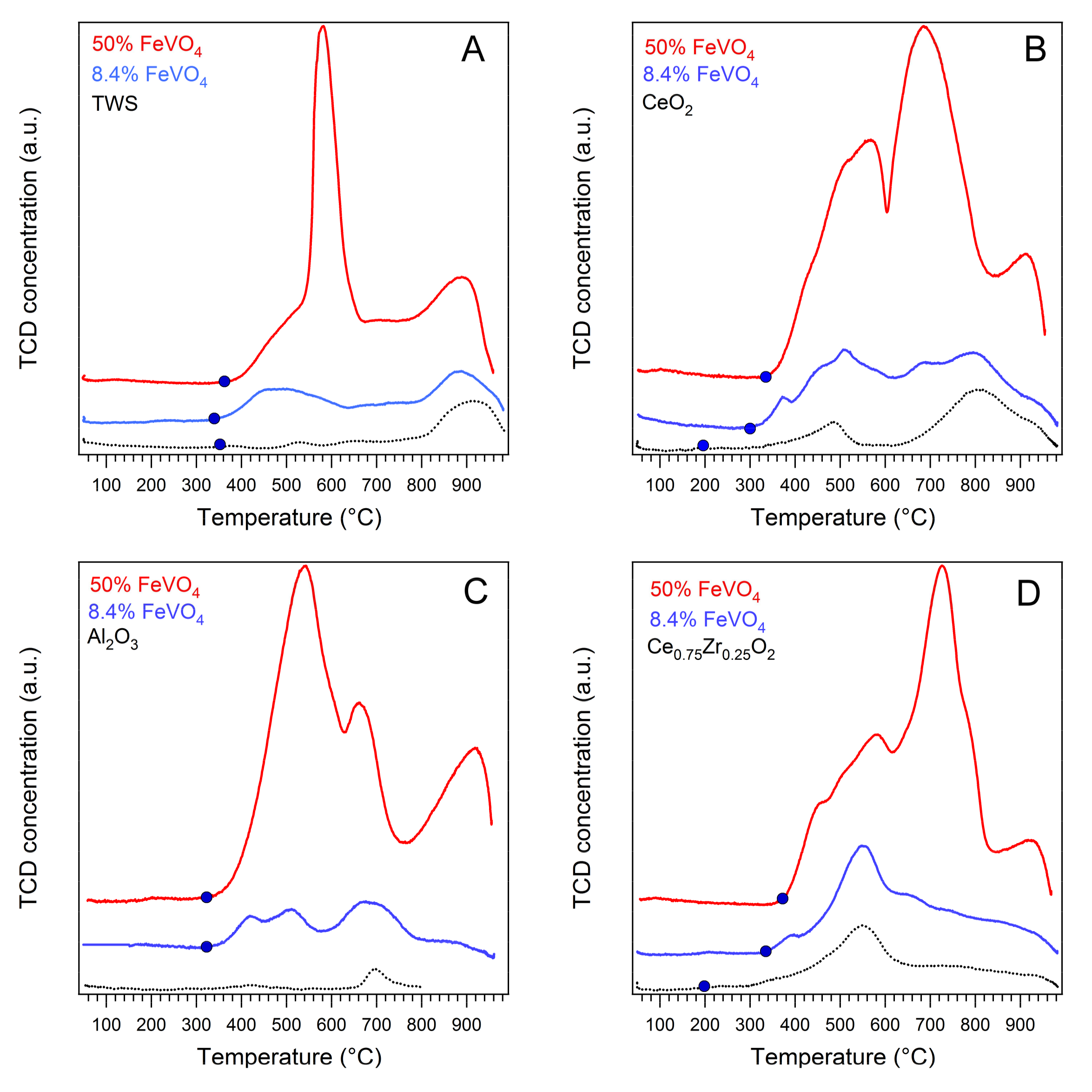
| Catalyst | B.E.T. (m2/g) | H2 Consumption (mmol/gcat) | H/V Atomic Ratio 1 | Onset of Reduction (°C) |
|---|---|---|---|---|
| TWS | 89 | 0.953 | / | 350 |
| 8.4% FeVO4/TWS | 68 | 2.184 | 8.88 | 344 |
| 50% FeVO4/TWS | 21 | 5.786 | 3.95 | 361 |
| CeO2 | 26 | 0.997 | / | 190 |
| 8.4% FeVO4/CeO2 | 12 | 1.926 | 7.83 | 285 |
| 50% FeVO4/CeO2 | 6 | 6.379 | 4.36 | 338 |
| Ce0.5Zr0.5O2 (CZ) | 52 | 1.404 | / | 186 |
| 8.4% FeVO4/CZ | 24 | 2.448 | 9.95 | 320 |
| 50% FeVO4/CZ | 8 | 6.694 | 4.57 | 348 |
| Al2O3 | 168 | 0.080 | / | / |
| 8.4% FeVO4/Al2O3 | 166 | 1.022 | 4.15 | 320 |
| 50% FeVO4/Al2O3 | 89 | 5.240 | 3.58 | 320 |
| Catalyst | T50 (°C) 1 air | Tp (°C) 2 NO/O2/N2 | Tp (°C) 3 NO/O2/N2/NH3 |
|---|---|---|---|
| 8.4% FeVO4/TWS | 520 | 490 | 480 |
| 8.4% Fe0.5Er0.5VO4/TWS | 567 | 548 | 541 |
| 8.4% FeVO4/CeO2 | 473 | 459 | 462 |
| 8.4% Fe0.5Er0.5VO4/CeO2 | 460 | 448 | 440 |
| 8.4% FeVO4/CZ | 459 | 459 | 447 |
| 8.4% Fe0.5Er0.5VO4/CZ | 423 | 419 | 411 |
| 8.4% FeVO4/Al2O3 | 602 | - | - |
| 8.4% Fe0.5Er0.5VO4/Al2O3 | 603 | - | - |
| FeVO4 | 450 | - | - |
| Fe0.5Er0.5VO4 | 450 | - | - |
| TWS | 580 | - | - |
| 50% FeVO4/TWS | 474 | - | - |
| 50% Fe0.5Er0.5VO4/TWS | 449 | - | - |
| CeO2 | 390 | - | - |
| 50% FeVO4/CeO2 | 493 | - | - |
| 50% Fe0.5Er0.5VO4/CeO2 | 493 | - | - |
| CeVO4 | 495 | - | - |
| Ce0.75Zr0.25O2 (CZ) | 385 | - | - |
| 50% FeVO4/CZ | 486 | - | - |
| 50% Fe0.5Er0.5VO4/CZ | 488 | - | - |
| Al2O3 | 612 | - | - |
| 50% FeVO4/Al2O3 | 546 | - | - |
| 50% Fe0.5Er0.5VO4/Al2O3 | 505 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, M.; Colussi, S.; Trovarelli, A. Investigation of Iron Vanadates for Simultaneous Carbon Soot Abatement and NH3-SCR. Catalysts 2018, 8, 130. https://doi.org/10.3390/catal8040130
Casanova M, Colussi S, Trovarelli A. Investigation of Iron Vanadates for Simultaneous Carbon Soot Abatement and NH3-SCR. Catalysts. 2018; 8(4):130. https://doi.org/10.3390/catal8040130
Chicago/Turabian StyleCasanova, Marzia, Sara Colussi, and Alessandro Trovarelli. 2018. "Investigation of Iron Vanadates for Simultaneous Carbon Soot Abatement and NH3-SCR" Catalysts 8, no. 4: 130. https://doi.org/10.3390/catal8040130





